Laparoscopy is widely accepted for numerous gynecologic oncology operative procedures. These operations have been associated with short hospital stays, quick recovery times, and rapid return to full activity. The assistance of video monitors makes laparoscopy a preferred technique because the surgeon can view the operation in real time. Laparoscopy continues to undergo evaluation of its feasibility, morbidity, cost-effectiveness, and long-term outcome for patients compared with the standard laparotomy.
Gynecologic oncology care provided the unique opportunity for prospective study of the use of operative laparoscopy because of the limited number of specialists performing the procedures and the need to perform pelvic and para-aortic lymphadenectomy to stage several gynecologic malignancies. As surgeons have adopted minimally invasive technologies, innovations in instrumentation from bipolar coagulation devices to large-scale robotically-assisted devices have been developed to facilitate laparoscopic approaches for increasingly complex surgical procedures (see Chapter 22).
The skills to manage gynecologic malignancies by laparoscopic techniques are acquired through a commitment on the surgeon’s part to learn the technique. It requires up-to-date equipment and a team familiar with the procedures. Hands-on experience in an animal laboratory and proctored learning in the operating suite are highly recommended. In the hands of experienced laparoscopic surgeons and with properly selected patients, laparoscopic surgery appears to result in outcomes comparable with laparotomy. Innovative technologies such as robotic-assisted surgical units may further expand the scope and use of minimally invasive techniques in the field of gynecologic oncology.
Laparoscopic Pelvic and Para-aortic Lymphadenectomy
The performance of a pelvic and para-aortic lymphadenectomy, either a partial lymphadenectomy (lymph node sampling) or complete lymphadenectomy, is the key procedure for the staging of gynecologic malignancies. Dargent and Salvat (1) used the laparoscope to perform limited pelvic lymphadenectomy in women with cervical cancer. This was not widely accepted because of its limited access to the pelvic lymph nodes and the inability to evaluate the lymph nodes in the common iliac and para-aortic chains. Childers and Surwit (2) described pelvic and para-aortic lymphadenectomy performed in conjunction with a laparoscopically assisted vaginal hysterectomy and bilateral salpingo-oophorectomy in two women with endometrial cancer. Nezhat et al. (3) published a case of laparoscopic radical hysterectomy and pelvic and para-aortic lymphadenectomy, although the dissection went only 2 cm above the aortic bifurcation, an inadequate evaluation. These early publications were limited case reports that gave no information on morbidity, mortality, or complications.
Querleu et al. (4) performed transperitoneal laparoscopic pelvic lymphadenectomy on 39 patients with cervical cancer. Five patients had metastatic lymph nodes and were treated with radiation therapy. Thirty-two patients underwent abdominal radical hysterectomy and evaluation of the completeness of the laparoscopic lymphadenectomy. The sensitivity for lymph node-positivity by laparoscopy was 100%. However, the number of additional lymph nodes found at laparotomy was not stated. Childers et al. (5) reported 59 patients with endometrial cancer who were staged laparoscopically, followed by vaginal hysterectomy and bilateral salpingo-oophorectomy. Of the 31 patients deemed candidates for staging based on criteria including high-grade or deep myometrial invasion, lymphadenectomy was completed in 29 patients (obesity precluded it in two patients), for a feasibility rate of 93%. Three major and three minor complications were reported. The surgical complications were experienced early in the series and led to alternative techniques as the series progressed. The average hospital stay was 2.9 days, but the operative time, lymph node counts, and cost analysis were lacking.
These early series emphasized pelvic lymphadenectomy, but it remained necessary to do para-aortic lymphadenectomy for laparoscopy to be fully accepted as a technique to stage all gynecologic malignancies. Childers et al. (6) reported their initial experience with pelvic and para-aortic lymphadenectomy extending from the duodenum to the bifurcation in 18 patients with cervical cancer. They subsequently summarized their experience in para-aortic lymphadenectomy with a report of 61 women with cervical, endometrial, or ovarian cancer (7). In three patients (5%), obesity prevented the completion of the surgery, and in one patient (0.8%), adhesions were responsible for failure. Lymph node counts were available in 23 patients: For the right-sided lymphadenectomy, there was an average lymph node count of three. The operating time for the six patients, who underwent a bilateral para-aortic lymphadenectomy ranged from 25 to 70 minutes, depending on whether or not a unilateral or bilateral procedure was performed (these times included only the time to complete the lymphadenectomy). The hospital stay for the 33 patients undergoing laparoscopic lymphadenectomy was 1.3 days. There was one vena caval injury that required transfusion and laparotomy, a complication rate comparable with that of open surgery. Spirtos et al. (8) reported 40 patients who underwent bilateral limited para-aortic lymphadenectomy (sampling). Five laparotomies were performed: Two to remove unsuspected metastases, two for control of hemorrhage, and one because of equipment failure. In two patients, the left-sided lymphadenectomy was judged to be inadequate, which was an overall failure rate of 12.5%. An average of eight para-aortic lymph nodes were removed: Four from the right side and four from the left side. Most of the patients also underwent a pelvic lymphadenectomy and hysterectomy. The mean operative time was 3 hours, 13 minutes, and the average hospital stay was 2.9 days. In these series (4–6,9), laparotomy was used to confirm the accuracy of the lymphadenectomy and in each report, all positive lymph nodes were identified.
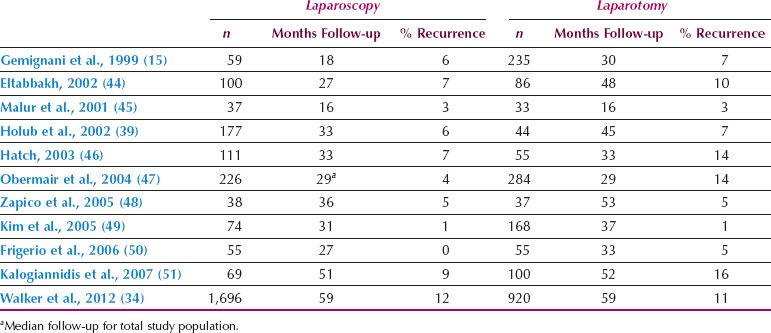
Possover et al. (10) reported 84 patients who underwent laparoscopic pelvic and para-aortic lymphadenectomy for cervical cancer. The surgeon visually classified the lymph nodes as positive or negative. The sensitivity and specificity of visualization was 92.3%. When frozen-section analysis was combined with laparoscopic assessment, 100% of the positive lymph nodes were identified. Based on these findings, in 13 of the 84 patients (15.5%), the treatment plan was altered during surgery. Possover et al. (11) analyzed videotapes of 112 para-aortic lymphadenectomies and detailed the ventral tributaries of the infrarenal vena cava (Fig. 21.1). They divided the vena cava into three levels based on the distribution of venous tributaries. This is a significant contribution to anatomic knowledge and is an important guide for beginning laparoscopic surgeons. A perforator of the inferior vena cava at the level of the bifurcation of the aorta is shown in Figure 21.1A. A diagram of the most common sites where perforators are encountered during a para-aortic lymphadenectomy is shown in Figure 21.1B.
Multiple studies report the adequacy and safety of laparoscopic pelvic and para-aortic lymphadenectomy in gynecologic cancer (12–19). In the experience by Kohler et al. reporting the laparoscopic para-aortic left-sided transperitoneal infrarenal lymphadenectomy, the number of lymph nodes removed could be doubled compared with an inframesenteric lymphadenectomy (12). In one of the largest series to date, they reported on 650 patients undergoing laparoscopic transperitoneal pelvic (n = 499) or para-aortic (n = 468) (combined pelvic and para-aortic n = 362) lymphadenectomies (19). The mean number of pelvic lymph nodes removed from 1994 to 2003 remained fairly constant (16.9 to 21.9). However, the mean number of para-aortic lymph nodes increased from 5.5 in 1994 to 18.5 in 2003, reflecting improvements in technique and extensive training. Intraoperative complications (bowel or vessel injury) occurred in 2.9% of patients while 5.8% had postoperative complications for an overall complication rate of 8.7%. The authors reported that no major intraoperative complications were encountered during the last 5 years of the study.
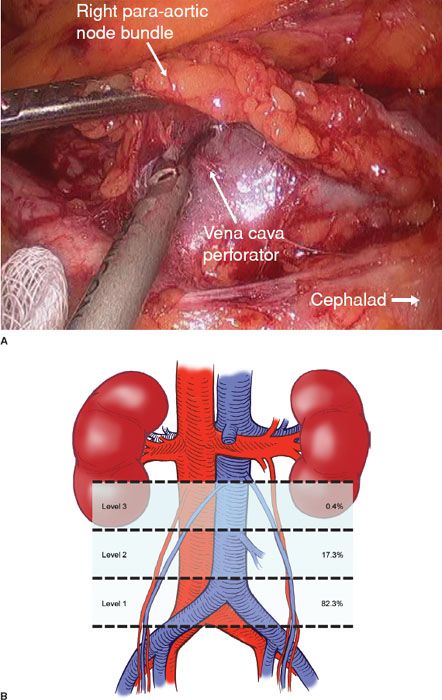
Figure 21.1 Perforators of the vena cava. A: A vena cava perforator at the level of the bifurcation of the aorta. B: Diagram of the most common sites where perforators are encountered during the performance of a para-aortic lymphadenectomy. The figure shows the anatomic distribution of 237 venous tributaries in 112 patients undergoing laparoscopic lymphadenectomy according to different levels of the inferior vena cava. (From Possover M, Plaul K, Krause N, et al. Left-sided laparoscopic para-aortic lymphadenectomy: Anatomy of the ventral tributaries of the infrarenal vena cava. Am J Obstet Gynecol. 1998;179:1295–1297, with permission.)
Querleu et al. subsequently reported on their experience with transperitoneal and extraperitoneal lymphadenectomy in 1,000 gynecologic cancer patients (18). This study included 777 pelvic (757 transperitoneal, 20 extraperitoneal) and 415 aortic lymphadenectomies (155 transperitoneal, 260 extraperitoneal) in patients with early cervical carcinoma (n = 456); advanced cervical carcinoma (n = 219); vaginal carcinoma (n = 4); endometrial carcinoma (n = 182); and ovarian carcinoma (n = 139). The mean number of pelvic lymph nodes removed was 18 via a transperitoneal approach; the mean number of para-aortic lymph nodes removed was 17 via a transperitoneal approach versus 21 via an extraperitoneal approach. The authors reported an increase in the number of lymph nodes removed with increasing experience, yielding an average of 24 pelvic and 22 aortic lymph nodes in 2003. Intraoperative complications occurred in 2% of patients including injury to vascular structures (1.1%); bowel (0.3%); ureter (0.3%); and nerves (0.3%). Five patients underwent conversion to laparotomy for completion of the lymphadenectomy, secondary to fixed lymph nodes or extensive adhesions. Conversion to laparotomy occurred in an additional two patients secondary to bowel or ureteric injury. Five patients required a second surgical intervention as a result of postoperative complications, most commonly bowel obstruction (n = 4).
Infrarenal Para-aortic Lymphadenectomy
Most of the early studies of para-aortic lymphadenectomy described dissection and removal of the right para-aortic lymph nodes to the duodenum and left-sided lymph nodes to the inferior mesenteric artery (IMA). Kohler et al. (12) were the first to report a transperitoneal technique for left infrarenal lymph nodes. Querleu et al. (18) staged patients for ovarian cancer, performing a left infrarenal lymphadenectomy and developing the left extraperitoneal para-aortic lymphadenectomy to surgically stage advanced cervical cancer prior to radiation treatment. Mariani et al. (20) at the Mayo Clinic reported their experience in the staging of endometrial cancer using either laparotomy or laparoscopic staging of the para-aortic lymph nodes including the infrarenal lymph nodes. They found that 63 (22%) of 281 patients had lymph node metastasis; 33% of these were in the pelvis alone; 51% had both pelvis and para-aortic; and 16% had para-aortic only. Most importantly, 77% of the patients with positive para-aortic lymph nodes had metastasis to the infrarenal lymph nodes. Those patients who were laparoscopically staged had left extraperitoneal lymphadenectomies. This report has stimulated gynecologic oncologists to remove the infrarenal lymph nodes when indicated by level of risk. The extraperitoneal approach has been the preferred method, because it can be used in obese patients or those with extensive intraperitoneal adhesions.
Extraperitoneal para-aortic lymphadenectomy for gynecologic cancer was first reported by Vasilev et al. (21) and Dargent et al. (22). The original goal of the retroperitoneal approach was to surgically stage cervical cancers so that radiotherapy could be directed to the appropriate fields without the risk of adhesion formation that accompanies transperitoneal surgery. The peritoneum forms a barrier between the lymphadenectomy and the bowel allowing for better visualization for laparoscopic removal of infrarenal lymph nodes especially in obese women. Querleu et al. (23) described the detailed technique in 42 patients with advanced cervical cancer where the positive lymph node rate was 32% and the mean number of lymph nodes removed was 20.7. Sonoda et al. (24) further characterized the results and complications in 111 women with cervical cancer—the mean age was 46 and the mean BMI was 24. The operative time was 157 minutes, the hospital stay was 2 days and the lymph node count was 19. The most common complication was lymphocele and the procedure was modified to include a peritoneal window at the end of the procedure to allow lymph to be absorbed by the peritoneum. They recommended this procedure in cervical cancer patients who would be candidates for extended-field radiation if positive lymph nodes were found. Twenty-seven women had positive lymph nodes and 21 patients underwent extended-field radiation with chemotherapy. Nine women underwent extended-field radiation alone. Mean survival was 38.6 months in the lymph node-negative group and 26.5 months in the lymph node-positive. The laparoscopic extraperitoneal lymphadenectomy has been reported by a number of authors and the results will be discussed below in the sections on cervical cancer and endometrial cancer (25–28).
Table 21.1 Recurrence Rates for Laparoscopic Surgery versus Laparotomy for Endometrial Cancer
Indications for Laparoscopic Surgery
Endometrial Cancer
Most women with endometrial cancer present with disease confined to the uterus. The treatment consists of total hysterectomy, bilateral salpingo-oophorectomy, and surgical staging, which includes peritoneal washings, inspection of the abdomen, and retroperitoneal lymphadenectomy. The early studies showed an advantage for laparoscopic staging compared with historical or matched controls in postoperative pain, blood loss, length of hospital stay, and postoperative complications (2,12–15,36,37). Long-term survival of patients reported in these studies is presented in Table 21.1 (15,39,44–51).
Surgical staging by pelvic and para-aortic lymphadenectomy followed by laparoscopically assisted vaginal hysterectomy or laparoscopic total hysterectomy has been established by randomized controlled trials comparing laparoscopy to laparotomy. Palomba et al. (29) summarized the first four of these trials. Two of these studies (30,31) had long-term follow-up of 202 patients with no difference in disease-free survival (87.3% in laparoscopy and 80% in laparotomy). The operative times were longer for laparoscopy; but the blood loss, transfusion, and postoperative complications were less. The lymph node count for both pelvic and para-aortic was the same. Malzoni et al. (32) reported 159 patients followed for a median of 38 months. He included patients with BMI up to 39. The operative times were 139 minutes for laparoscopy and 123 for laparotomy. The blood loss was 50 mL versus 145 mL and the mean length of stay was 2.1 days versus 5.1 days. There were 8.6% recurrences in the laparoscopy group, and 11.5% in the laparotomy group. Janda et al. (33) published the results of a multi-institutional trial in Australia, New Zealand, and Hong Kong. A total of 332 patients were randomized and filled out quality-of-life questionnaires for up to 6 months to identify early and late complications. There were no restrictions on weight: 40% of the total laparoscopic hysterectomy and 36% of the total abdominal hysterectomy patients were above BMI 35. Operating times were longer in the total laparoscopic hysterectomy group (138 minutes vs. 109 minutes). Lymphadenectomy was not required and 40.5% of the total laparoscopic hysterectomy and 67.6% of the total abdominal hysterectomy patients had lymph nodes removed. Hospital stay was longer than 2 days in all but 2% of the total abdominal hysterectomy patients and 37% of the total laparoscopic hysterectomy. The number of intraoperative adverse events was the same in both groups, but the late adverse events were doubled in the total abdominal hysterectomy group. These included wound dehiscence, bleeding, and infection. The QOL analysis favored the total laparoscopic hysterectomy from baseline through the 6-month period.
The Gynecologic Oncology Group (GOG) conducted the LAP 2 trial, a large, prospective, randomized trial designed to determine equivalency in early-stage endometrial cancer outcomes in laparoscopically assisted vaginal hysterectomy and bilateral salpingo-oophorectomy with surgical staging, when compared with traditional open surgery (34). It is the only randomized controlled trial that required pelvic and para-aortic lymphadenectomy. Results of this important study help answer many questions regarding feasibility, appropriate patient selection, and short-term and long-term oncologic outcomes of laparoscopy in the management of endometrial cancer. This study enrolled 2,616 patients and there were 1,696 patients randomized to laparoscopy and 920 to laparotomy in a two-to-one randomization ratio. Length of stay was shorter in the laparoscopic arm (median 3 days, range 0 to 95) versus laparotomy arm (median 4 days, range 1 to 49). Operative time was increased with laparoscopic procedures (3.3 hours vs. 2.2 hours) and approximately 23% of patients who were randomized to laparoscopy required laparotomy to complete staging. The BMI initially was limited to 34 and below; however, 388 patients were enrolled with BMI greater than 35. There were 434 (23%) women in the laparoscopy group who were converted to laparotomy for the following reasons: poor exposure 14.6%, resection of metastatic disease 4.1%, bleeding and other 4.3%. Laparoscopic operative times were significantly longer—204 minutes versus 130 minutes. Pelvic and para-aortic lymphadenectomy was accomplished in 92% of the laparoscopic group and 96% of the laparotomy group. Detection of advanced stage was the same in both groups—17%. There was no difference in initial blood loss.
With a median follow-up of 59 months, the recurrence and survival results from GOG LAP2 were reported. The estimated recurrence rate was 11.4% in the laparoscopy group and 10.2% in the laparotomy group. The estimated 5-year survival was 89.9% in both arms. There was no difference in the postoperative treatments or the site of first recurrence, which would seem to dispel the concern that laparoscopy did not detect disease in the infrarenal lymph nodes or caused more intra-abdominal recurrence by spreading malignant cells via the intrauterine manipulator. There were four port site recurrences (0.24%) unique to the laparoscopic arm and three of these patients had advanced disease at the time of surgery. Therefore, surgical staging with operative laparoscopy followed by vaginal hysterectomy or laparoscopic total hysterectomy has been proposed as an alternative to laparotomy (2,12–15,36–38).
The other randomized trials showed less blood loss in the laparoscopic group, but they did not require lymphadenectomy in all patients. Only one study by Tozzi et al. had more than 50% of patients who had para-aortic lymphadenectomy (29). The quality of life in this trial reported by Kornblith et al. (35) noted less pain, earlier resumption of normal activities, earlier return to work, and better body image, which was sustained for 6 months.
Women with endometrial cancer are often obese with BMI greater than 35 (40–42,44). This was thought to be a limiting factor in using laparoscopy to stage and treat endometrial cancer. As surgical skills have grown, laparoscopy has been used successfully in these women. Holub et al. (41) completed staging and hysterectomies successfully in 94.4% of 33 patients with BMIs of 30 to 40. After hysterectomy, the pelvic and para-aortic lymphadenectomy was performed based on grade and depth of invasion. Women who had BMI from 30 to 40 were compared to those who were under 30. Two women in the obese group were converted to laparotomy to complete staging. Lymph node staging was completed in 94.4% of the women in whom it was indicated. Scribner et al. (43) reported 55 patients who had planned laparoscopic staging for endometrial cancer. The BMI ranged from 28 to 60. Those women with BMI greater than 35 had a decreased success rate (44%) versus those under 35 (82.1%). In the GOG LAP2 trial, 322 patients had a BMI of greater than 35 (34): The number of patients converted to laparotomy was 17.5% in patients with BMI 25 to 30; 26.5% from 30 to 35; and 57.1% in patients with BMI over 40. Failure to complete laparoscopic staging was greater with increasing BMI, metastatic disease, and age.
The concept of sentinel node removal has been studied in endometrial cancer in several small studies. Techniques utilized for the detection of sentinel lymph nodes include injection of blue dye, injection of a radiocolloid, or both. Studies in endometrial cancer have focused on injection of the tracer into the uterine corpus (subserosal or myometrial), the cervix, the endometrium using hysteroscopy, or combined sites (52). The sentinel lymph node detection rates using injection of the subserosal myometrium alone ranged from 0–92% (53–58). Altgassen reported the highest detection rate (92%) utilizing eight injection sites, in contrast to the one to three sites reported by several other authors. Similarly, Li et al. (55) reported a 75% detection rate using three subserosal myometrial sites and two subserosal isthmic sites. Reported detection rates using cervical injection alone ranged from 80–100% (59–62). Holub et al. reported a detection rate using cervical and subserosal myometrial injections of 84% (63). Hysteroscopic injection of the endometrium has yielded detection rates of 50–100% (64–67). While the possibility of laparoscopic assessment of sentinel lymph nodes and targeted sampling is interesting, sentinel lymph node detection in endometrial cancer remains investigational.
Cervical Cancer
The use of laparoscopy in the treatment of cervical cancer was initially limited by the fact that there was no apparent advantage to laparoscopic lymphadenectomy, because the standard operation for the primary cervical tumor was radical abdominal hysterectomy. Dargent (68) first suggested that laparoscopic pelvic lymphadenectomy could be followed by a Schauta radical vaginal hysterectomy and has published long-term results, reporting a 95.5% 3-year survival rate in 51 patients with negative pelvic lymph nodes. Querleu (69) reported on eight patients and demonstrated an average blood loss of less than 300 mL, an average hospital stay of 4.2 days, and decreased pain from the elimination of an abdominal incision.
Hatch et al. (70) reported 37 patients treated by laparoscopic pelvic and para-aortic lymphadenectomy followed by radical vaginal hysterectomy. The mean operative time was 225 minutes, the mean blood loss was 525 mL and the average hospital stay was 3 days. Blood transfusion was required in 11% of the patients, compared with the range of 35–95% reported in the literature for radical abdominal hysterectomy. Complications occurred early in the series and included two cystotomies repaired at surgery without an increase in hospital stay. In two patients (5.4%), ureterovaginal fistulae developed that were treated by ureteral stents. These were removed 6 weeks later without further operative intervention.
Schneider et al. (71) reported 33 patients in whom bipolar techniques were used for lymphadenectomy and to transect the cardinal ligaments and uterine vessels. Hysterectomy was completed by the Schauta–Stoeckel technique. There were five (15%) intraoperative injuries managed successfully without sequelae. Four patients required transfusion. Numerous retrospective studies comparing laparoscopically assisted radical hysterectomy versus radical abdominal hysterectomy have reported increased operative time but decreased blood loss, decreased transfusion rates and hospital length of stay in patients undergoing the minimally invasive procedures (72–76).
Studies have shown that the complication rates decrease as the operator’s experience increases (77,78). Long-term survival has been reported by Hertel et al. (14) for 200 patients, with a mean follow-up of 40 months. The projected 5-year survival was 83%. For the 100 patients who were stage I, lymphovascular space-negative and lymph node-negative, the survival was 98%.
Laparoscopic Radical Hysterectomy
Although most initial reports in the literature detailed some form of laparoscopically assisted radical vaginal hysterectomy, there are increasing reports of laparoscopic radical hysterectomy. Spirtos et al. (13) reported laparoscopic radical hysterectomy (type III) with aortic and pelvic lymphadenectomy in 78 patients. The average operative time was 205 minutes, length of hospitalization was 3.2 days, and blood loss was 225 mL; one transfusion was necessary. There were acceptable intraoperative and postoperative complications. With a minimum of 3 years follow-up, the disease-free survival was 95%. Numerous authors have reported similar findings (57,79–83). In one of the largest series, Puntambekar et al. (81) reported on 248 patients with early stage cervical cancer, noting a median operative time of 292 minutes, median number of resected pelvic lymph nodes of 18, median blood loss of 165 mL, and a median length of stay of 3 days. Other studies have reported longer operating times ranging from 196 to 344 minutes (57,76,83,84).
The issue of blood loss and transfusion has become very important to patients and surgeons since the identification of the human immunodeficiency virus and other blood-borne pathogens. Every report on laparoscopic lymphadenectomy and radical hysterectomy has noted a significant decrease in blood loss and transfusion rates. Other societal advantages are the decreased hospital stay and rapid return to normal function, even with radical surgery.
Laparoscopic Nerve-sparing Radical Hysterectomy
Preservation of the superior hypogastric nerve plexus, the hypogastric nerve, and the inferior hypogastric plexus is important for function of the bladder and rectum. The superior hypogastric plexus is composed of sympathetic nerves that allow the bladder to store urine. If this is damaged, the bladder will have a small-volume and high pressure under parasympathetic control. The inferior hypogastric plexus is composed of parasympathetic nerves that initiate urination. If the inferior hypogastric plexus is damaged, the patient will have lack of sensation and be unable to initiate urination. The hypogastric nerve connects the two plexuses. If it is severed, the patient will have a mixed pattern, consisting of an initial small-volume, high-pressure phase followed by a hypotonic high-residual state. This may lead to the need for chronic self-catheterization.
Damage to the superior hypogastric plexus can occur at the time of para-aortic lymphadenectomy or presacral lymphadenectomy. Injury to the hypogastric nerve may occur when clamping the cardinal or uterosacral ligaments. Injury to the inferior hypogastric plexus may occur with lateral dissection of the cardinal ligaments, dissection of the posterior vesicouterine ligament, or with removal of paravaginal tissue.
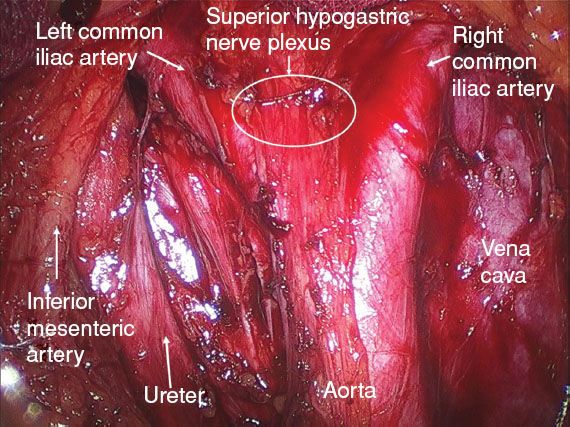
Figure 21.2 The superior hypogastric nerve plexus is preserved during the para-aortic lymphadenectomy.
Recent description of the anatomy of these nerves and the surrounding blood vessels has established the role of nerve-sparing techniques when performing radical hysterectomy (85). The laparoscope is an excellent tool to identify and preserve the nerves, because it magnifies small vessels and nerves so that the surgeon can more easily identify them.
The technique performed is described below (Figs. 21.2–21.8):
1. The patient is placed in the lithotomy position with Trendelenburg tilt to the table. A nasogastric tube is placed to reduce the distention of the stomach. The four trocars are placed in the lower abdomen in a diamond shape.
2. The para-aortic lymphadenectomy is performed first when the tumor is 2 cm or greater in size. This allows for exposure of the superior hypogastric plexus (Fig. 21.2).
3. The hypogastric nerve is located by opening the peritoneum between the ureter and the sigmoid colon mesentery (Fig. 21.3). The hypogastric nerve is dissected down into the pelvis and its location dorsal to the uterine artery is shown (Fig. 21.4).
4. The uterine artery is divided and the hypogastric nerve is dissected lateral and dorsal to the ureter (Fig. 21.5).
5. The anterior vesicouterine ligament with its vessels and connective tissue is divided and the ureter dissected laterally (Fig. 21.6). The branch of the nerve following the ureter into the base of the bladder is preserved in the posterior vesicouterine ligament (Fig. 21.7).
6. The cardinal ligament can be divided medial to the nerve and ligament (Fig. 21.8).
The operation can be completed by the vaginal route or with further dissection through the laparoscope. The vaginal route allows more precise removal of the vaginal margin.
The urethral catheter is left in place for 48 hours. Then the patient is allowed to void and a postvoid residual is obtained. If she had good sensation of bladder fullness and a residual urine amount less than 60 cc, the catheter is left out. If she is unable to void, has no sensation of filling, or if the residual urine is over 60 cc, the catheter is left in place for 7 days. The nerve-sparing operation has been performed in a total of 33 patients. Twenty-one of these patients had a laparoscopic lymphadenectomy and radical vaginal trachelectomy. Twelve had a laparoscopic lymphadenectomy and radical hysterectomy completed by laparoscopy or by vaginal assistance. Eight of the patients were unable to void with residual of less than 60 cc at 7 days. All of the patients were able to void at 21 days.
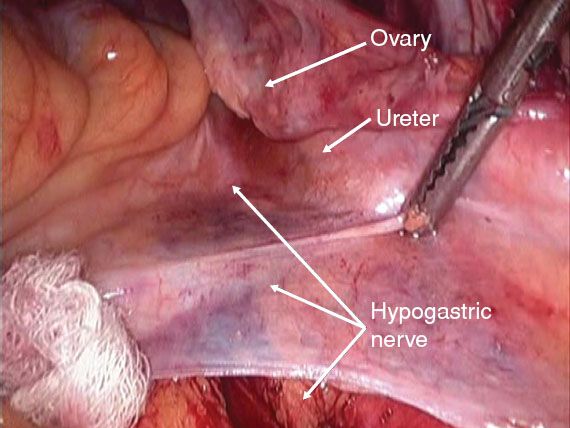
Figure 21.3 Traction on the peritoneum overlying the bifurcation of the aorta helps identify the course of the hypogastric nerve 2 to 3 cm medial to the ureter at the pelvic brim.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree