Traditionally, nutritional support has been viewed as an intervention to preserve lean body mass, maintain immune function, and avoid metabolic complications. As understanding of the biologic effects of nutrition has improved, the focus has shifted from nutrition as a supportive intervention to a therapy. Delivering early and appropriate nutrition attenuates the metabolic stress response, prevents oxidative cellular injury, and modulates the immune response (1). The beneficial impact of nutritional support on the outcome and quality of life of the gynecologic oncology patient has been widely recognized. To facilitate the appropriate institution of nutritional support, an understanding of the classification of malnutrition and its diagnosis is essential. Physicians should be familiar with enteral and parenteral nutrition, their means of delivery, and their associated complications. There should also be appropriate monitoring of the response to therapy. A team approach by physician, nutritionist, and pharmacist is ideal for achieving these goals.
Normal Body Metabolism
According to the first law of thermodynamics, the energy derived from ingested food must equal the energy expended or stored in the body at equilibrium. Although the quantity of energy intake and the amount expended and stored in any 24-hour period do not correspond exactly, body weight eventually reflects the balance between energy intake and energy expenditure.
Calories
The unit of energy exchange is the calorie, which is the amount of heat required to raise the temperature of 1 mL of water 1°C at 1 atmosphere of pressure.
Dietary Calories
The dietary calorie equals 1,000 calories. Thus, 1,500 dietary calories are equal to a 1,500-kilocalorie diet. This notation is used to describe body stores of energy and the quantity of food ingested.
Body Stores
Although diets are variable, all foods are broken down through digestion into monosaccharides, amino acids, fatty acids, and glycerol. These are redistributed to body stores or metabolized for energy.
The body stores of energy are very different from the composition of the diet. The average diet has from 30–50% fat calories, 40–60% carbohydrate calories, and 15–20% protein calories. Roughly 1,200 carbohydrate calories are stored as glycogen in muscle and liver, whereas 130,000 to 160,000 calories are contained in fat; one pound of fat represents 3,500 stored calories. The body also contains approximately 54,000 calories as protein in muscle and organs, but only 30–50% of this is available to be burned for energy. Greater than a 50% depletion of total body protein is incompatible with life (2).
Metabolism During Starvation
During starvation, the body adapts to spare vital protein stores. Carbohydrate stores are depleted within 3 days of total starvation at rest, or more rapidly if the metabolic effects of catabolic illnesses elevate the requirements. Many organs use glucose in large amounts, obligating the breakdown of 75 g/d of muscle early in starvation (3). If the muscle were to continue to be broken down at this rate, starvation would lead to death in 45 to 60 days, but adaptation from a fed to a fasting state occurs over 2 to 3 days (Fig. 19.1). In this adaptation, peripheral tissues and organs use ketone bodies, a breakdown product of fat, in place of glucose. Because the fat stores contain an average of 160,000 calories, survival can be extended to 140 to 160 days. Some muscle breakdown continues, limiting survival, because the brain and the red blood cells require glucose, necessitating the breakdown of 20 g/d of muscle even after full adaptation.

Figure 19.1 The metabolic adaptation to starvation. (From Cahill GF, Owen OE. Some observations on carbohydrate metabolism in man. In: Dickens F, Randle PJ, Whelan WI, eds. Carbohydrate Metabolism and its Disorders. New York, NY: Academic Press; 1968:497, with permission.)
Malnutrition
Malnutrition is caused by an imbalance of energy, protein, and other nutrients. It adversely affects body composition, function, and clinical outcome. If left untreated, it invariably leads to cachexia, a syndrome characterized by severe body weight, fat, and muscle loss (4). About 40–55% of adult hospitalized patients in the United States are malnourished or at risk for malnutrition and 12% are severely malnourished (5–8). Similar percentages of malnutrition have been documented in thousands of hospitalized patients throughout the world (9). Between 40% and 80% of cancer patients are malnourished (10,11) and a study of gynecologic oncology patients found a prevalence of 54% (95% confidence interval, 41–66%) (8). In 2004, the same investigators evaluated gynecologic oncology patients in a prospective cohort trial (12). Subjective assessment using the Subjective Global Assessment (SGA) and objective assessment using the Prognostic Nutrition Index (PNI) found the incidence of malnutrition in this population to be 61% and 70%, respectively.
Nutrition screening to identify patients at risk for malnutrition is an important component of caring for gynecologic oncology patients. Malnutrition is associated with decreased tolerance to cancer therapies, increased morbidity and mortality, decreased quality of life, and increased health care costs (13,14). Early recognition and treatment affords the best opportunity to prevent these complications. The appropriate time to identify a patient as needing nutritional support is at the time the diagnosis of cancer is made (6,15).
Risk Factors
Predisposing conditions frequently found in malnourished hospitalized patients include the following:
1. heart failure
2. chronic obstructive pulmonary disease
3. infection
4. gastrointestinal (GI) disorders
5. psychiatric disorders
6. renal insufficiency
7. malignancy
It is typical for undernourished patients to have more than one predisposing condition (2).
Undernourished patients also commonly have vitamin and trace element deficiencies, particularly of vitamins A, D, E, B12, and iron. Decreased stores of these vitamins can be detected in early malnutrition. Because vitamins are stored in small amounts, the provision of only dextrose and water intravenously leads to their rapid depletion, abnormal enzyme function, and clinical signs of vitamin deficiency.
Etiology of Malnutrition in the Cancer Patient
Many systemic illnesses, including cancer, predispose patients to malnutrition (Fig. 19.2). Although abnormalities of metabolism, digestion, absorption, and utilization of nutrients all contribute to malnutrition in such patients, decreased nutrient intake is still an almost universal finding in malnourished patients, with the exception of those with uncomplicated hyperthyroidism. Many changes that occur in cancer patients are similar to those seen in inflammatory diseases, such as autoimmune disorders, chronic heart failure, infections such as human immunodeficiency virus, and prolonged critical illness. In particular, tumor necrosis factor (TNF), interleukins 1 and 6 (IL-1 and IL-6), and interferon-γ are etiologic agents of anorexia and cachexia (16,17).
Anorexia
Anorexia, is the major factor contributing to decreased intake in many disease processes. During tumor growth, anorexia and reduced food intake markedly contribute to the development of malnutrition. Serotonin plays a key role in the control of appetite and there is evidence that administration of neutral amino acids can counteract anorexia mediated by the increased tryptophan concentrations observed in cancer patients with anorexia (18).
Although anorexia can be a feature of cancer, it can also be a side effect of many drugs, including antineoplastic drugs. A number of other commonly used drugs (e.g., anticholinergics, antihistamines, methyldopa, sympathomimetics, clonidine, and tricyclic antidepressants) may cause a dry mouth, which decreases sensation and food palatability. Another common type of anorexia is a learned aversion to food when it is known to cause adverse physical symptoms. GI diseases, including reflux esophagitis, gastritis, and peptic ulcer, frequently cause dyspepsia. Irritable bowel syndrome, food allergies, lactose intolerance, diverticulae, and biliary disease can cause diarrhea or flatulence that may contribute to anorexia.

Figure 19.2 Impact of disease factors on nutrition.
All of these GI problems cause patients to avoid foods altogether or to ingest an unbalanced diet. Improvements in the pharmacotherapy of nausea have lessened the anorexia associated with chemotherapy. The pharmacologic classes for possible cancer cachexia treatment include appetite stimulants, metabolic inhibitors, anabolic agents, and anticytokine agents. Megestrol acetate, a progestational appetite stimulant, is an FDA-approved treatment for anorexia. This progestational steroid increases appetite through the central nervous system and peripheral mechanisms, analogous to the increased appetite that women note during the luteal phase of the menstrual cycle. Body weight gains are from fat alone and may not improve survival. Megestrol dosing begins at 160 mg/d and may advance to 480 to 800 mg/d. There may be a trend toward an increased risk of thromboembolism or adrenal insufficiency. American Society for Parenteral and Enteral Nutrition (ASPEN) guidelines recommend consideration of megestrol as a first-line agent. If this fails, a corticosteroid trial for several weeks may be warranted (19).
Intestinal Dysfunction
Mechanical malfunction of the bowel is a particularly common problem among patients who have undergone abdominal radiation or extensive abdominal surgery. Postoperative or postirradiation adhesions can lead to partial or complete bowel obstruction. In patients with a disseminated intra-abdominal malignancy such as ovarian cancer, an adynamic ileus, or intestinal pseudoobstruction can result in a nonfunctional GI tract. Impaired capacity for self-feeding can markedly decrease food intake. Imaging or exploratory laparotomy may be indicated to define the nature, severity, and location of intestinal dysfunction.
Metabolic Disturbances
Cancer specifically affects nutrient metabolism. Patients with metastatic and localized cancer develop a chronic systemic inflammatory state that results in a hypermetabolism, including increased rates of hepatic glucose production, insulin resistance, whole-body glucose metabolism, lipolysis, and whole-body protein breakdown (20,21). These processes distinguish cancer associated weight loss from starvation, where weight loss is not accompanied by inflammatory and maladaptive metabolic changes. Improved nutrition often fails to correct such abnormalities when severe malnutrition is present, despite continuous parenteral or enteral alimentation with adequate nutrients (21–23). Specific metabolic disturbances and their consequences are presented in Table 19.1.
Table 19.1 Metabolic Consequences of Cancer

Clinical Features
Regardless of the metabolic features of malnutrition, weight loss is usually the presenting sign. Severe malnutrition can be defined as >10% weight loss over 2 to 3 months or a history of inadequate oral intake longer than 7 days. It is essential to know the patient’s usual weight and ideal body weight (IBW).
Ideal Body Weight
For the purposes of assessment for malnutrition in gynecologic patients, a practical formula that can be used for determining the IBW is the following:
Hamwi equation for women: Ideal body weight = 100 lb for 5 ft + 5 lb/in > 5 ft
For example, a woman whose height is 5 ft, 4 in would have an IBW of 120 pounds. This equation has never been validated, but was compared to the body mass index (BMI) of 4.2 million people from life insurance tables in 1964. Overall it is equivalent to other predictive formulas of IBW (24). Some sources recommend that for small frames, 10 pounds should be subtracted and for large frames, 10 pounds should be added, although no definition of frame measurement has been made.
For many common cancers, loss of as little as 6% of usual body weight can have significant prognostic effects on survival (25), and weight loss often mirrors declining performance status. Therefore, it’s not only important to consider a patient’s weight in terms of their IBW, but also to appreciate a change or loss in weight relative to their baseline.
Weight Loss
Weight loss results from loss of body fat, body protein, or body water. Each liter of body water lost represents a weight loss of 2.2 pounds, but this weight loss can be corrected rapidly with rehydration. The degree to which losses of body protein or fat dominate the clinical picture is a reflection of the body’s ability to adapt to a fat fuel economy in the face of inadequate nutrition (26). There are three basic types of malnutrition: Kwashiorkor, marasmus, and a combination of the two, cachexia (Fig. 19.3).
Kwashiorkor
This form of malnutrition is variously termed protein caloric malnutrition, hypoalbuminemic malnutrition, protein energy malnutrition, or kwashiorkor-like malnutrition of the adult. If malnutrition is rapid and occurs in the face of disease factors that affect nutrition, a rapid depletion of protein stores can occur out of proportion to the loss of body weight. Kwashiorkor originally referred to a tropical pediatric disease and meant “separation from the breast” in Swahili.
In hospitalized patients, the major signs of protein depletion are the following:
1. Decrease in serum albumin to less than 3.5 mg/dL
2. Decrease in absolute lymphocyte count (ALC) to less than 1,500/mm3
3. Decrease in serum transferrin to less than 150 mg/dL
4. Loss of reactivity to common skin test antigens
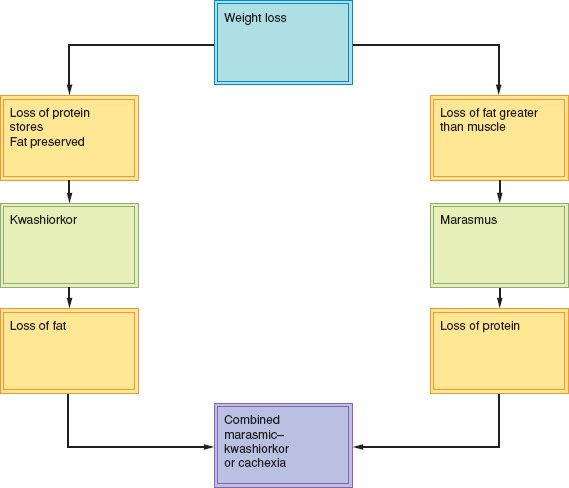
Figure 19.3 Classification of malnutrition.
It is possible for this form of malnutrition to occur in the absence of weight loss if the hypoalbuminemia leads to ascites or edema.
Marasmus
The other major form of malnutrition in adults is called marasmus, starvation, or chronic inanition. Primary malnutrition due to anorexia or dietary inadequacy is the most common form. It is characterized by a depletion of fat stores and the obvious appearance of malnutrition with visible loss of muscle and fat in the arms and legs. Although weight loss is often significant in these thin patients, protein stores can be remarkably preserved. It is not uncommon for the starved patient to have normal serum albumin and transferrin levels, a normal lymphocyte count and normal skin test responses.
Cachexia
When the two major forms of malnutrition occur together in patients with advanced malnutrition, the condition is called cachexia. Cachexia is a life-threatening condition and has also been termed combined marasmic–kwashiorkor or mixed-form malnutrition of the adult.
Cancer cachexia, also referred to as cancer cachexia syndrome (CCS), is related to proinflammatory cytokines such as IL-1, IL-2, IFNγ, and TNFα. Cytokines serve to decrease protein synthesis and increase proteolysis. Increased proteolysis releases amino acids to be utilized by the liver for energy production, and as precursors in the formation of C reactive protein and serum amyloid peptide. Cytokines also stimulate release of cortisol and catecholamines. Cachectic behavior further decreases a patient’s activity (20). In this advanced condition, there is depletion of body fat stores and body protein stores, which produce visible emaciation with loss of body muscle and fat, as well as decreased serum proteins.
Table 19.2 Physical/Biochemical Markers of Malnutrition
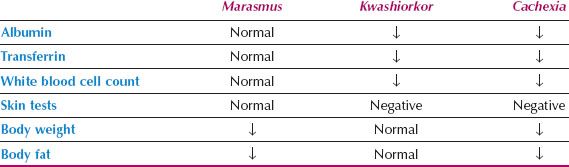
The exact contribution of malnutrition to mortality in hospitalized gynecologic oncology patients is difficult to quantify. The additive effects of malnutrition include impaired immunity, poor wound healing, and cardiorespiratory dysfunction, which all impact negatively on patient survival (27).
Diagnosis
To diagnose malnutrition, all patients should be at least minimally screened or preferably assessed, for malnutrition upon diagnosis of cancer (15). Low levels of circulating serum proteins can reflect impaired function of the liver and other organs, even in the absence of marked depletion of visceral and muscle protein (18). This usually occurs in the setting of excessive metabolic demands caused by specific illnesses that impair the body’s ability to conserve protein. Similarly, protein and fat stores can be depleted markedly, while circulating proteins remain in the normal range. This in turn reflects a gradual adaptation to starvation in adults with anorexia and primary malnutrition.
A nutritionist can provide the nutritional assessment, which should include a history, physical examination, and laboratory evaluation. The assessment should include current medical problems/treatments; social support and functional status; anthropomorphic measurements; calculation of IBW; BMI; and visceral protein measurement. The nutritional assessment can identify the current degree of malnutrition, determine the nutritional support required, and set the goals of therapy. Assaying immune function also contributes to an understanding of the nutritional status.
Anthropometry, in which body stores are estimated by direct measurements, and biochemical markers that assess circulating proteins, must be used in concert to determine the specific type of malnutrition in any given patient (Table 19.2).
Anthropometric techniques include the measurement of body weight and height in adults. The patient’s normal weight can be obtained from the history. The Hamwi formula described above is used to calculate the percentage of IBW. The BMI is calculated by dividing weight in kg by height in meters squared (kg/m2). Normal BMI is 18.5 to 25, overweight is 25 to 29.9, with obesity being >30, and underweight being <18.5.
Fat stores can be measured by assessing skin-fold thickness. The most commonly used skin fold in practice is the triceps. For this measurement, the patient sits with the right arm hanging freely at the side. For bedridden patients, the right arm is flexed at the shoulder while the forearm crosses the chest. The midpoint between the acromion and the olecranon posteriorly over the triceps muscle is marked. The skin and subcutaneous tissue at the midpoint are then pinched and pressure-regulated calipers are applied for 3 seconds before a reading is taken (28). The calipers are designed to deliver a pressure of 10 g/mm2 regardless of the fold thickness and can be used to compare the same patient’s progress over time and to assess the severity of malnutrition.
There are a number of other means of body fat assessment such as bioelectrical impedance analysis and submersion measurements, but each institution should be capable of providing at least one type of assessment.
Protein Store Assessment
Midarm circumference and midarm muscle circumference can help to estimate lean body mass/protein stores. Midarm muscle circumference (cm) = midarm circumference (cm) – (π × triceps skin-fold thickness (cm)). It is an attempt to estimate lean body mass. Protein stores can also be assayed by a number of circulating proteins, most of which are secreted by the liver (29,30). Their synthesis and secretion are inhibited rapidly in the presence of protein malnutrition and they decrease to a variable extent in the circulation according to their metabolic half-lives. The most widely used markers are albumin and transferrin. Each has advantages and disadvantages (29).
Table 19.3 Serum Half-life of Circulating Proteins Decreased in Malnutrition

Albumin
Albumin has a half-life of 21 days, so that significant decreases may not occur for up to 1 month after the onset of starvation. Albumin may be decreased by rapid loss of serum proteins (e.g., excessive losses from the GI tract), by dilution by volume resuscitation, or by fluid shifts into ascites. Restoration of the serum albumin to normal levels by nutritional means is slow and often lags behind clear improvement in nutritional status by other criteria.
Transferrin
Transferrin is synthesized in the liver and other sites, where it can act as a growth-promoting peptide. In the liver, synthesis is modulated by the iron stores in the hepatocytes and by the overall protein status. The half-life of the protein is 9 days and the body pool is only 5 g. The synthetic rate is the major factor determining serum levels and serum transferrin increases within 9 days of nutritional repletion. The problems with the interpretation of transferrin levels are that degradation rates increase during illness and iron deficiency falsely elevates the serum levels.
For these reasons transferrin and albumin must be interpreted within the context of anthropometric determinations of body weight and triceps skin-fold thickness.
Retinol- and Thyroxine-binding Proteins
Retinol-binding protein and thyroxine-binding prealbumin also are synthesized in the liver, with half-lives of 10 hours and 2 to 3 days, respectively. Their levels drop acutely with metabolic stress and retinol-binding protein is also filtered and broken down by the kidney. These factors complicate the interpretation of serum levels for the diagnosis of malnutrition, but they can be used in a research setting to assess more quickly the response to nutritional support.
Inflammation causes a ≥25% decrease in all of the aforementioned serum transport proteins (31). The serum half-lives of these circulating proteins are listed in Table 19.3.
Immune Function
The total lymphocyte count and delayed cutaneous hypersensitivity responses to skin test antigens are nonspecific markers of impaired immune function in malnourished patients (32):
1. Depressed levels of complement components, including C3
2. Reduced amounts of secretory immunoglobulin A in external body secretions
3. Abnormal T-cell function
4. Impairment of nonspecific defenses, including decreased epithelial integrity, decreased mucus production, and decreased ciliary motility.
Most patients with protein and caloric malnutrition have multiple deficiencies and almost any single nutritional deficiency, if severe enough, can affect immune function (33) (Figure 19.4). Correction of malnutrition improves immune function; this is especially true in the gynecologic oncology patient, whose immune function can be impaired by therapy as well as by the tumor itself.
Absolute Lymphocyte Count
The absolute lymphocyte count (ALC) is calculated by multiplying the percentage of lymphocytes by the total white blood cell count. The ALC and skin tests are the most widely used immune markers of nutritional status. The normal lymphocyte count is greater than 2,000/mm3 in patients who are not receiving chemotherapy. The ALC is not considered valid unless the white blood cell count is normal.
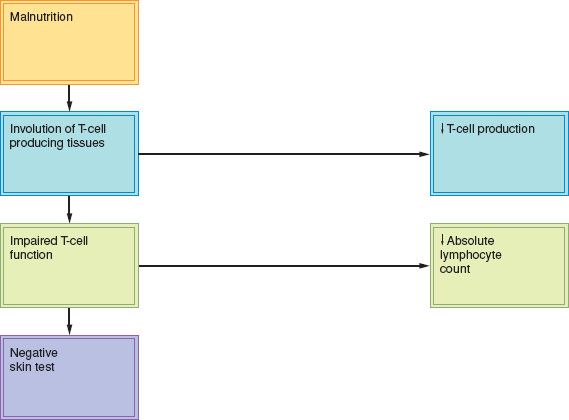
Figure 19.4 Immunologic alterations associated with malnutrition.
Most circulating lymphocytes are T cells and involution of the tissues producing T cells occurs early in the course of malnutrition. The delayed hypersensitivity skin test response reflects three processes:
1. Processing of the antigen by macrophages resulting in the generation of both effector and memory T cells
2. Recognition of antigen rechallenge resulting in blast transformation, cellular proliferation, and generation of lymphokine-producing effector cells
3. Production of a local wheal and flare, secondary to the actions of lymphokines and chemotactic factors at the skin site.
Antigens that are frequently tested include purified protein derivative, streptokinase-streptodornase, mumps, Candida, Trichophyton, and coccidioidin. The prevalence of nonreactivity to skin test antigens is approximately 50% in patients whose serum albumin level is less than 3 g/dL, but it can be as high as 30% in patients whose serum albumin level exceeds 3 g/dL. Other problems with interpretation of skin tests include the following:
1. Only about 60% of healthy patients respond to most of the antigens, so that failure to respond to one or two antigens may not be predictive
2. Primary illnesses, including sarcoidosis and lymphoma, as well as immunosuppressive drugs, produce energy.
The delayed hypersensitivity (DH) and ALC are useful in uncomplicated nutritional deficiencies, but are not typically considered useful in this patient population.
The assessment of malnutrition by means of clinical examination in combination with routinely available laboratory tests provides an accurate estimation in more than 70% of patients (17). Difficulties with each of these tests have kept the nutritional assessment from becoming part of the routine database for every hospitalized patient.
Subjective Global Assessment
This is the most commonly utilized tool by nutritionists in both surgical and medical hospitalized patients to assess the risk for malnutrition. It is subjective in that it does not rely on calipers or laboratory values, but rather on the patient’s history of weight change, dietary change, GI symptoms, functional status, and primary disease, coupled with the nutritionist’s physical examination and global rating. A variant of this, the Patient Generated SGA (PGSGA) has been developed for assessment of the oncology patient, and is considered highly sensitive (>90%) and specific for detection of malnutrition (34,35).
Prognostic Nutritional Index
The Prognostic Nutritional Index (PNI) combines anthropometric and laboratory tests to calculate a single index number. It is a linear predictive model of increased morbidity and mortality after surgical procedures and uses serum albumin (A) in g/dL; triceps skin fold (TSF) in mm; serum transferrin (TFN) in mg/dL; and delayed hypersensitivity (DH) response (0–2). The formula is:
PNI% = 158 − 16.6 × A − 0.78 × TSF − 0.2 × TFN − 5.8 × DH
For example, a well-nourished patient with A = 4.8, TSF = 14, TFN = 250, and DH = 2 has a PNI of 158 to 152.2, or a 5.8% chance of complications. On the other hand, a malnourished patient with abnormal indexes (A = 2.8, TSF = 9, TFN = 180, and DH = 1) has a PNI of 158 to 95.3, or a 62.7% chance of complications. A PNI of <40 is taken as well nourished, whereas a PNI of >40 is taken as evidence of malnutrition. In one study of 76 gynecologic oncology patients, serum albumin could be substituted for PNI to detect malnutrition (8). The PNI describes surgical risk reliably based on nutritional status.
Nutritional Support
Nutritional support is an adjunct to primary therapy for the gynecologic oncology patient. The aim is to prevent deterioration of nutritional status during planned primary therapy, such as radiation, surgery, and chemotherapy. Early initiation of nutritional support is desirable. This goal necessitates early evaluation, the proper choice of nutritional therapeutic modalities, and an accurate assessment of requirements.
After protein deficiency occurs, it is difficult to reverse, because less than 5% of the protein is replaced per day, regardless of the amount of substrate provided. Vitamins and minerals are replaced more easily, but there is no substitute for adequate planning to meet caloric and protein requirements essential for nutritional maintenance of vital functions.
The first intervention is dietary counseling. Modification of a patient’s diet to small and frequent meals with liquids taken only between meals may help address early satiety and anorexia. Commercially prepared fortified foods can improve the intake of energy and nutrients. For those who remain malnourished after these interventions, other means of support may be considered (36).
Caloric Requirements
The protein and caloric requirements can be estimated at 0.8 g/kg/d and 20 to 35 kcal/kg/d for healthy adults, respectively (12). If malnutrition exists or if the patient’s metabolism is elevated by infection or other metabolic stresses, then 1.5 to 2.5 g/kg/d of protein and 35 to 45 kcal/kg/d should be supplied. More exact formulas are available for pediatric patients and patients at the extremes of height and weight.
Need for Nutritional Therapy
Prior to initiating nutritional therapy, it is important to consider the overall treatment goals (palliative or curative) and the associated risks of each intervention. The decision to initiate nutritional therapy in patients with advanced cancer must take into account the patient’s wishes. The palliative use of nutritional therapy in terminally ill cancer patients is rarely indicated (37).
There are two key aspects of the patient’s nutritional status that affect decisions about nutritional therapy:
1. The degree of prior malnutrition at the time of assessment
2. The degree of hypermetabolism or metabolic abnormality expected to interfere with nutritional rehabilitation.
If the degree of prior malnutrition is minimal and the patient has only mild hypermetabolism after elective surgery, a temporary form of nutritional support can be used. On the other hand, if the patient requires additional calories to restore pre-existing severe malnutrition, forced intake of calories by an enteral or parenteral route can be used. The following guidelines should be applied:
1. If a patient is to be without nutrition for a period of 7 days, some form of nutritional therapy should be used.
2. If nutritional therapy is to be continued enterally for more than 4 weeks, a permanent intestinal access should be considered. If more than 2 weeks of parenteral nutritional support is required, long-term central access should be placed. Arrangements should be made for home enteral or parenteral nutrition (38).
Method of Support
The choice between parenteral and enteral therapy should be made on the basis of the availability and functional status of the GI tract (Fig. 19.5). If the GI tract is functioning normally, the expense and complications of parenteral nutrition are not warranted. Swallowing evaluation and nutritional assessment are useful in determining whether the enteral route is the best choice. Intake may also be improved by the pharmacologic therapies described previously. If the GI tract is functional, but oral intake remains inadequate, a feeding device should be placed to assist intake. Patients complaining of depression or pain should have these symptoms addressed concurrently.
Enteral Feeding
In view of the difficulties inherent in the use of parenteral nutritional support, every effort should be made to use the enteral route. Enteral access may be obtained at the bedside by placement of a nasal feeding tube, endoscopically by a gastroenterologic consultant, by an interventional radiologist under fluoroscopic or ultrasonic guidance, or by a surgeon in the operating room, depending upon the anticipated complexity of placement. If a long-term nasal feeding tube is required, it should be changed to alternating nostrils every 4 to 6 weeks (39). If enteral access is required beyond 4 weeks, consideration should be given to a gastrostomy or jejunostomy.
A gastrostomy port can be used at night for enteral support therapy by continuous infusion of isotonic enteral supplements at a rate no greater than 100 kcal/hr. The next day, the patient can cover the port with a dressing and go through her usual daily activities. This approach is often more acceptable to patients than a nasogastric tube, which is visible and irritating.
In some patients, the gastrostomy port has the added advantage that the stomach can be used as a reservoir for bolus feeding, which is more convenient. In cases of abnormal gastric motility, esophageal reflux, or possible aspiration of gastric contents, continuous slow infusion of supplement should be used, or a tube passed beyond the pylorus into the jejunum. The gastrostomy tube may also be used as a venting port should nausea and vomiting develop.
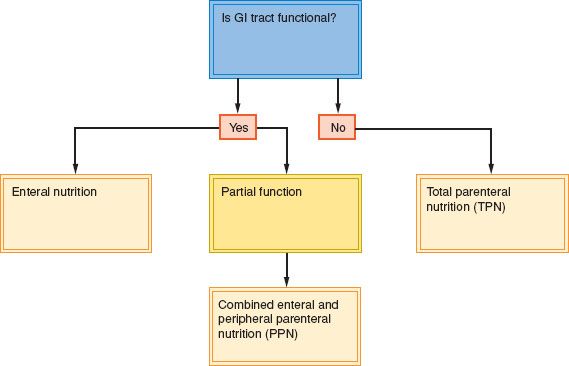
Figure 19.5 Parenteral versus enteral nutrition.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree