Cancer is diagnosed in 1 per 1,000 to 2,000 pregnancies, and is likely to increase with childbearing being delayed until higher maternal age in developed countries (1,2). Pregnancy does not predispose to cancer and cancers occurring during pregnancy are those typical of women of reproductive age. The most frequently encountered cancers during pregnancy include breast, cervical, hematologic malignancies, and melanoma (2,3). Lung and gastric cancers are less frequent, but are associated with a worse prognosis (4,5). Cancer diagnosis and treatment during pregnancy is a challenge for the obstetrician, the oncologist, and the perinatologist. Treatment decisions must take into account maternal and fetal concerns. Whether or not to terminate the pregnancy is an individual decision and depends on the parental opinion, the oncologic prognosis, and the urgency of cancer treatment initiation.
Diagnosis
Time between symptoms and cancer diagnosis may be delayed by physiologic gestational symptoms (6,7). Pregnant patients with fatigue, anemia, vaginal bleeding, vaginal discharge, or breast lumps deserve a careful clinical examination. Complaints of pregnant patients are not necessarily physiologic, and careful examination and further investigation are mandatory. In the absence of symptoms, palpation of regional lymph nodes or breasts, and visualization and Pap smear of the cervix may help in early detection.
The maternal prognosis per stage does not seem to be worse during pregnancy. Historical data are underpowered to provide valid data for most tumor types. In contrast, studies comparing breast cancer in the pregnant and nonpregnant states, stratified for the most important prognostic factors, have shown no significant differences in survival rates (8,9).
Staging
Staging examinations are performed as in nonpregnant women and are important for their impact on therapeutic decisions. Nonionizing examinations, including ultrasound and magnetic resonance imaging (MRI), should be preferred during pregnancy (10). If needed, diagnostic ionizing imaging modalities can be performed with appropriate fetal shielding. Ionizing examinations of distant parts of the maternal body expose the fetus to low dosages, and the accumulated radiation dose may harm the fetus. The general rule when performing radiologic and nuclear medical examinations during pregnancy is that the radiation doses should be kept as low as reasonably achievable (ALARA-principle), and where possible should be avoided.
In the absence of safety data, contrast agents should not be used, which is unlikely to alter the diagnostic accuracy. For the evaluation of adnexal masses, contrast administration is mainly used to assess the presence of solid components in a cystic mass, and to a lesser extent, to evaluate nonenhancement in a torted mass. The presence of solid components in a cystic mass can generally be derived from gray-scale and Doppler sonography. It is unlikely that intravenous gadolinium would be considered critical for MRI of an adnexal mass in pregnancy (11). For cervical cancer, MRI is the reference diagnostic examination to determine tumor size in three dimensions. Stromal invasion, vaginal, and parametrial extension can be visualized without the use of gadolinium (12).
During normal pregnancy, tumor markers including CA15-3, squamous cell carcinoma (SCC) antigen, and CA125 can be elevated (13). A recent review reported that elevated titers were found in 3–20% of patients for CA15–3 (maximum 56 unit/mL in the third trimester), 3–10.5% for SCC (maximum 4.3 μg/L in the third trimester), and up to 35% for CA125 (maximum 550 unit/mL in the first trimester). Inhibin B, anti-müllerian hormone (AMH), and lactate dehydrogenase (LDH) levels are not elevated in maternal serum during normal pregnancy (13).
Interdisciplinary Review
The treatment of pregnant cancer patients is not a typical daily practice, and the experience is diluted among specialists and centers. Apart from this limited experience per clinician, the complexity of conflicting maternal and fetal interests deserves an interdisciplinary discussion, to include radiologists and obstetricians/perinatologists (Fig. 17.1). An initial interdisciplinary discussion on the choice of imaging modality is a good strategy to avoid overlapping examinations and accumulation of radiation exposure (14).
A second interdisciplinary discussion should individualize the treatment, which should adhere as much as possible to standard care (14). Fetal concerns are assessed by the obstetricians and perinatologists, who address the long-term morbidity of prematurity. Where possible, the standard maternal treatment and the prevention of prematurity should be the goal. An interdisciplinary setting is the best guarantee to maximize fetal and maternal outcomes.
Surgery during Pregnancy
A stable maternal condition is the best guarantee for fetal well-being. Abdominal surgery is preferably planned for the second trimester, because the risk of miscarriage is decreased and the size of the uterus still allows a certain degree of access. Monitoring of hemodynamic parameters is crucial to prevent hypoxia, hypotension, and hypoglycemia. From 20 weeks gestational age onward, the pregnant patient is positioned in the “left lateral tilt” position.
Pregnant patients may undergo laparoscopic surgery for pelvic conditions safely up to about 28 weeks (15,16). Specific risks in pregnancy include hypercapnia related to the use of carbon dioxide, perforation of an enlarged uterus, and reduced blood flow caused by the increased intraabdominal pressure. A maximum laparoscopic procedure time of 90 minutes, a pneumoperitoneum with a maximum intra-abdominal pressure of 10 to 13 mm Hg, an open introduction, and an experienced surgeon are prerequisites. Apart from adequate analgesia, tocolytic agents are indicated postoperatively when manipulation of the pregnant uterus has been unavoidable. After surgery on upper parts of the body, tocolytics should not be routinely prescribed.
Radiation during Pregnancy
Fetal exposure to radiation is associated with deterministic and stochastic effects. Radiotherapy exposes the fetus to higher radiation doses than diagnostic procedures. Deterministic effects occur when a certain threshold has been exceeded, shortly after the radiation has been given. Possible deterministic effects during pregnancy include fetal malformations, growth restriction, microcephaly, mental retardation, and fetal demise. Stochastic risk of radiotherapy cannot be exactly predicted, and there is no threshold for toxic effects. These manifest years later, and cannot definitively be associated with the radiation exposure. Radiation exposure during fetal life results in a slightly greater risk of childhood cancer and leukemia. A fetal dose of 0.01 Gy will increase their incidence from 2 to 3 per 1,000 to 3 to 4 per 1,000 (17).

Figure 17.1 Management of cancer during the three trimesters of pregnancy.
When applied during the first trimester, pelvic radiotherapy causes spontaneous abortion. When applied during the second trimester, pelvic radiotherapy evokes death of the fetus within the first month after external beam therapy (18). The advantages and disadvantages of hysterotomy before radiotherapy should be assessed on an individual basis. When hysterotomy is performed before initiating radiotherapy, there is no need to alter the radiation fields, there is less psychological distress, and fewer obstetrical complications (bleeding, diffuse intravascular coagulation) (19). Abdominal surgery may potentially delay treatment should a wound infection occur, and there is a small risk of wound metastasis. Postoperative adhesions may enhance radiation toxicity (18).
Chemotherapy during Pregnancy
Pharmacokinetics
During pregnancy multiple changes in physiology occur that affect the major pharmacokinetic processes, including absorption, distribution, metabolism, and excretion. Changes in drug metabolism begin at 4 weeks of gestation and progressively increase, mainly under the influence of progesterone and estrogen. These alterations are more pronounced in the third trimester of pregnancy and may alter the efficacy and toxicity of antineoplastic drugs administered during pregnancy (20,21).
The maternal gastrointestinal absorption of drugs may be altered because of changes in gastric secretion and motility. Changes in gastric pH influence the degree of ionization and solubility of many drugs, modifying their absorption rate and altering drug bioavailability. During normal pregnancy, maternal drug metabolism may be altered by elevation of endogenous hormones such as progesterone and estrogen. Their increased secretion can induce or inhibit the hepatic microsomal oxidase system and elevated or reduced rates of hepatic metabolism may result in altered transformation of drugs. The cholestatic effect of estrogen may interfere with biliary drug clearance (20–22).
Therapeutic concentrations of the active drug may be affected by hemodynamic changes that take place throughout pregnancy. Cardiac output and blood volume increase by 50%, primarily as a result of an increase in plasma volume of 45%, leading to an increase in hepatic and renal perfusion, and an increased glomerular filtration rate, which may cause increased renal elimination. Total body water increases by 5–8%, resulting from the expansion of the extracellular fluid space and the growth of new tissue. Body water accumulates in the fetus, placenta, and amniotic fluid, which collectively contribute to an increase in the distribution volume and this may lower the concentration of drugs and increase their elimination half-life (20–22).
As pregnancy advances, the plasma volume expands at a greater rate than the increase in albumin production, creating a dilutional hypoalbuminemia, which may increase the fraction of unbound drugs (20).
Physiologic pharmacokinetic changes in humans result in a decrease in plasma drug exposure, as measured by area under the curve (AUC) and peak plasma concentrations, because of the increased plasma volume and renal clearance (20).
Theoretically, a reduced plasma drug concentration could lead to suboptimal treatment. The largest follow-up study in breast cancer showed no significant difference in survival between women with cancer during pregnancy and stage-matched nonpregnant women (8,9). Therefore, despite the dilution of chemotherapy during pregnancy, data have not shown a worse outcome in pregnant women who receive chemotherapy, so doses should be based on actual weight and height during pregnancy.
Transplacental Passage of Chemotherapeutic Drugs
The placenta is an active organ providing nutrition and protection for the fetus. Transplacental transfer of substances is mediated by passive diffusion, active transport, facilitated diffusion, and phagocytosis and pinocytosis (23,24).
The placenta, and more specifically, the syncytiotrophoblast and fetal capillary endothelium, express ATP-binding cassette (ABC) efflux transporters, including P-glycoprotein (P-gp: MDR 1/ABCB1), breast cancer resistance protein (BCRP/ABCG2), and multidrug resistance protein 1 to 3 and 5 (MRP 1 to 3 and 5/ABCC 1 to 3 and 5), which actively extrude substrates into the maternal circulation. One of the major roles of these efflux transporters in the placenta is to limit fetal overexposure to toxins, drugs, or xenobiotics, thus protecting the fetus pharmacokinetically and pharmacodynamically. Most of the chemotherapeutic drugs (e.g., anthracyclines, taxanes) are substrates of these ABC drug transporters.
The data on transplacental passage are in agreement with the expectations based on physicochemical drug characteristics. Because all drugs that are substrates of ABC transporters have a limited transplacental transfer, a major role for the active transporters in the protection of the fetus from toxic agents is suggested (25).
The placenta reduces transplacental passage of all studied drugs, albeit at variable rates. Transplacental transfer of doxorubicin and epirubicin averages less than 10% (26). Taxanes display a favorable toxicity profile during the second and third trimesters, because less than 2% of maternal plasma concentrations are measured in fetal plasma (27,28). Approximately 20% of the vinca alkaloids and the active metabolite of cyclophosphamide cross the placenta (26). In contrast, fetal plasma concentrations of carboplatin reach up to 60% of maternal plasma levels (27).
Obstetric Management
A pregnancy complicated by cancer is a high-risk pregnancy and should be managed as such. A checklist for obstetricians is available (14). A 3-week interval between the last cycle of chemotherapy and the delivery will avoid problems associated with hematopoietic suppression in the mother and child, and the accumulation of cytotoxic drugs in the neonate. For all patients, a term delivery (37 weeks’ gestation or longer) should be the aim whenever possible. Chemotherapy during pregnancy followed by term delivery is preferable to late preterm delivery without chemotherapy. A vaginal birth should also be the objective whenever possible. It is acceptable in cases of cervical intraepithelial neoplasia (CIN) (29), but in the presence of invasive cervical cancer, an operative delivery is preferable. The literature has documented seven fatal recurrences in the episiotomy site after vaginal delivery (18).
A classical (corporeal) uterine incision, avoiding the lower uterine segment in order to prevent wound metastasis, is preferable. Cesarean section enables additional surgical treatment of the cancer, when indicated. After surgery for vulvar cancer, vulvar scarring and the risk of vulvar laceration may be an indication for cesarean section. In the presence of vulvar cancer, tumor location and diameter will determine the safest route for delivery.
Fetal Outcome after Antenatal Exposure to Chemotherapy
Timing of oncologic treatment is a crucial factor in determining the fetal outcome. During the period of organogenesis, the fetus is especially vulnerable to cytotoxic agents. After the period of organogenesis, different developmental steps still take place, for example, the development of the central nervous system. Therefore, the fetal outcome in the short and long term is of particular interest.
Short-term Outcome
Spontaneous abortion, fetal death, and major malformations (toes, eyes, ears, and palate) are associated with the administration of chemotherapy during the first trimester. If chemotherapy is administered after the first trimester, there is no increased risk of congenital malformations compared to the background population (2,30,31). In utero exposure to chemotherapy during the second and the third trimester of pregnancy is related to low birth weight and intrauterine growth restriction (2,3,8). In a series of 413 women exposed to chemotherapy for breast cancer during pregnancy, 50% had a spontaneous or induced premature delivery (8).
Long-term Outcome
There are few published studies concerning the long-term outcome of in utero exposure to chemotherapy or radiotherapy. In a series of 40 children for whom parents completed a questionnaire, health and cognitive outcomes were reassuring after antenatal exposure to chemotherapy (32). In a prospective study of 70 children with in utero exposure to chemotherapy, general health, cognitive development, and cardiac outcome showed levels comparable to the general population, with a median follow-up of 22 months (31). A bias related to prematurity was suggested, because the IQ-scores increased with gestational age. Prematurity is associated with impaired cognitive functioning, and should be avoided whenever possible (33,34).
A longer follow-up and more children are needed, but the available data suggest that fear of the long-term effects in children should not be a reason to withhold chemotherapy during pregnancy (31).
Management of Cervical Intraepithelial Neoplasia in Pregnancy
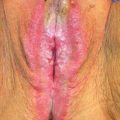
The American Society for Colposcopy and Cervical Pathology (ASCCP) has provided consensus guidelines for the management of abnormal cervical cytology and biopsies during pregnancy, which we have summarized below (35,36). The main treatment strategy for CIN during pregnancy is observation. Pregnancy does not influence cervical lesions, and progression to invasive disease during pregnancy is very rare (0–0.4%) (37). When progression does occur, a higher stage than microinvasion has not been documented. Colposcopy and directed biopsies can be safely performed during pregnancy, but endocervical curettage is contraindicated (36). Physiologic cervical changes during pregnancy, such as increased vascularity, hypertrophy, and hyperplasia of the endocervical glands, may mimic CIN, so colposcopy should be performed by an experienced colposcopist. Several physiologic cellular changes, such as degenerated decidual cells (Arias-Stella phenomena) and trophoblastic cells with variably staining cytoplasm and an enlarged nucleus may mimic high-grade squamous intraepithelial lesions (HSIL), and lead to false-positive Pap smears if the pathologist is unaware of the pregnant state.

Figure 17.2 Management of pregnant women with low-grade squamous intraepithelial lesion (LSIL) according to the 2013 American Society for Colposcopy and Cervical Pathology (ASCCP) guideline. CIN, cervical intraepithelial neoplasia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree