BRAIN ABSCESS
MATTHIAS KLEIN, HANS-WALTER PFISTER, ALLAN R. TUNKEL, AND W. MICHAEL SCHELD
DEFINITION
Brain abscess is a focal intracerebral infection that begins as a localized area of cerebritis and develops into a collection of pus surrounded by a well-vascularized capsule. It continues to be a diagnostic and therapeutic challenge to the clinician.
HISTORY
The first reference to a brain abscess is attributed to Hippocrates in the fifth century BC (1). He described a syndrome of purulent otorrhea and fever associated with cerebral symptoms and stressed that the chances of survival depended on the external draining of purulent material: “Chills, pain, and fever throughout the head, especially in the ear, temples, and bregma. . . . If anyone moves him, he vomits copiously and easily. . . . If, in this patient, a watery discharge breaks out through the nostrils or ears, it runs out mixed with pus, and he recovers; if not, he usually dies in seven days.” The Corpus Hippocraticum even advocated surgical therapy: “Then incise the head at the bregma; bore right through to the brain, and heal the wound as you would one made by sawing.” The first documented successful drainage of a brain abscess (of otitic origin) is attributed to the French surgeon S.F. Morand in 1752 (2). In 1872, the army surgeon J. F. Weeds reported on the successful drainage of a traumatic frontal brain abscess and reviewed the American and European medical literature (2). Of 214 cases of brain abscess on record, only 4 cases resulted in recovery. Weeds noted that “not a single case recovered without the abscess being opened and its contents evacuated, either by nature or by the knife of the surgeon.” In 1893, Sir William MacEwen published his famous monograph Pyogenic Infective Diseases of the Brain and Spinal Cord (2). Nineteen patients operated on for a cerebral or cerebellar abscess were reported in that work; eighteen of these recovered and only one patient died. This publication established MacEwen as the “father” of modern brain abscess management. Diagnostic delay was the major obstacle in the success of therapeutic intervention, as Victor Horsley put it in 1888, “These cases are fortunately simple, perhaps the simplest, examples of intracranial surgery, but there remains yet the method of diagnosis” (3). In the twentieth century, refined surgical techniques, introduction of antibiotics, and finally the advent of computed tomography (CT) have contributed to a significant improvement in the mortality and morbidity rates associated with brain abscess (e.g., 4% mortality from 1981 to 1986 in a study by Mampalam and Rosenblum [4]). Among the major problems in the twenty-first century are postoperative intracranial infections due to antimicrobial-resistant bacteria and growing numbers of immunosuppressed patients who have an increased risk of developing a brain abscess, mostly due to “atypical” pathogens such as fungi, and excessive mortality rates of up to 97% (5,6). Further progress in the therapy of brain abscess is therefore necessary.
EPIDEMIOLOGY
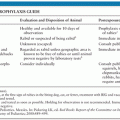
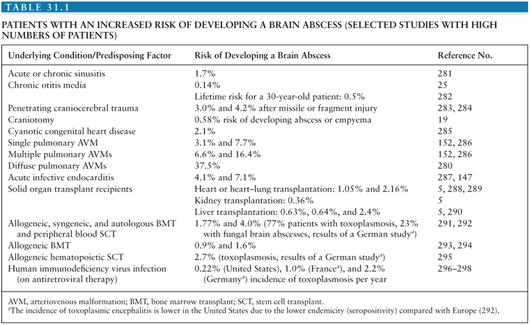
The incidence of brain abscess is estimated at 0.3 to 1.3 cases per 100,000 people per year (7,8) with a male-to-female ratio of 2:1 to 4:1 (4,9,10). One important factor in the incidence of brain abscess (as is true for most infectious diseases) seems to be the general health status of the population: In a study on 973 patients from South Africa with brain abscess from 1983 to 2002, the incidence dropped by 50% from 1983 to 2002, mainly due to improvements of socioeconomic standards and increased availability of primary health care services (9). In the total population, the incidence of brain abscess is relatively low; however, the risk is markedly elevated in certain patient groups (Table 31.1).
ETIOLOGY
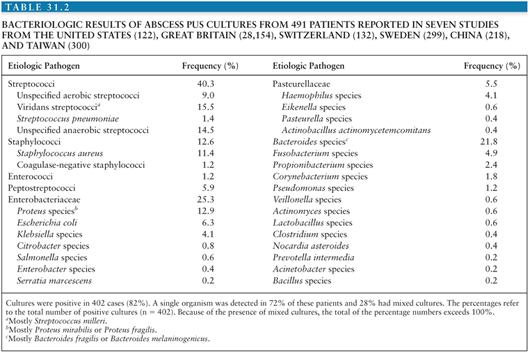
In the preantibiotic era, analysis of intracranial pus revealed Staphylococcus aureus in 25% to 30% of patients, streptococci in 30%, coliforms in 12%, and no growth in approximately 50% (11,12). With proper attention to techniques, the role of anaerobic agents in brain abscess has become apparent. In one earlier study (13), 14 of 18 abscesses grew anaerobes on culture—predominantly streptococci in 66%, with Bacteroides species in 60%. Series from the United Kingdom have stressed the role of anaerobic bacteria in brain abscesses, especially of otitic origin (11,12). The results of seven studies (in adults and children) demonstrate a particularly important role for streptococci, Enterobacteriaceae (in particular Proteus species), and Bacteroides species (Table 31.2). The organisms and their frequency of isolation in exclusively pediatric-based series of brain abscess are similar to these figures except in the neonatal setting: In a large study on neonatal brain abscess, Proteus mirabilis was identified in 27 (90%) of 30 cases, Escherichia coli in two, and Serratia marcescens in another newborn (14).
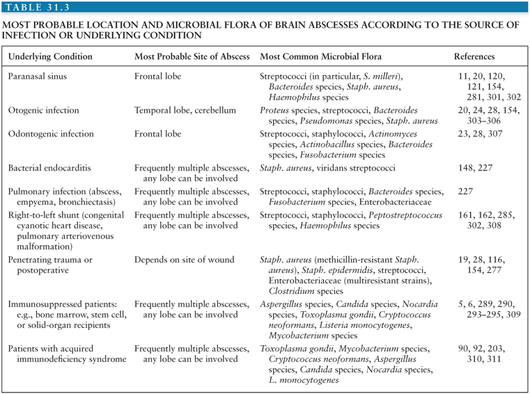
The location of a given brain abscess or its predisposing cause often suggests the most likely etiologic agents (Table 31.3).
Bacteria
The streptococci represent a diverse group of organisms; however, those most frequently isolated in patients with brain abscess belong to the Streptococcus milleri group (Streptococcus anginosus, Streptococcus constellatus, Streptococcus intermedius), which has a recognized predilection for causing focal suppurative disease (15–17). Streptococci from this group are often microaerophilic. Streptococcus pneumoniae, despite being the leading cause of bacterial meningitis in adults, is only rarely cultured from patients with brain abscesses (18).
S. aureus is the most common cause of traumatic and postoperative brain abscesses, often isolated in pure culture (9). After craniotomy, S. aureus accounted for almost 50% and Staph. epidermidis for 5% of intracranial infections in a large prospective study of 2,944 patients (19) and the proportion of methicillin-resistant Staph. aureus (MRSA) was very high (41%). In a recent study from Argentina, MRSA was even identified in 10% of 80 patients with brain abscess (15).
Enterococcus faecalis has been reported as a rare cause of otogenic, metastatic (e.g., due to endocarditis), meningitis-associated, and neonatal brain abscess (20–22). Peptostreptococci, which are a common oropharyngeal flora, have been occasionally found in odontogenic, otorhinogenic, postoperative, and metastatic (e.g., following esophageal dilation) brain abscess (20,23).
Enterobacteriaceae are usually found in mixed cultures. Proteus species are the most commonly isolated organism; they exhibit a particular association with otogenic disease (17,24,25) and neonatal brain abscess (26). Brain abscesses due to Proteus species have also been reported after neurosurgical procedures (27). E. coli is sometimes found in otogenic or metastatic brain abscesses (28). It is also a cause of neonatal brain abscesses (29). In two studies from Taiwan (30,31), Klebsiella species (mostly Klebsiella pneumoniae, less often Klebsiella oxytoca) were common (some with a gas-forming appearance) in patients with debilitating diseases (in particular, diabetes mellitus). Citrobacter species, especially Citrobacter diversus, are often implicated in brain abscesses that arise as a complication of neonatal meningitis (32,33), but they are very rare in adults with brain abscesses (34). Salmonella (S. typhi, S. typhimurium, and S. enteritidis of group B, C, or D) brain abscess is also uncommon (35). This disease is most likely to occur in adults with certain predisposing factors like meningitis, tumor, ischemia, trauma, intracranial hematoma, and human immunodeficiency virus (HIV) infection (36–38). Enterobacter species, though another rare cause of brain abscess (39), must be considered in postoperative cases (27). Serratia marcescens is another rare cause of neonatal brain abscess (40,41). In adults, only few cases with opportunistic S. marcescens brain abscess are reported (42,43). In general, Enterobacteriaceae must be taken into account especially in postoperative deep brain infections (9,19,35).
Aggregatibacter aphrophilus and Aggregatibacter paraphrophilus are most often isolated in brain abscesses due to Haemophilus species (16,17,44,45). Brain abscess secondary to H. influenzae is rare (46). Eikenella corrodens, which belongs to the oropharyngeal flora, has been occasionally detected in particular in otorhinogenic or odontogenic brain abscess (47,48). Pasteurella multocida brain abscess has been reported as a rare complication of animal scratches or bites (49–51). Actinobacillus actinomycetemcomitans is sometimes encountered in polymicrobial odontogenic brain abscesses, often in conjunction with Actinomyces infection (52,53).
Pseudomonas aeruginosa is mainly associated with otogenic brain abscess but has also been detected as a postoperative, posttraumatic, or as a hematogenous complication (9,20,35,54). Anaerobes are significant causative pathogens of brain abscess, often found in mixed culture (16,35). Bacteroides and Fusobacterium species are most frequently isolated. Brain abscesses due to Clostridium species (typically with a gas-forming appearance) may develop in particular after penetrating head trauma with soil or fecal contamination of the wound. Clostridium infections must also be considered postoperatively (55). In particular, cases due to Clostridium welchii, Clostridium perfringens, Clostridium septicum, Clostridium tertium, and Clostridium bifermentans have been reported (56–58). Propionibacterium acnes has also gained attention as a cause of postoperative and posttraumatic brain abscess, being reported to occur up to 10 years after surgery (59–61). Few cases of a brain abscess due to corynebacteria have been reported (62–64). Actinomyces species are anaerobic human commensals of low pathogenicity, which have been repeatedly recovered from brain abscesses (65). Two thirds of the patients with central nervous system (CNS) actinomycosis have an obvious primary site of infection distant from the CNS, most commonly the lung or cervicofacial region. When no obvious source is apparent, occult dental infection is often suspected (66).
Especially in immunocompromised hosts with brain abscess, Listeria monocytogenes or Nocardia species can be found as causative pathogens (more than 90% of patients with listerial brain abscess and 34% with nocardial brain abscess are immunocompromised [9,30,35,67]). N. asteroides was isolated in 98% of nocardial brain abscesses; single cases were due to N. farcinica and N. caviae (67). N. asteroides infections of the brain often had evidence of a pulmonary portal of entry (38% of cases). Bacillus cereus (68,69), Rhodococcus equi (70,71), and Gordona terrae (72) are other recognized pathogens of brain abscess in immunocompromised hosts. In approximately 5% to 10% of cases of meningitis due to Mycobacterium tuberculosis, tuberculous granulomas (tuberculomas) can be found (73,74); they usually resolve with medical therapy. When the caseous core of a tuberculoma liquefies, a tuberculous abscess results; tuberculous brain abscesses are usually larger and less common than tuberculomas, may be multiloculated, and often have greater mass effect and edema (73). Depending on the prevalence of tuberculosis, a considerable proportion of focal intracranial infections may be due to M. tuberculosis (9,30), reaching 56% in a Saudi Arabian study (75). In addition to M. tuberculosis, other mycobacteria were observed to cause focal CNS lesions; these include Mycobacterium bovis (bacille Calmette-Guérin) in an immunocompromised child (76), Mycobacterium kansasii in patients with acquired immunodeficiency syndrome (AIDS) (77), and Mycobacterium avium complex in patients with and without AIDS (78).
Yeasts and Fungi
Fungi have assumed an increasing role as the etiologic agents of focal intracranial infections. Many pathogenic fungi, such as Cryptococcus neoformans, Candida species, Sporothrix schenckii, Aspergillus species, and Zygomycetes species are ubiquitous (79). Others are geographically restricted, such as Coccidioides immitis (southwest United States, northern Mexico, and Argentina), Histoplasma capsulatum (greater Mississippi Valley, along the U.S.–Mexico border, in multiple regions of Central and South America, and in scattered areas around the world), and Blastomyces dermatitidis (midwestern and mideastern United States, along the St. Lawrence River, and in certain countries in Africa, the Middle East, and India). Most cases occur in immunocompromised patients, and mortality remains high.
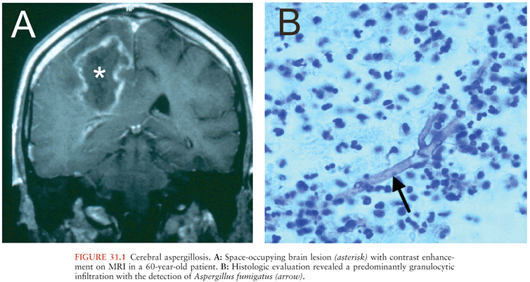
Cerebral aspergillosis occurs in 10% to 20% of all cases of invasive aspergillosis (Fig. 31.1) and only rarely is the brain the sole site of infection (80). Without therapy, mortality is high, but survival rates of up to 31% are reported in patients receiving voriconazole therapy (81,82). Among the most important risk factors for invasive aspergillosis are immunosuppressive therapy (including high-dose steroids), hematologic malignancies, solid-organ and bone marrow transplantation, and HIV infection (80,83). A report of brain abscesses in bone marrow transplant recipients observed that 33 (58%) of 62 cases were due to Aspergillus species (mostly Aspergillus fumigatus, with a few cases of Aspergillus flavus and Aspergillus terreus) (6). Fungi of the Zygomycetes (formerly Phycomycetes) class can also invade the CNS. Rhino-(orbital-)cerebral mucormycosis usually affects immunocompromised patients, in particular patients with diabetes mellitus, hematologic malignancies, or stem-cell and solid organ transplantation (84–86). Isolated cerebral mucormycosis has been observed mostly in injection drug users with or without AIDS (87), but also in patients with hematologic malignancies (88) or pharmacologic immunosuppression (89). Candidiasis is the fungal infection most often observed at autopsy to be involving the CNS (90). Cases of diffuse cerebritis with miliary microabscesses, large parenchymal abscesses, meningitis, and cerebrovascular complications have been described (90). Risk factors for the development of CNS candidiasis are immunosuppression, treatment with antibiotics or corticosteroids, intravascular catheters, recent abdominal surgery, prematurity (in neonates), recent neurosurgery or cerebrospinal fluid (CSF) diversion, and injection drug abuse (90). Cryptococcus neoformans usually causes meningitis when it invades the CNS, but mass lesions due to this organism have been observed. Neuroradiologic studies have shown cryptococcoma (granuloma) in 11% of patients with cryptococcal meningitis studied by CT (91) and in 50% of patients studied by magnetic resonance imaging (MRI) (92).
Scedosporium apiospermum (formerly known as Allescheria boydii, Pseudallescheria boydii, and Petriellidium boydii) has received increased attention as a potential cause of CNS infection (93). In addition to immunosuppression, near drowning in polluted water and rarely direct inoculation (orbital trauma, lumbar puncture) were reported as predisposing conditions (94,95). Cladosporium trichoides (Xylohypha bantianum, Cladosporium bantianum) is the most common cause of cerebral phaeohyphomycosis, accounting for 28 of 53 reviewed cases (96).
Protozoa and Helminths
Various protozoa and helminths may cause brain abscesses, with their incidence varying with geographic location. Toxoplasmosis is one of the most common parasitic infections of the brain, particularly in the setting of AIDS (97) (see later discussion) but also in patients after bone marrow transplantation (98,99). Entamoeba histolytica infections rarely involve the brain (100). The lesions are usually multiple and are most often associated with another focal site of infection, usually hepatic. Free-living amebas (Naegleria species, Acanthamoeba species, and Balamuthia species) are preferentially neurotropic (101,102). CNS infection with Naegleria fowleri typically presents as acute fulminant meningoencephalitis (primary amebic meningoencephalitis) in immunocompetent children and adults after exposure to polluted water (water-related sport activities in the tropical and subtropical climates) (102). Balamuthia mandrillaris and Acanthamoeba species cause granulomatous amebic encephalitis, which has a subacute or chronic course and is usually seen in immunocompromised individuals (in particular, in patients with AIDS) (102,103). Amebic CNS infections are usually fatal.
Neurocysticercosis (due to Taenia solium larvae) is the most common parasitic infection of the human nervous system (104–107). It is endemic in many parts of the world, particularly Latin America, Africa, and Asia, and still relatively common in Portugal, Spain, and Eastern European countries. In many developed countries with high rates of immigration from endemic areas, neurocysticercosis is an imported disease (107,108). Cysticercosis is probably the single most important cause of acquired epilepsy in the developing world (109). Focal brain lesions in neurocysticercosis usually present as (solid-enhancing) granulomas, calcified nodules, or (ring-enhancing) cystic lesions with or without a scolex (109). Other helminthic infections that occasionally lead to focal intracranial lesions include schistosomiasis (110), echinococcosis (111), paragonimiasis (112), trichinosis (113), sparganosis (114), and infections due to Angiostrongylus cantonensis (115) or Strongyloides stercoralis (116).
PATHOGENESIS
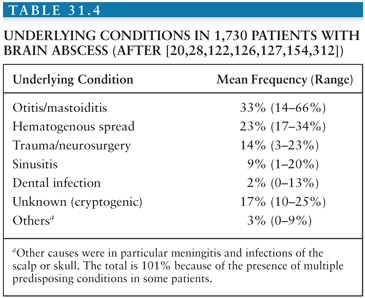
Brain abscesses occur most commonly in association with one of three distinct clinical settings: (a) a contiguous focus of infection, (b) cranial trauma or neurosurgical procedures, or (c) hematogenous spread from a distant focus. No predisposing factors are recognized in approximately 10% to 25% of reported cases (Table 31.4).
Contiguous Infectious Foci
Most patients with brain abscess demonstrate a contiguous focus of infection, usually sinusitis or otitis.
Otitis/Mastoiditis
The incidence of otogenic brain abscess (Tables 31.1 and 31.4) seems to decrease in developed countries. However, in areas where otitis media continues to be neglected or where therapy is delayed, intracranial complications of this process still present a particularly serious threat (117). Chronic otitis media and/or mastoiditis leads to intracranial extension much more often than acute disease. In particular, cholesteatoma is a common associated finding in otogenic brain abscesses, being found in 38% to 98% of patients (24,25). Most otogenic brain abscesses are solitary lesions. The majority of otogenic brain abscesses (55% to 75%) are located in the temporal lobe (24,25). The cerebellum is the next most commonly affected area (20% to 30%), and it has been observed that approximately 90% of cerebellar abscesses are secondary to otogenic infections (118). Otogenic intracranial suppuration most often develops in patients younger than 30 years and shows a male predominance (24,25).
Sinusitis
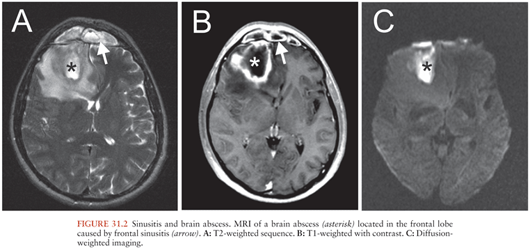
Sinugenic brain abscesses are almost exclusively located in the frontal lobe (Fig. 31.2), reflecting the distribution of the associated sinusitis (119–121). Most cases are in the setting of frontoethmoidal disease followed by maxillary disease (122). Sphenoid sinusitis, despite its relative rarity (approximately 3% of all sinusitis cases), has a relatively high rate of intracranial complications (123). Intracranial suppuration secondary to sinusitis predominates in men in their second or third decade of life (119,120).
Dental Infection
Dental infections have less often been documented as a predisposing cause of a brain abscess in the past (Table 31.4), but a recent study indicated an important role for dental infection as a source of brain abscess in up to 12% of patients (16). Given the frequency of dental infections, however, intracranial complications of this process are rare. The site of an associated brain abscess is most commonly the frontal lobe, but temporal lobe localization can also occur by direct extension. A large majority of intracranial infections in this setting follow a recent tooth extraction or dental manipulation. Bacteremia has been observed in 100% of patients after dental extraction, in 70% after dental scaling, in 55% after third molar surgery, and in 20% after endodontic treatment (124). Another study reported that 9% of children with extensive tooth decay were bacteremic before treatment (125). Hygiene procedures such as brushing of the teeth increased the prevalence of bacteremia to 40%, and anesthetic and surgical procedures increased it to 97%. In both studies, the bacteria isolated from the blood were mostly facultative species indigenous to dental plaque, in particular viridans streptococci (124,125). Many cases of cryptogenic brain abscess are believed to be secondary to asymptomatic dental foci of infection.
Facial and Scalp Infections
Facial and scalp infections were reported in 1% to 4% of patients with brain abscess (126,127). Septic thrombosis of the cavernous sinus is a possible link, and Staph. aureus is the most common pathogen in this setting (128).
Meningitis
Brain abscess rarely complicates bacterial meningitis; however, it should be strongly considered as an associated possibility in the neonate with purulent meningitis, particularly when meningitis is due to gram-negative organisms (29). The vast majority of neonatal brain abscesses that complicate meningitis are due to P. mirabilis or C. diversus. Neonatal meningitis due to Enterobacter species or Serratia species is also often complicated by brain abscesses (39,40). Although P. mirabilis is a rare cause of neonatal meningitis, 27 of 30 neonatal brain abscesses diagnosed over a 12-year period were due to this organism (14). Additionally, abscess formation has been associated with more than 70% of cases of C. diversus meningitis in the infant (129). The increased propensity for these two organisms to cause a brain abscess is incompletely understood (130,131).
Cranial Trauma and Neurosurgical Procedures
Penetrating Cranial Injury
The risk of a brain abscess is increased in the setting of a penetrating cranial injury. Combat series reported a risk of 3% to 4.2% of developing a brain abscess after missile or fragment injuries (Table 31.1). Risk factors were extensive brain injury, coma, trajectory through an air sinus, wound infections, CSF fistula, inadequate initial débridement, retained (bone or metallic) fragments, and incomplete dural closure. Several series have noted the role of head trauma as the cause of brain abscess in civilians. Thus, head trauma was reported as an underlying cause of brain abscess in 3% to 11% of patients in most series (4,28,132,133). Whereas the incidence of brain trauma as the cause for cerebral abscess formation declined in a study from the United Kingdom (16), it was found to be the most common cause (33%) for brain abscess in 973 patients from South Africa (9), reflecting the incidence of brain trauma (due to accidents or violence) in a society. Various types of cranial trauma have been implicated, including compound depressed skull fractures (133,134), dog bites (135), injuries due to rooster pecking (136), and cranial penetration with lawn darts, pencil tips, or paint brushes (137,138). Several cases of brain abscess as a complication of cervical traction with tongs or halo fixation have been described (139,140). Associated pin-site cellulitis is usually observed.
Craniotomy
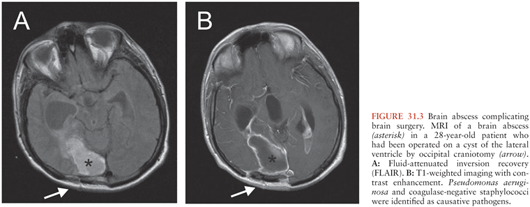
Brain abscess after neurosurgery is common (Fig. 31.3). In a series of 2,944 patients who underwent neurosurgery, neurosurgical site infections developed in 117 (4%) (19). Forty-four patients (1.5%) had incisional infections (30 with scalp infections and 14 with bone flap osteitis), and seventy-three (2.5%) had deep wound infections (meningitis in 56 and mass lesions in 17 patients). Given the high number of neurosurgical operations at certain centers, it was reported among the most frequent causes for brain abscess in some series (16,141). Risk factors for deep wound infections were a Glasgow Coma Scale score of less than 10 on admission, total hair removal, emergency surgery, CSF drainage, CSF leakage, and early subsequent operations. Interestingly, wound contamination, surgical duration, and the absence of antimicrobial prophylaxis were not significant risk factors for deep wound infections (19). The authors, therefore, hypothesized that the risk of contaminating CSF or brain parenchyma begins during surgery but persists in the postoperative period if a CSF drainage or leakage is present. This hypothesis was supported by a predominance of hospital-acquired antibiotic-resistant bacteria in deep wound infections.
Hematogenous Spread
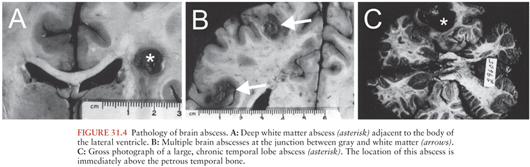
Hematogenous brain abscesses often share the following characteristics: (a) a distant focus of infection, most often within the chest; (b) location in the distribution of the middle cerebral artery (Fig. 31.4), (c) initial location at the gray matter–white matter junction, (d) poor encapsulation, and (e) high mortality. These abscesses are more often multiple and multiloculated as compared with those that have an origin from a contiguous focus of infection. Thirty-seven percent to 50% of multiple brain abscesses were of hematogenous origin in several studies (142–144), whereas metastatic spread accounted only for 17% to 34% of all brain abscesses (Table 31.4).
Pulmonary Infections
Pyogenic lung diseases, especially lung abscess and bronchiectasis, accounted for 7% to 18% of total brain abscess cases in four studies (4,9,122,127). Underlying chronic pulmonary diseases such as cystic fibrosis, however, are surprisingly infrequently complicated by a brain abscess (145). When observed, it most often occurs in older adolescents or adults (146). The organisms cultured from the sputum only occasionally matched those cultured from the brain abscess and in some patients, associated sinus disease was found. These observations suggest that the lung is not necessarily the source of brain abscess in patients with cystic fibrosis and that contiguous spread of infection should also be considered.
Endocarditis
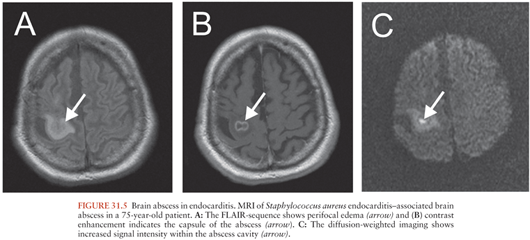
Brain abscess occurs in 5% to 7% of patients in the setting of bacterial endocarditis (Table 31.1) (147,148), despite the presence of persistent bacteremia (see Chapter 34). The highest incidence of brain abscess (5.4%) was reported in infective endocarditis of the left-sided valves due to Staph. aureus (148). The development of a brain abscess depends on the level and duration of the bacteremia, the virulence of the organism, and the occurrence of preceding emboli. A greater incidence of brain abscess or meningitis is observed in cases of acute, rather than subacute bacterial endocarditis (Fig. 31.5).
Right-to-Left Shunt
Abnormal venous-arterial channels which increase the risk of developing a brain abscess probably because of septic microemboli that can escape the pulmonary capillary filter (right-to-left shunt) include pulmonary arteriovenous malformations (AVMs), congenital cardiac defects, patent ductus arteriosus, and patent foramen ovale (PFO) (149,150). Hereditary hemorrhagic telangiectasia (Rendu-Osler-Weber disease) is complicated by brain abscess, with striking regularity (151). Approximately 25% of patients with this condition have pulmonary AVMs and 70% of the patients with pulmonary AVMs have hereditary hemorrhagic telangiectasia (152). The incidence of brain abscess seems to increase with the number of pulmonary AVMs (Table 31.1). Clinical findings, such as cyanosis, clubbing, polycythemia, and hypoxemia, may be absent (asymptomatic AVMs). The diagnostic workup of patients with brain abscess should therefore include a search for pulmonary AVMs (153). Congenital cyanotic heart disease (CCHD) was found in 0% to 10% of patients with brain abscess (122,154). Some pediatric series suggest that CCHD is the most common cause of brain abscess in children (150,155). In many studies, 50% or more of all brain abscesses were attributed to CCHD in this age-group (155–160), and in two large series of brain abscess in those with cyanotic heart disease, more than 50% of patients were younger than 10 years of age (161,162). Tetralogy of Fallot is consistently the most commonly cited defect (161,162). Transposition of the great arteries, double outlet right ventricle, single ventricle, tricuspid atresia, Ebstein disease, and Eisenmenger complex were the next most frequently observed disorders in the largest series (161). Several reports showed PFO in patients with brain abscesses (163). However, the prevalence of PFO in adults is high (20% to 35%). Therefore, a population study will be necessary to document whether the prevalence of PFO is higher in patients with brain abscess with a presumed spread from a distant infectious focus than in the general population.
Other Foci of Metastatic Spread
Other distant foci of infection may be associated with brain abscess and have included wound and skin infections, osteomyelitis, pelvic infection, cholecystitis, and other forms of intraabdominal sepsis. Brain abscess has also been described as an unusual complication of esophageal dilation of caustic strictures and endoscopic ligation of varices (164,165). Brain abscess in these cases is probably due to bacteria of the oropharyngeal flora that entered into the circulation through lesions of the mucous membrane. Transient bacteremia develops in up to 100% of cases of esophageal dilation (mostly due to viridans streptococci, Staph. aureus, and Staph. epidermidis) and in up to 50% of cases involving sclerotherapy (mostly due to Pseudomonas species and α-hemolytic streptococci) (164,166).
Location
In approximate decreasing order of frequency, a solitary abscess may involve the following brain regions: frontal, temporal, parietal, and occipital lobes and cerebellum (Table 31.5). This distribution reflects the associated, often contiguous, focus of infection (Table 31.3). Intrasellar, brainstem, basal ganglia, and thalamic abscesses are rare.
Pituitary Abscesses
Pituitary abscess is caused either by hematogenous seeding of the pituitary gland or by direct extension of an adjacent infection, such as meningitis, sphenoid sinusitis, cavernous sinus thrombophlebitis, or a contaminated CSF fistula (167). Besides infection by pyogenic bacteria, pituitary infections have been caused by Treponema pallidum, M. tuberculosis, Entamoeba histolytica, and a variety of fungi, including Histoplasma capsulatum, Blastomyces dermatitidis, Sporothrix schenckii, Candida species, and Aspergillus species (167,168).
Brainstem Abscesses
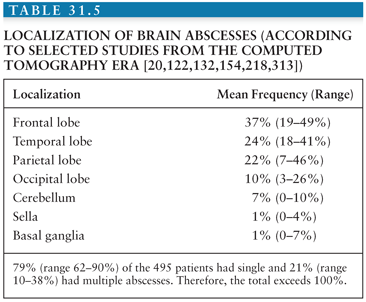
Brainstem abscesses (Fig. 31.6) often arise from hematogenous spread or, less frequently, in association with a contiguous infection, such as otitis (169,170). Brainstem abscesses have a relatively poor prognosis. This is primarily because their anatomic location can lead to catastrophic neurologic complications and often preclude definitive drainage. Brainstem abscesses tend to elongate in the brainstem rather than expand laterally (170). Therefore, the clinical findings can be confusing.
Abscesses in the Thalamus and Basal Ganglia
Inflammatory lesions (especially when solitary) of the thalamus and basal ganglia are also somewhat unusual (Table 31.5). Most often they are hematogenous (171) and they are frequently associated with hydrocephalus, most likely due to close proximity to the ventricles (172).
General Pathogenetic Considerations
The final common pathway for brain abscess development in the preceding conditions appears to require a compromised area of brain. Experimental data suggest that infection is extremely difficult to establish in normal brain tissue (173). Brain abscess may develop from a contiguous infection via direct extension through areas of associated osteitis or osteomyelitis and retrograde thrombophlebitic spread via diploic or emissary veins into the intracranial compartment. Additional possibilities in the case of otogenic infection include spread through preexisting channels (the internal auditory canal, cochlear, and vestibular aqueducts) or between temporal suture lines. Hematogenous dissemination is occasionally implicated with contiguous foci, particularly in cases of sinus or odontogenic origin. None of these hypotheses explains the relative rarity of intracranial infection with sinusitis or otitis, how bacteria traverse an intact dura, or what determines the form of intracranial complication that eventually evolves (e.g., meningitis, epidural abscess, subdural empyema, or brain abscess) in the individual with the same predisposing condition.
The development of a brain abscess in the setting of neonatal meningitis deserves special comment. The pathogenesis appears to follow the initiation of a necrotizing vasculitis (particularly in the small penetrating vessels), leading to subsequent hemorrhagic necrosis and liquefaction of the subcortical white matter, an area highly susceptible to changes in cerebral perfusion (174). Others disagree with this formulation, contending that the evidence favors an initial ventriculitis followed by ependymal disruption and subsequent direct extension of the infection into the brain parenchyma. Similar findings have been noted in an infant rat model of Citrobacter diversus meningitis and brain abscess (175) and support the latter hypothesis.
In hematogenous cases, the polycythemia and systemic hypoxia observed in CCHD and hereditary hemorrhagic telangiectasia increase blood viscosity, with an associated reduction in brain capillary flow, perhaps leading to microinfarction and reduced tissue oxygenation in the brain. Septic microemboli that escape the pulmonary capillary filter through right-to-left shunts during bacteremic episodes might then seed already compromised brain tissue. This hypothesis is in good agreement with the development of abscesses in focal brain damage due to, for example, brain tumor, hemorrhage, or ischemia. In 1997, a review reported 15 cases of abscess formation in brain neoplasms (176). Six of the brain abscesses developed in a meningioma, glioma, or craniopharyngioma and were associated with bacteremia due to Salmonella typhi, Staph. aureus, Bacteroides oralis, or E. coli (most of the other abscesses developed in pituitary tumors or craniopharyngiomas and were attributed to contiguous spread from an adjacent infected sinus). It was proposed that tumor-associated blood–brain barrier breaching favored the development of hematogenous brain abscesses as eight such cases of abscess development at the site of an intracerebral hemorrhage were reported (21). A similar mechanism has been proposed for the observation of brain abscess formation in ischemic brain tissue (177,178) and after endovascular treatments of cerebral aneurysms, AVMs, or dural arteriovenous fistulas (38,179,180).
The immune response that is elicited to combat the invading pathogen is an essential part of abscess formation as it also destroys surrounding normal brain tissue (181). This is suggested by the finding that lesions of the brain are much greater than the area of bacterial growth in experimental brain abscesses (182,183). The most extensive work on the pathogenesis of brain abscess has been performed in a mouse model of S. aureus brain abscess. S. aureus has been shown to activate parenchymal microglia and astrocytes (in a toll-like receptor 2 [TLR2], TLR4, and MyD88-dependent fashion [184,185]). In turn, they produce a variety of cytokines and chemokines such as interleukin-1β, tumor necrosis factor-α (TNF-α), and macrophage inflammatory protein-2 (MIP-2) (186–189) that attract peripheral inflammatory cells. If the initial immune response is unable to clear the infection (which can be related to the presence of bacterial virulence factors such as α-toxins in S. aureus–related brain abscess or the result of an insufficient immune responses), a continuing inflammatory response is initiated and upheld. Among the stimuli for such an ongoing inflammation are bacterial toxins (pathogen-associated molecular patterns [PAMPs]) and endogenous agents that are set free as a consequence of tissue damage (damage-associated molecular patterns [DAMPs]) and stimulate the immune response (189,190). As a consequence, the development of a chronic disruption of the blood–brain barrier and final abscess formation is found. These experimental findings support the idea that an intervention with both antiinfective and antiinflammatory compounds might be an effective strategy to minimize damage to brain parenchyma in brain abscess (181).
PATHOLOGY
Histopathologic Stages of Brain Abscess Development
The evolution of a brain abscess includes four histopathologic stages (191). This staging process, described in animal models of brain abscess, correlates well with human brain abscess evolution (192).
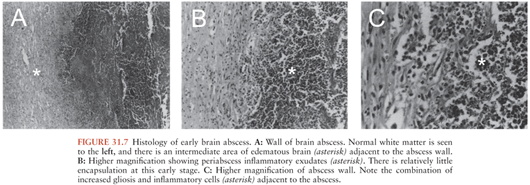
The first stage is an early cerebritis (days 1 to 3 following intracerebral inoculation in animals), which progresses to a perivascular inflammatory response surrounding the developing necrotic center by the third day. Profound edema in the surrounding white matter develops concurrently (Fig. 31.7).
In the second stage of late cerebritis (days 4 to 9), development of a well-formed necrotic center reaches its maximum size. Additionally, fibroblasts appear, setting the stage for capsule formation and a marked increase in neovascularity at the periphery of the necrotic zone. These newly formed capillaries often lack tight junctions and leak proteinaceous fluid. Surrounding this is the beginning of a reactive astrocyte response, along with persistent white matter edema.
The third stage, early capsule formation (days 10 to 13), is characterized by a slight decrease in the size of the necrotic center (Fig. 31.8). At this point, there is a well-developed layer of fibroblasts, with significantly more reticulin deposition on the cortical side than on the ventricular side of the lesion. Outside this developing capsule is a region of persistent cerebritis and neovascularity, with a further increase in reactive astrocytes.
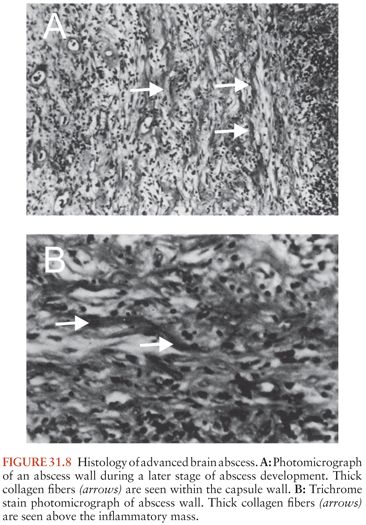
These processes continue in the fourth and final stage, late capsule formation (day 14 and later). The capsule continues to thicken, with an abundance of reactive collagen present by the third week.
Some studies have criticized the preceding model’s utility in describing a uniform mode of brain abscess evolution (193). These authors, using the same dog model and inoculum, could not detect viable organisms in the brain lesions after 3 days and, in all animals, the lesions spontaneously resolved. Further work is necessary to reconcile this debate. Nonetheless, a brain abscess model in rats supports the previously mentioned histologic progression (194). Two other experimental models of brain abscess using organisms other than α-hemolytic streptococci also indicate that this view of abscess evolution is either overly stereotyped or affected by inadequate growth of the microorganisms. In a model of Bacteroides fragilis (inoculated with Staph. epidermidis) brain abscess (195), the same stages of evolution were observed; however, the early and late capsule stages could not be differentiated, because of a delay in encapsulation when compared to abscesses following streptococcal challenge. These abscesses enlarged more quickly, were prone to early ventricular rupture (25%), and exhibited incomplete encapsulation. This suggests the extreme virulence of B. fragilis, an important pathogen in brain abscess, in brain tissue when part of a mixed infection. In an experimental model using S. aureus (196), there were quantitative and qualitative differences in abscess evolution when compared with inoculation of α-streptococci. S. aureus inoculation resulted in larger lesions, earlier ependymitis, and delayed progress toward healing, with longer periods required for the infection to reach a stable size. The same approximate stages were present, but their separation was also not as distinct as previously described. The white matter appeared more susceptible to destruction than the gray matter, and the spread of infection was centrifugal, rather than along particular white matter tracts. In contradistinction, a model of E. coli brain abscess exhibited expansion of the abscess along white matter tracts (197). In addition, results from the S. aureus experimental model raised some questions regarding the previously held assumption that the capsule serves to contain infection. The abscess reached its maximum size in the late cerebritis stage, before any significant capsule formation had taken place, suggesting that the host was able to contain the infection before capsule formation. Also, even in the late capsule stage, inflammation, necrosis, and edema extended well beyond the well-formed capsule.
Two observations regarding encapsulation deserve special attention: (a) capsule formation is usually more complete on the cortical side of the abscess than on the ventricular side (191), and (b) encapsulation is less extensive in abscesses resulting from hematogenous spread than in those arising from a contiguous focus of infection (198). The preferential deposition of collagen on the outer edge of the abscess is thought to be due to the better vascularization of the gray matter (197). This discrepancy probably explains the propensity for abscesses to rupture medially rather than into the subarachnoid space. Similarly, cerebral ischemia from a septic embolus might impede optimal collagen formation (198). Therefore, brain abscess formation is a continuum from cerebritis to a collagen-encapsulated necrotic focus; however, its maturity is dependent on many factors, including local oxygen concentration, the offending organism, and the host’s immune response.
CLINICAL MANIFESTATIONS
Signs and Symptoms
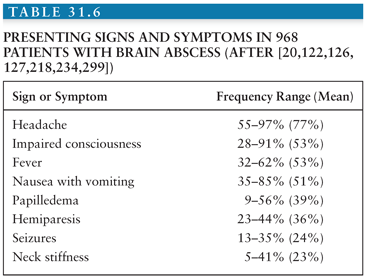
The clinical manifestations of a brain abscess (Table 31.6) can vary greatly among patients. Additionally, symptoms from the primary site of infection (i.e., otitis, sinusitis, or distant suppurative foci) may predominate. The evolution of symptoms of a brain abscess may range from indolent to fulminant; the average duration of symptoms until hospital admission is 11 to 12 days (range, hours to several weeks) (122,132). The classic triad of fever, headache, and a focal neurologic deficit is present in less than 50% of patients. In most patients, the prominent clinical manifestations of brain abscess are due to an expanding intracerebral mass rather than infection; neurologic signs, such as headache and hemiparesis, predominate in patients with multiloculated brain abscess as compared with patients with uniloculated brain abscess (199). The clinical presentation of patients with cerebellar abscesses is different, because signs of raised intracranial pressure (ICP) (e.g., headache, vomiting, and papilledema, >90%), fever (90%), meningismus (>70%), and depressed consciousness (>50%) are particularly common (118,200). Cerebellar signs are present in 25% to 50% of patients, whereas hemipareses are evident in only 10% of patients. The clinical presentation of frontal lobe abscesses is often dominated by headache, drowsiness, inattention, and a generalized deterioration in mental function. Hemiparesis with unilateral motor signs and a motor speech disorder are the most common focal findings. A temporal lobe abscess may present with early ipsilateral headache. If the abscess is in the dominant hemisphere, an aphasia or dysphasia may be present. An upper homonymous hemianopia may also be demonstrated and may be the only sign of a right temporal lobe abscess. Intrasellar abscesses often simulate a pituitary tumor, presenting with headache (>90%), abnormal pituitary function (>50%), and visual field defects (50%) (167). The clinical presentation of brainstem abscess is usually a rapidly progressing neurologic deficit involving cranial nerves and long tracts.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree