Bart J. Currie
Burkholderia pseudomallei and Burkholderia mallei
Melioidosis and Glanders
The genus Burkholderia is currently composed of many species, but only three are notable pathogens for humans or animals: the former cepacia complex (described in Chapter 222), pseudomallei (the agent of melioidosis), and mallei (the agent of equine glanders). All three are aerobic, nonsporulating, straight or slightly curved gram-negative bacilli that were formerly placed in the genus Pseudomonas.
Melioidosis
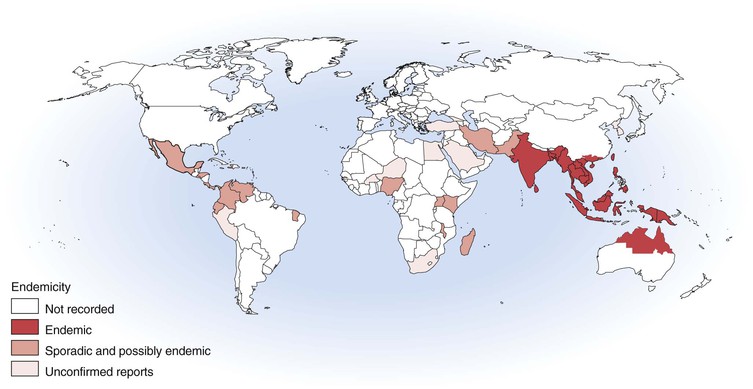
Melioidosis is a disease of humans and animals; it has enormous clinical diversity, spanning asymptomatic infection, localized skin ulcers or abscesses, chronic pneumonia mimicking tuberculosis, and fulminant septic shock with abscesses in multiple internal organs. Most disease is from recent infection, but latency with reactivation is described up to 62 years after exposure. Most cases are reported from Southeast Asia and northern Australia, but melioidosis is increasingly being recognized in other tropical and subtropical locations and in people infected in an endemic region who return or travel to Europe and the United States (Fig. 223-1).1 The causative bacterium, Burkholderia pseudomallei, is also considered a potential biological warfare agent.
History
In 1912, Whitmore and Krishnaswami2 described cases of a newly recognized septicemic disease in morphine addicts in Rangoon, Burma. Fatal cases were characterized by widespread caseous consolidation of the lung and abscesses in liver, spleen, kidney, and subcutaneous tissues. The bacillus isolated from tissues was similar to that causing glanders (Burkholderia mallei) but was motile. Whitmore noted the clinical similarity to glanders, and Stanton and Fletcher2a subsequently proposed the name melioidosis, derived from the Greek melis (distemper of asses). Various names were used for the causative bacterium, including Bacillus whitmori and, for many years, Pseudomonas pseudomallei.3 In 1992, seven Pseudomonas species were moved to a new genus, Burkholderia. B. cepacia is the type species in the genus, which includes the organisms causing melioidosis (B. pseudomallei) and glanders (B. mallei).
Etiology
B. pseudomallei is a small, gram-negative, oxidase-positive, motile, aerobic bacillus with occasional polar flagella. On staining, a bipolar “safety pin” pattern is seen. The organism is easily recovered on standard culture medium but may be misidentified as B. cepacia, B. thailandensis, P. stutzeri, or other Pseudomonas species.
The organism is present in soil and surface water in endemic regions. Humans and animals are infected by percutaneous inoculation, inhalation, aspiration, or ingestion. Occasional laboratory-acquired infections are described, but person-to-person spread and zoonotic infection are very uncommon.
Epidemiology
After the initial account in Burma, melioidosis was documented in humans and animals in Malaysia and Singapore from 1913 and then Vietnam from 1925 and Indonesia from 1929.4 Thailand has reported the largest number of cases,5–7 with an estimated 2000 to 3000 cases of melioidosis each year.8 Melioidosis is also common in Malaysia9 and Singapore.10,11 Other countries in the region where melioidosis is recognized in humans and animals include China (especially Hong Kong), Taiwan, Brunei, Vietnam, Cambodia, and Laos.12–18 Melioidosis is also likely to occur in the Philippines.8,19 Melioidosis has been increasingly recognized in India, although reports that some of the “plague” scares of 1994 may have been cases of melioidosis have been disproved.20,21 Cases have been reported from Sri Lanka, Bangladesh, and Pakistan.19 Despite the early documentation of melioidosis in Burma and Indonesia, recent cases had not been reported from Indonesia until after the 2004 Asian tsunami.22 Cases of melioidosis have also been documented from Papua New Guinea, Fiji, and New Caledonia,23 but the extent of endemicity in the Pacific islands remains to be defined.
Cases of melioidosis are increasingly being documented from outside the classic endemic region of Southeast Asia, Australasia, the Indian subcontinent, and China. These include sporadic human or animal cases or environmental isolates of B. pseudomallei from the Middle East, Africa, the Caribbean, and Central and South America. Although some of these reports are from incorrect species diagnosis, others are confirmed, making the endemic limitations of melioidosis very unclear.19 Sporadic cases and occasional case clusters have recently occurred in Brazil and elsewhere in the Americas.24 Despite recent cases from Madagascar and Nigeria,25,26 the true extent and magnitude of the presence of B. pseudomallei in Africa remains entirely unknown. Global warming may well result in expansion of the endemic boundaries of melioidosis.
The two locations where melioidosis is arguably the single most important bacterial pathogen for humans are some northeast provinces in Thailand and the Top End of the Northern Territory of Australia. In northeast Thailand, 20% of community-acquired septicemic cases are caused by melioidosis, which accounts for 39% of fatal septicemias6 and 36% of fatal community-acquired pneumonias.27 In the Top End of the Northern Territory, melioidosis has been the most common cause of fatal community-acquired bacteremic pneumonia.28
In addition to endemic melioidosis, there are several documented situations where melioidosis became established in nontropical locations. In France, in the 1970s, cases of melioidosis occurred in animals in a Paris zoo, with spread to other zoos and equestrian clubs.29 In addition to fatal animal and human cases, there was extensive soil contamination persisting for some years. B. pseudomallei is considered likely to have been introduced by importation of infected animals. A cluster of cases occurred over a 25-year period in southwestern Western Australia (31° S), involving animal cases and one human infection in a farmer. Ribotyping of the farm animal and human isolates and one isolate from the soil showed identical patterns.30 This supports the suggestion of clonal introduction of B. pseudomallei into this temperate region, probably via an infected animal, with environmental contamination, local dissemination, and persistence over 25 years.
Melioidosis was an important cause of morbidity and mortality in foreign troops fighting in Southeast Asia. Dance29 has noted that at least 100 cases occurred among French forces in Indochina between 1948 and 1954. By 1973, 343 cases had been reported in American troops fighting in Vietnam. Concerns of reactivation of latent infection in soldiers returning from Vietnam, with estimates from serology studies of approximately 225,000 potential cases, resulted in melioidosis being called the “Vietnamese time bomb.”31 However, although occasional cases of reactivation of B. pseudomallei still occur in Vietnam veterans, it is rare in comparison to the numbers of troops exposed.
Transmission
Figure 223-2 summarizes the natural history of infection with B. pseudomallei. Studies from Malaysia, and more recently from Thailand32 and Australia,33 have found that the organism is more common in cleared, irrigated sites, such as rice paddies and farms. It has been suggested that the increase in melioidosis cases in Thailand may partly be the consequence of the increased number of bacteria in such environments and partly the consequence of increased exposure to bacteria resulting from changes in behaviors, such as farming techniques.12 In Australia, B. pseudomallei has been found most commonly in clay soils to a depth of 25 to 45 cm, and it has been proposed that the bacteria move to the surface with the rising water table during the wet season.34 An alternative explanation for the variable bacterial presence found is that during times of stress, such as in prolonged dry seasons, B. pseudomallei may persist in soil in a viable but nonculturable state.35 Differential gene activation may allow such environmental bacteria to respond and adapt to different environmental conditions. This possibility is also relevant to the pathogenicity, latency, and reactivation of infection with B. pseudomallei in humans. Melioidosis endemic locations may vary in their specific ecological niches for B. pseudomallei. B. pseudomallei colonizes and thrives in the rhizosphere and aerial parts of native and imported grasses in northern Australia, raising implications for global epidemiology and potential dispersal.36 The role of biofilms in the persistence of B. pseudomallei in the environment, as well as in animal and human hosts, requires further study.37
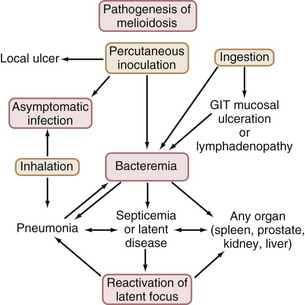
In most endemic regions, there is a close association between melioidosis and rainfall. In northeast Thailand38 and northern Australia,4 75% and 81% of cases, respectively, have occurred in the wet season. Although early animal studies showed infection with B. pseudomallei through oral or nasal exposure and from ingestion, more recent reviews have considered that most human cases are from percutaneous inoculation of B. pseudomallei after exposure to muddy soils or surface water in endemic locations.3,19,28 Ingestion and sexual transmission have been suggested as unusual modes of transmission of B. pseudomallei. Nevertheless, a recent study from Thailand raised the possibility that ingestion of water contaminated with B. pseudomallei may be a more common infecting event than previously thought, especially in endemic regions with unchlorinated water supplies.38a Presentations of melioidosis pneumonia after presumptive inoculating skin injuries have been documented in patients with soil-contaminated burns and are also common in tropical Australia.28 This suggests hematogenous spread to the lung rather than inhalation or spread from the upper respiratory tract. However, under certain epidemiologic conditions, the inhalation route may predominate, as suggested for soldiers exposed to dusts raised by helicopter rotor blades in Vietnam.39 Melioidosis after near-drowning is well documented, with the probable infecting event being aspiration.13 Intensity of rainfall is an independent predictor of melioidosis, manifesting as pneumonia and of a fatal outcome,40 suggesting that heavy monsoonal rainfall and winds may result in a shift toward inhalation as the mode of infection with B. pseudomallei. Currently, the overall proportion of melioidosis cases resulting from inhalation rather than percutaneous inoculation remains uncertain.1,41 Several outbreaks of melioidosis in Australia have been linked to contamination of potable water with B. pseudomallei.42,43 The water supplies involved were unchlorinated or the chlorination was below standard. The contamination of the water supply has been attributed to soil disturbance during excavations.
The incubation period for melioidosis is influenced by inoculating dose, mode of infection, host risk factors, and probably differential virulence of infecting B. pseudomallei strains. Onset of melioidosis within 24 hours has been seen in presumed aspiration after near-drowning and, in some cases, after severe weather events. In 25 cases of acute melioidosis in which a clear incubation period could be determined between the inoculating injury and the onset of symptoms, the incubation period was 1 to 21 days (mean, 9 days),44 which is consistent with a series of nosocomial cases from Thailand, in which the incubation period was 3 to 16 days (mean, 9.5 days).45
Pathogenesis
Serology studies have shown that most infection with B. pseudomallei is asymptomatic.46,47 In northeast Thailand, most of the rural population is seropositive by indirect hemagglutination (IHA),38 with most seroconversion occurring between 6 months and 4 years of age.47 Although melioidosis occurs in all age groups, severe clinical disease, such as septicemic pneumonia, is seen mostly in those with risk factors such as diabetes, renal disease, and hazardous alcohol use.
In addition to infection by inhalation, bacterial load on exposure (inoculating dose) and virulence of the infecting strain of B. pseudomallei are also likely to influence the severity of disease. However, it has been noted that despite the large bacterial load in severely ill patients with septicemic pulmonary melioidosis, person-to-person transmission is extremely unusual. This, together with the rarity of fulminant melioidosis in healthy people, supports the primary importance of host risk factors for development of melioidosis. Furthermore, although it is clear from laboratory studies of isolates of B. pseudomallei from animals, humans, and the environment that virulence differs among B. pseudomallei isolates,48 the importance of this variation in virulence in determining clinical aspects of melioidosis remains uncertain. Molecular typing that shows clonality of isolates in animal and human clusters has revealed that the same outbreak strain can cause different clinical presentations, with host factors being most important in determining the severity of disease.43 Whole-genome sequencing and subsequent molecular studies have shown that B. pseudomallei has two chromosomes, with a complex accessory genome that includes multiple genomic islands that are variably present in different strains and have a great propensity for horizontal gene transfer.49,50 Further studies are required to unravel the global phylogeny and evolutionary history of B. pseudomallei and related species and to determine which genes or gene clusters may be critical for pathogenesis and disease presentation and outcome.1,51
B. pseudomallei is a facultative intracellular pathogen that can invade and replicate inside various cells, including polymorphonuclear leukocytes and macrophages and some epithelial cell lines.52 Resistance to human serum (conferred by lipopolysaccharide [LPS])53 and the ability of B. pseudomallei to survive intracellularly (conferred in part by capsular polysaccharide) appear to be critical in the pathogenesis of melioidosis.54,55 Type III and type VI secretion systems in B. pseudomallei have also been found to be important in cell invasion and intracellular survival.56,57 Quorum sensing may play an important role in many aspects of virulence of B. pseudomallei, including cell invasion, cytotoxicity, and antimicrobial resistance.37,52,58 Other putative virulence factor candidates include flagella, type IV pili and other adhesins, a siderophore, and secreted proteins, such as hemolysin, lipases, and proteases.52 Burkholderia lethal factor-1 is similar to Escherichia coli cytotoxic necrotizing factor-1 and interferes with initiation of translation, leading to alteration of the actin cytoskeleton and ultimately cell death.59
Intracellular survival of B. pseudomallei in human and animal hosts is likely to explain the ability for latency. After internalization, B. pseudomallei escapes from endocytic vacuoles into the cell cytoplasm, and induction of actin polymerization at one bacterial pole leads to membrane protrusions, with cell-to-cell spread involving these actin tails.60 An additional survival factor for B. pseudomallei is the ability for phenotypic switching with a change in colony morphology, resulting in changes in the expression of putative virulence factors, such as biofilm and flagella.61
There have been a number of studies showing elevated levels of various endogenous inflammatory mediators and cytokines to be associated with severity and outcomes of melioidosis. Nevertheless, whether these elevated cytokines are a cause or result of severe disease is not established. In Thailand, there was an association of severe melioidosis with tumor necrosis factor-α (TNF-α) gene allele 2, which is linked to higher constitutive and inducible production of TNF-α.62 However, in a mouse model of melioidosis, neutralization of TNF-α or interleukin (IL)-12 increased susceptibility to infection in vivo, and interferon-γ (IFN-γ) was found to be important for survival, with mice treated with monoclonal anti–IFN-γ dying more quickly.63 A role for Toll-like receptors in the innate immune response in melioidosis has been proposed.64,65 There are, therefore, important host protective mechanisms against B. pseudomallei in cytokine responses as well as potentially detrimental ones, with the timing of cytokine release and the balance between proinflammatory and anti-inflammatory responses likely to determine the severity of disease and outcome of infection.52,66 The extent to which host polymorphisms in immune response contribute in comparison to differences in organism virulence, infecting dose of B. pseudomallei, and defined host risk factors, such as diabetes, remains to be clarified. Nevertheless, the predominant association with fatal melioidosis is the presence of defined patient risk factors.
Although a vigorous cell-mediated immune response may protect against disease progression,67 there is no definitive evidence for the development of immunity from melioidosis after natural exposure to B. pseudomallei, and reinfection can occur with a different strain of B. pseudomallei after successful treatment of melioidosis.68
Table 223-1 summarizes the risk factors for melioidosis. The most important risk factors are diabetes, hazardous alcohol use, and renal disease.3,4,69 In Thailand, the adjusted odds ratios for diabetes and renal disease (chronic renal impairment or renal or ureteric calculi) in cases of melioidosis versus control subjects were 12.9 (95% confidence interval [CI], 5.1 to 37.2) and 2.9 (95% CI, 1.7 to 5.0), respectively.69 Other risk factors for melioidosis include chronic lung disease (including cystic fibrosis), thalassemia (odds ratio in Thailand, 10.2; 95% CI, 3.5 to 30.8), malignancies, steroid therapy, iron overload, and tuberculosis.69 Severe disease and fatalities are uncommon in those without risk factors who are diagnosed and treated early, with only two deaths in 106 patients without risk factors, in one study.4 Risk factors are less common in children than in adults.70,71
TABLE 223-1
Risk Factors for Melioidosis
| RISK FACTOR* | THAILAND (% of cases) | AUSTRALIA (% of cases) |
| Diabetes | 23-60 | 37 |
| Alcohol excess | 12 | 39 |
| Renal disease | 20-27 | 10 |
| Chronic lung disease | NR | 27 |
| Thalassemia | 7 | 0 |
| No risk factors | 24-36 | 20 |
* Not listed: malignancy, steroid therapy, iron overload, cardiac failure.
NR, not reported.
Australia data from Currie BJ, Fisher DA, Howard DM, et al. Endemic melioidosis in tropical northern Australia: a 10-year prospective study and review of the literature. Clin Infect Dis. 2000;31:981-986.
Thailand data from Punyagupta S. Melioidosis: review of 686 cases and presentation of a new clinical classification. In: Punyagupta S, Sirisanthana T, Stapatayavong B, eds. Melioidosis. Bangkok: Bangkok Medical; 1989:217-229; Chaowagul W, White NJ, Dance DA, et al. Melioidosis: a major cause of community-acquired septicemia in northeastern Thailand. J Infect Dis. 1989;159:890-899; Suputtamongkol Y, Chaowagul W, Chetchotisakd P, et al. Risk factors for melioidosis and bacteremic melioidosis. Clin Infect Dis. 1999;29:408-413; and Limmathurotsakul D, Chaowagul W, Chierakul W, et al. Risk factors for recurrent melioidosis in northeast Thailand. Clin Infect Dis. 2006;43:979-986.
Evidence suggests that the predisposition to melioidosis in those with diabetes, hazardous alcohol use, or chronic renal disease, may reflect impairment of innate immune function, especially neutrophil and other phagocytic cell functions, such as mobilization, delivery, adherence, ingestion, and killing.4,72 Melioidosis has also been described in chronic granulomatous disease.73
Clinical Manifestations
The earliest descriptions of melioidosis documented the fulminant end of the clinical spectrum, with abscesses throughout both lungs and in many organs.2 At the other end of the spectrum are asymptomatic infections and localized skin ulcers or abscesses without systemic illness. Howe and colleagues39 classified melioidosis as acute, subacute, and chronic. The Infectious Disease Association of Thailand summarized 345 cases in these categories3,5:
1. Multifocal infection with septicemia (45% of cases, 87% mortality)
2. Localized infection with septicemia (12% of cases, 17% mortality)
3. Localized infection (42% of cases, 9% mortality)
Bacteremia and overall mortality rates have been, respectively, 60% and 44% in Thailand,38 55% and 14% in Australia,4 and 43% and 39% in Singapore.19
Table 223-2 summarizes the clinical manifestations in patients with melioidosis in northern Australia. Pneumonia is the commonest clinical manifestation in patients with melioidosis in all studies, accounting for about half of cases. Secondary pneumonia after another primary presentation occurs in about 10% of cases. Acute melioidosis pneumonia has a spectrum from fulminant septic shock (mortality up to 90%; Figs. 223-3 to 223-6) to mild undifferentiated pneumonia, which can be acute or subacute in nature, with little mortality.74 Septicemic patients present acutely unwell with high fevers and prostration and often little initial cough or pleuritic pain. On chest radiographs, diffuse nodular infiltrates often develop throughout both lungs, and they coalesce, cavitate, and progress rapidly, consistent with the caseous necrosis and multiple metastatic abscess formation seen at autopsy. Nonsepticemic patients with pneumonia and some with septicemic pneumonia have a more predominant cough, with productive sputum and dyspnea, and their chest radiographs show discrete but progressive consolidation in one or more lobes (Fig. 223-7). In endemic regions, acute pneumonia with upper lobe consolidation warrants consideration of melioidosis, although lower lobe infiltrates are also common.
TABLE 223-2
Clinical Presentations and Outcomes of Melioidosis in Northern Australia
| TOTAL | BACTEREMIC | NONBACTEREMIC | ||||
| Number | Deaths (Mortality) | Number | Deaths (Mortality) | Number | Deaths (Mortality) | |
| Septic Shock Present | 116 (21%) | 58 (50%) | 103 | 48 (47%) | 13 | 10 (77%) |
| Pneumonia | 88 | 43 (49%) | 78 | 35 (45%) | 10* | 8 (80%) |
| No evident focus | 13 | 8 (62%) | 12 | 7 (58%) | 1† | 1 (100%) |
| Genitourinary | 10 | 5 (50%) | 9 | 4 (44%) | 1‡ | 1 (100%) |
| Osteomyelitis/septic arthritis | 4 | 2 (50%) | 4 | 2 (50%) | 0 | 0 (0%) |
| Soft tissue abscess | 1 | 0 (0%) | 0 | 0 | 1 | 0 (0%) |
| Not Septic Shock | 424 (79%) | 19 (4%) | 195 | 13 (7%) | 229 | 6 (3%) |
| Pneumonia | 190 | 12 (6%) | 89 | 9 (10%) | 101 | 3 (3%) |
| Skin infection | 68 | 0 (0%) | 1 | 0 (0%) | 67 | 0 (0%) |
| Genitourinary | 66 | 2 (3%) | 41 | 2 (5%) | 25 | 0 (0%) |
| No evident focus | 52 | 2 (4%) | 47 | 2 (4%) | 5 | 0 (0%) |
| Soft tissue abscess(es) | 18 | 0 (0%) | 4 | 0 (0%) | 14 | 0 (0%) |
| Osteomyelitis/septic arthritis | 16 | 0 (0%) | 10 | 0 (0%) | 6 | 0 (0%) |
| Neurologic | 14 | 3 (21%) | 3 | 0 (0%) | 11 | 3 (27%) |
| Total | 540 | 77 (14%) | 298 (55%) | 61 (20%) | 242 (45%) | 16 (7%) |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








