- Musculoskeletal disorders may cause pain and functional impairment, and adversely affect management of diabetes.
- Fibroproliferative disorders of soft tissue such as limited joint mobility, frozen shoulder, Dupuytren contracture, trigger finger and carpal tunnel syndrome occur more commonly in diabetes and can lead to upper limb disability.
- Charcot joint is an infrequent but serious complication of diabetic peripheral neuropathy, and is characterized by disordered osteoclas-togenesis. Bisphosphonate therapy may be a useful adjunct to standard treatment.
- Patients with diabetes are at high risk of developing gout, particularly in the presence of renal impairment and diuretic use.
- The prevalence of diffuse idiopathic skeletal hyperostosis is increased in patients with type 2 diabetes (T2DM).
- Fracture risk is increased in both type 1 (T1DM) and T2DM.
- Bone mineral density is decreased in T1DM, and increased in T2DM.
- Disease complications increase fracture risk by increasing risk of falls and causing regional osteopenia.
- Thiazolidinediones decrease bone formation and bone mineral density, and increase fracture risk in T2DM.
- Fracture healing may be impaired in diabetes.
Musculoskeletal disease in diabetes
Background
A variety of musculoskeletal disorders are associated with diabetes. These disorders may cause pain and functional impairment, and influence the ability of patients to adhere to other aspects of diabetes treatment, particularly exercise and weight management. Therapies commonly used in the treatment of rheumatic disease, particularly corticosteroids and non-steroidal anti-inflammatory drugs (NSAIDs), may be particularly problematic in patients with diabetes. The important bone and joint disorders associated with diabetes discussed in this chapter are outlined in Table 48.1.
Table 48.1 Bone and joint disorders in patients with diabetes.
| Fibroproliferative disorders of soft tissue Cheiroarthropathy Frozen shoulder Dupuytren contracture Stenosing tenosynovitis (trigger finger) Carpal tunnel syndrome Disorders of joint tissue Charcot joint Gout Osteoarthritis * RA genetic risk and type 1 diabetes * Disorders of bone Diffuse idiopathic skeletal hyperostosis Osteoporosis and fractures Disordered fracture healing |
RA, rheumatoid arthritis.
* Direct association not proven.
Fibroproliferative disorders of soft tissue
Limited joint mobility (cheiroarthopathy)
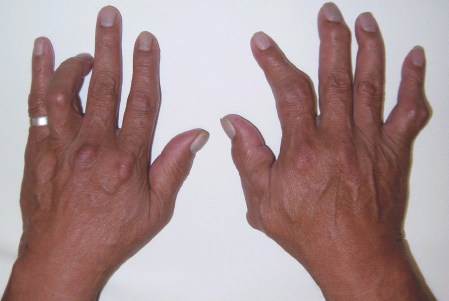
Limited joint mobility refers to a syndrome of joint contractures resulting in decreased passive mobility of the joints in patients with diabetes [1]. Flexion contractures of the proximal inter-phalangeal (PIP) joints and metacarpophalangeal (MCP) joints of the hands are characteristic, with the fifth PIP joint affected first (Figure 48.1). The skin on the dorsum of the hands typically appears tight and waxy [2]. Large joints such as the wrists, elbows, ankle and cervical spine can also be affected, and reduced lung volumes have been reported in severe cases [3,4]. Pain is usually mild or absent early in disease, and features of synovitis such as joint swelling, effusion, warmth and tenderness are typically absent. The disorder can be readily differentiated from systemic sclerosis by lack of Raynaud phenomenon and other systemic features, normal nailfold capillary examination and negative autoantibodies [5,6].
Figure 48.1 Limited joint mobility with flexion contractures affecting the finger proximal interphalangeal joints. Courtesy of Dr. Tim Cundy.

The presence of limited joint mobility of the hands is detected clinically by assessing for the prayer sign or the table-top sign. The prayer sign is positive if patients are unable to oppose the palmar surfaces at any interphalangeal or MCP joints when the hands are placed in the prayer position. For assessment of the table-top sign, the patient places both hands on a table top with the palms down and the fingers fanned out. The fingers are then viewed at table level. In stage 0, the entire palmer surface of the fingers makes contact with the table. In stage 1, one finger is affected (usually the fifth PIP joint of one or both hands). In stage 2, two or more fingers of both hands are affected (usually the fourth and fifth PIP joints). In stage 3, there is involvement of all the fingers and also restricted movement in a larger joint, usually the wrist or elbow [7]. Passive joint movement should also be assessed to confirm limitation of joint mobility [8].
Prevalence estimates for limited joint mobility in type 1 diabetes mellitus (T1DM) range 9–58%, and 25–75% in type 2 diabetes mellitus (T2DM) [1,9–11]. There is some evidence that prevalence rates have declined in patients with T1DM in the last 20 years because of improvements in glycemic control [12].
Limited joint mobility is an important entity primarily because of its clinical associations. It is one of the earliest complications of diabetes and is strongly associated with the presence of microvascular complications such as retinopathy and nephropathy in T1DM, [1,12–15], and macrovascular complications in T2DM [10]. It is also associated with other fibroproliferative disorders affecting the upper limb such as frozen shoulder, Dupuytren contracture and carpal tunnel syndrome [15–18]. In general, limited joint mobility does not severely impact on hand function, but combinations of these upper limb disorders may cause upper limb disability [19–21]. In addition to microvascular complications, risk factors for the development of limited joint mobility in patients with diabetes include older age, puberty (in T1DM), disease duration and cigarette smoking [10,22–24].
Advanced imaging techniques have demonstrated thickening of skin, tendons and tendon sheaths in patients with limited joint mobility [25,26]. Histologic examination of the skin shows altered mucopolysaccharide distribution, elastin and collagen, and reduced vascular lumen [27]. Non-enzymatic glycosylation and accumulation of collagen have been implicated in the pathogenesis [28]. Disordered glycosaminoglycan (GAG) metabolism is also a feature; skin biopsies from patients with severe limited joint mobility show pronounced hyaluronan expression in the epidermis and diminished expression in the dermis and basement membrane compared with skin from controls without diabetes and controls with diabetes but without limited joint mobility [29]. In addition, increased urinary GAG excretion has been reported in patients with limited joint mobility [30]. Reduced circulating insulin-like growth factor I (IGF-I) is associated with limited joint mobility, implicating the growth hormone–I GF-I axis in the pathogenesis of this complication [31]. Microvascular abnormalities also contribute to disease, with reports of disordered palmar microvascular flow in response to thermal challenge [32].
The mainstay of therapy remains obtaining excellent glycemic control, and reduced prevalence of this disorder has been reported with such interventions [12,33]. Physiotherapy, particularly hand therapy, may be of benefit to improve joint contractures and function. Corticosteroid injection of flexor tendon sheaths has been reported to lead to resolution of finger contractures in almost two-thirds of cases related to limited joint mobility, and should also be considered [34].
Frozen shoulder
This disorder is characterized by shoulder pain, stiffness and severely restricted range of motion in all planes [35]. Three phases of the disorder are well-recognized: first, the painful freezing stage with associated nocturnal pain (lasting 4–8 months), followed by the adhesive phase with improvement in pain but severely restricted range of motion (lasting 8–24 months), and finally the resolution phase [36]. The mean time to resolution is 30 months [36]. Plain radiographs of the shoulder are typically normal. Although the condition is usually self-limiting, some patients have persistent shoulder pain and restricted range of motion many years after assessment [37,38].
Imaging and histologic studies have demonstrated that the pathologic features of frozen shoulder are thickening of the capsule and synovium with contracted joint volume. Affected tissue is characterized by dense type I and type III collagen deposition with proliferating fibroblasts and a chronic inflammatory infiltrate comprising T cells, macrophages and mast cells [39,40]. Disordered collagen synthesis and vascular endothelial growth factor 1 (VEGF-1) mediated angiogenesis have also been implicated [41,42].
Treatment is tailored to the stage of the disorder [35]. In the painful freezing stage, analgesics, including NSAIDs if tolerated, are indicated. Early use of intra-articular corticosteroids is associated with improved outcomes, and physiotherapy with exercise within the limits of pain is of greater benefit than more intensive physiotherapy such as stretching and mobilization [43,44]. Although oral corticosteroids provide short-term relief in the painful freezing stage, they are not routinely recommended because of lack of long-term benefit and risk of adverse events [45]. In the adhesive phase, more intensive physiotherapy is indicated. For those who fail to respond to physiotherapy and have persistent shoulder restriction, interventions such as radio-graphic-guided hydrodilatation, manipulation under anesthesia or arthroscopic release should be considered [46,47].
Diabetes is a major risk factor for frozen shoulder. The prevalence of frozen shoulder is 11–19% of patients with diabetes, compared with 2–3% of age-matched controls [16,19,48,49]. Patients with diabetes are also more likely to have bilateral disease. Key risk factors for frozen shoulder in patients with diabetes are older age, duration of diabetes, previous myocardial infarction, retinopathy and peripheral neuropathy [50]. The presence of other fibroproliferative musculoskeletal disorders such as limited joint mobility and Dupuytren contracture is strongly associated with frozen shoulder in patients with diabetes [50]. Furthermore, frozen shoulder in patients with diabetes is more difficult to treat because of persistent disease and worse outcomes following surgical interventions [47,51,52].
Dupuytren contracture
Dupuytren contracture is a fibroproliferative disorder of the palmar fascia leading to formation of palmar nodules, development of a palmar aponeurosis cord with tethering of the overlying skin and eventually flexion contractures, particularly affecting the ring and little fingers [53]. Elderly men of northern European ancestry are most frequently affected. Disordered fibroblast and myofibroblast function has been described, with deposition of type III collagen, potentially mediated by growth factors such as transforming growth factor β (TGF-β) and basic fibroblast growth factor [54–56].
Surgical treatment is the mainstay of therapy, although non-surgical options, particularly local injection of collagenase, are promising [57]. Splinting and intralesional corticosteroids may be considered, but are frequently ineffective [58]. Surgical referral should be considered in the presence of contracture. Various surgical approaches are available, including fasciotomy (division of the affected palmar fascia) or fasciectomy (excision of the affected palmar fascia). Percutaneous needle fasciotomy is a minimally invasive technique with good short-term outcomes, although recurrence is a frequent problem [59,60].
Risk factors for Dupuytren contracture include advanced age, male sex, cigarette smoking, manual labor and alcohol consumption. Diabetes is also an important risk factor for Dupuytren contracture, which is present in up to 26% of patients with diabetes [19,20,61]. Age and disease duration are the major risk factors for development of Dupuytren contracture in patients with diabetes [62]. Dupuytren contractures are also associated with microvascular complications in T1DM and macroalbuminuria in T2DM [63,64]. Rapidly progressive contractures are less frequently seen in patients with diabetes [62]. Coexistent fibroproliferative disease is frequent in patients with diabetes-associated Dupuytren contracture, with higher rates of limited joint mobility [64].
Stenosing tenosynovitis (trigger finger)
Trigger finger is “a condition in which the flexor tendon is prohibited from gliding through the tendon sheath because of thickening of the synovial sheath over the tendon” [65]. This disorder most frequently affects the ring finger, but can also affect the other fingers and the thumb. The patient may report a clicking sensation when moving the finger, discomfort over the palm or overt triggering when the finger is locked in flexion [66]. Nodular or diffuse flexor tendon sheath swelling may be palpable.
The syndrome occurs as a result of a discrepancy between the flexor tendon and its sheath in the A1 pulley at the level of the metacarpal head [66]. The pulley becomes thickened with increased extracellular matrix and fibrocartilage metaplasia [67]. These pathologic changes may be induced by repetitive trauma.
Corticosteroid injection into the tendon sheath is an effective therapy for the majority of patients, particularly in the presence of nodular disease. For patients with nodular disease of less than 6 months’ duration, local injection has a reported success rate of 90% [68]. Splinting and hand therapy are useful adjuncts to local injection. If conservative therapy fails, release using a percutaneous needle approach or open surgery is indicated [69].
Patients with diabetes are at higher risk of trigger finger, with a lifetime risk of 10% compared to 2.6% of the general population [66]. Patient age, diabetes duration and presence of microvascular complications are associated with increased risk of trigger finger in diabetes [62,70]. Outcomes are typically worse when trigger finger is associated with diabetes, with lower responses to corticosteroid injection and greater need for surgery [71–73]. Furthermore, T1DM is associated with higher prevalence of disease, more affected digits, greater need for surgery and higher risk of recurrence [62,71,73].
Carpal tunnel syndrome
Carpal tunnel syndrome is a common compressive neuropathy affecting the median nerve as it traverses with the flexor tendons through the carpal tunnel, an anatomical space comprised of the carpal bones and the transverse carpal ligament [74]. The most common histologic appearance is non-inflammatory tenosynovial fibrosis, with increased fibroblast number and type III collagen deposition, most likely mediated by TGF-β [75]. Compression within the carpal tunnel leads to disordered microvascular supply of the nerve, causing demyelination and axonal degeneration. The typical presentation is hand parasthesia, particularly affecting the thumb, index finger and middle finger. Paresthesia is often more frequent at night, and may wake the patient from sleep. Wrist and hand pain may also occur, and patients frequently report hand clumsiness.
Clinical examination may be normal, but in the presence of severe and prolonged disease there may be features of median nerve denervation, including thenar wasting, weakness of thumb abduction and sensory loss over the median nerve distribution. Provocative tests including Phalen and Tinel tests may be positive, and if present have relatively high specificity for carpal tunnel syndrome. The Phalen test is positive if paresthesia in the median nerve distribution is reported following flexion of the wrist at 90° for 60 seconds. The Tinel test is positive if paresthesia is reported after tapping the volar wrist over the carpal tunnel. The diagnosis is confirmed by nerve conduction testing, with the typical findings of prolonged latencies and delayed conduction velocities affecting the median nerve across the wrist [76].
Treatment consists of maintaining the wrist in a neutral position using a removable wrist splint. Splinting is particularly useful for nocturnal symptoms, and may be sufficient to treat mild disease [77]. Although oral corticosteroids have short-term efficacy, side effects are usually unacceptable [78]. Local corticosteroid injection provides good short-term relief [79]. Surgical release under local anesthesia is a well-tolerated and effective therapy, which should be considered in patients who have failed conservative therapy or have severe symptoms and signs of nerve compression [80]. Open release and endoscopic approaches have similar clinical outcomes [81].
Carpal tunnel syndrome may be caused by a number of factors including non-specific flexor tenosynovitis affecting the wrist, rheumatoid arthritis and other inflammatory synovial arthropathies, obesity, pregnancy and disordered wrist anatomy [74]. Diabetes is one of the most common metabolic disorders associated with carpal tunnel syndrome, being present in 16% of affected patients [82]. Most studies have shown increased risk of carpal tunnel syndrome in patients with both T1DM and T2DM [62,83,84]. A recent survey using clinical and neurophysiologic assessment reported a prevalence of carpal tunnel syndrome of 2% in a reference population without diabetes, 14% in patients with diabetes but no diabetic polyneuropathy, and 30% in patients with diabetic polyneuropathy [85]. Carpal tunnel syndrome is associated with duration of diabetes, and is more frequently present in patients with microvascular complications such as retinopathy, nephropathy and polyneuropathy [62,86]. Carpal tunnel syndrome is also more common in patients with limited joint mobility, and it has been postulated that this disorder occurs at higher frequency in diabetes because of accelerated thickening and fibrosis of the flexor tendon sheaths within the carpal tunnel [18]. Glycosylation of collagen may also reduce compliance of connective tissue within the carpal tunnel [84]. In addition, the presence of existing microvascular disease may further increase the risk of endoneural ischemia as the median nerve travels through the carpal tunnel. Carpal tunnel syndrome may be more difficult to assess in patients with coexistent diabetic neuropathy, because of atypical presentation and neurophysiologic assessment [85,87]. Treatment options for patients with diabetes and carpal tunnel syndrome are similar to those for patients without diabetes, and responses to surgery are usually good [88,89]. One study reported positive results in symptoms scores and neurophysiologic testing using local insulin injections in women with T2DM and carpal tunnel syndrome, in combination with corticosteroid injection [90].
Disorders of joints
Charcot joint
Charcot joint is a destructive arthropathy, most commonly affecting patients with diabetes in the presence of severe peripheral neuropathy. This disorder affects 0.1–0.4% of patients with diabetes and may lead to severe foot deformity and disability, ulceration and limb amputation [91].
Several stages of disease are described [92,93]. The developmental stage presents as acute inflammation with swelling, warmth and erythema of the foot. Pain may be a feature, despite the presence of peripheral sensory neuropathy. Peripheral pulses are usually easily palpable. Gradually worsening deformity occurs, with bone resorption, fracture and dislocation, leading to instability of the foot and the classic rocker-bottom dislocation of the midfoot. Plain radiographs may appear normal early in the acute phase of disease (Stage 0), but magnetic resonance imaging (MRI) scans show florid bone marrow edema, subchondral cysts and microfractures, and bone scintiscan shows increased uptake on the bony phase [93]. As deformity develops, radiographs show severe osteolysis, bone fragmentation and disordered architecture (Stage 1). In the coalescence phase (Stage 2), hyperemia resolves, swelling reduces and skin temperature normalizes. Bone debris is resorbed and bone sclerosis may occur. The reconstructive stage (Stage 3) is characterized by remodeling of bone, ankylosis and proliferation of bone, and formation of a stable foot. The acute phase (Stages 0 and 1) typically lasts 2–6 months, and the reparative phase (Stages 2 and 3) lasts up to 24 months. During both the acute and the reparative phases of disease, bony deformity may lead to abnormal load bearing, with ulceration of overlying skin and secondary osteomyelitis.
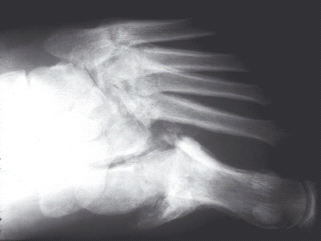
Five separate patterns of foot involvement are identified in patients with diabetes [94]: I, affecting the forefoot with osteolysis of the MTP and IP joints of the feet, leading to the “sucked candy” appearance on plain radiography; II, affecting the tarsometatarsal (Lisfranc) joint leading to instability, subluxation and fracture (Figure 48.2); III, dislocation and fracture affecting the midtarsal and naviculocuneiform joints; IV, affecting the ankle and subtalar joints, often with severe osteolysis; V, affecting the calcaneus. The most common patterns are II and III, and combinations of patterns may be present. Bilateral disease is present in one-quarter of patients. Rarely, other joints such as the knees, elbows and shoulders are affected.
Figure 48.2 Plain radiograph of Charcot foot. Note the osteolysis, bone fragments, subluxation and fracture affecting the tarsometatarsal joints of the foot. Courtesy of Dr. Tim Cundy.
The etiology of the disease remains controversial [95]. Minor trauma frequently precipitates onset of disease, and may lead to subclinical bone injury that triggers an aberrant inflammatory response [96]. It is likely that disordered weight-bearing in joints affected by peripheral neuropathy leads to repetitive injury and instability (the neurotraumatic hypothesis). Additionally, autonomic dysfunction causing vasodilatation, arteriovenous shunting and hyperemic bone resorption has been implicated (the neurovascular hypothesis). Development of osteopenia and osteolysis increases risk of fractures in the presence of abnormal load-bearing with a cycle of joint instability and fracture development, causing further abnormal load-bearing [97]. Recent work has focused on the role of local inflammation in disease pathogenesis. Advanced imaging and histologic analysis have demonstrated that inflammation of synovium and bone is evident in Charcot joint, and is characterized by increased expression of the pro-inflammatory cytokines tumor necrosis factor α (TNF-α) and interleukin 1 [98–101]. Large numbers of osteoclasts are present within affected bone, and patients with Charcot joint have increased ability to form peripheral blood derived osteoclasts in vitro compared with diabetic and non-diabetic controls [101,102]. Markers of bone resorption are increased in patients with acute Charcot joint [103]. Interestingly, acute phase markers are not significantly elevated, indicating an apparent dissociation between local and systemic inflammatory disease [104]. These > data implicate receptor activator of nuclear factor κB ligand (RANKL) mediated osteoclastogenesis, driven locally by proinflammatory cytokines, and provide a rationale for the use of agents that target the osteoclast in treatment of the disease.
Management of Charcot joint depends on the stage of disease. Treatment during the acute phase consists of immobilization which reduces inflammation, prevents abnormal load-bearing and stabilizes the foot in a position of least deformity. The standard immobilization method during the acute phase is a non-weightbearing total contact cast. This treatment requires close monitoring and regular adjustment, and should be maintained until swelling and temperature normalize, and radiographs show no further bony destruction [105]. Some recent uncontrolled reports have indicated that use of a weightbearing total contract cast may be an acceptable alternative to the non-weightbearing option, but controlled trials are not yet available [106,107].
The recognition that the acute phase of Charcot joint is associated with excessive osteoclast activity has led to the testing of agents targeting bone turnover for treatment of this condition. Two randomized controlled trials of bisphosphonates have been reported, and both show efficacy. A single intravenous infusion of 90 mg pamidronate in a study of 39 patients with acute Charcot joint led to significant improvements in symptoms and bone turnover markers [108]. In a study of 20 patients with acute Charcot joint, weekly oral administration of 70 mg alendronate for 6 months was associated with significant improvements in pain scores, bone turnover markers and foot bone density [109]. A randomized trial of intranasal calcitonin demonstrated efficacy with respect to bone turnover markers, but no differences in clinical variables were reported [110]. The efficacy of TNF-inhibitors and other antiresorptive agents such as the RANKL inhibitor denosumab has not yet been studied in Charcot joint, although the potential use of these agents has been recently highlighted [96].
Surgery is generally not considered first-line therapy, although one study has reported good surgical outcomes following débridement, open reduction and internal fixation with autologous bone grafting in the acute phase of disease [111]. In general, surgical management is currently recommended for patients in the reparative (rather than the acute) phase of disease, and particularly for patients with deformities associated with chronic foot ulcers and joint instability. Various surgical approaches to arthrodesis may be used, including open reduction with both internal and external fixation, depending on the presence of local infection and other anatomic variables [112]. Other surgery includes exostotomy, osteotomy, intramedullary rodding and amputation. Infection, non-union and triggering of an acute Charcot reaction are important postoperative complications, and careful postoperative management is essential.
Outcomes in patients with Charcot foot are frequently poor. A recent analysis of 115 patients reported that non-operative management was associated with a 2.7% annual rate of amputation, a 23% risk of requiring bracing for more than 18 months and a 49% risk of recurrent ulceration. The presence of open ulcers at initial presentation or chronically recurrent ulcers is associated with increased risk for amputation [113].
Gout
Gout is an inflammatory arthritis caused by intra-articular deposition of monosodium urate (MSU) crystals [114]. This disorder is the most common form of inflammatory arthritis affecting men, and affects 1–2% of the Caucasian adult population [115]. In early disease, gout presents as recurrent episodes of self-limiting acute inflammatory attacks (“flares”) of arthritis. These attacks most often affect the first MTP joint, midfoot and ankle. In the presence of prolonged hyperuricemia, some patients develop recurrent polyarticular attacks, chronic tophaceous disease and erosive arthritis (Figure 48.3).
The key risk factors for gout are hyperuricemia, male sex, chronic renal impairment, hypertension, obesity, diuretic use, coronary heart disease, and seafood, meat and alcohol intake [116–118]. The relationship between gout and metabolic syndrome is well-recognized. Serum urate concentrations and gout are strongly associated with abdominal adiposity, and have been shown to predict the development of T2DM [119–121]. Patients with gout have high rates of the metabolic syndrome and T2DM compared to individuals without gout [122]. Promotion of renal tubular reabsorption of uric acid by insulin is thought to mediate this relationship [123]. Recent identification of the glucose and fructose transporter SLC2A9 as a key regulator of serum urate concentrations suggests a further etiologic link between hyperuricemia and hyperglycemia [124]. A recent study reported a prevalence of gout of 22% in patients with T2DM treated in secondary care [125]. Key risk factors for gout in this population were male sex, renal impairment and diuretic use. Fewer than half of the patients with gout and diabetes in this study were prescribed urate-lowering therapy, and only 8% had a serum urate of <0.36 mmol/L. Interestingly, severe hyperglycemia may reduce urate concentrations, as glycosuria has a uricosuric effect. Thus, as glycemic control improves in patients initiating treatment for diabetes, there is a potential risk of worsening gout attacks [126].
Options for treatment of acute gout flares include NSAIDs, corticosteroids and/or colchicine [127]. Long-term urate-lowering therapy is indicated for patients with gout who have recurrent flares, gouty arthropathy, tophi or radiographic damage. Serum urate lowering to a concentration of <0.36 mmol/L (6mg/dL) is needed to dissolve MSU crystals, prevent flares and achieve tophus regression. Allopurinol is the mainstay of urate-lowering therapy, but may be ineffective at recommended doses. If the serum urate target is not achieved with allopurinol alone, further options include dose escalation of allopurinol, addition of a uricosuric agent such as probenecid or benzbromarone, or consideration of the new xanthine oxidase inhibitor febuxostat [128]. Initiation of urate-lowering therapy is frequently associated with exacerbation of gout flares; this side effect can be avoided by commencement of urate-lowering therapy once the acute flare has resolved, gradual introduction of the urate-lowering drug, and co-prescription of low dose colchicine.
The presence of coexistent gout has several implications for individuals with T2DM. Poorly controlled gout may hinder attempts at exercise and weight loss. In addition to the dietary restrictions required for glycemic control, these patients also need to avoid alcohol and purine-rich foods. Diuretic therapy may exacerbate hyperuricemia and should be avoided in patients with gout unless absolutely required. Drugs such as losartan and fenofibrate have weak urate-lowering effects and may be of particular benefit in patients with diabetes and gout if antihypertensive or lipid-lowering therapy is required [129,130].
Osteoarthritis
Increased load-bearing of articular cartilage is an important risk factor for development of osteoarthritis, and a strong positive relationship has been reported between obesity and the risk of developing osteoarthritis [131–134]. Although some studies have reported an association between T2DM and osteoarthritis, most have not adequately controlled for body mass index (BMI). In most (but not all) studies that have adjusted for BMI, T2DM has not been shown to be an independent risk factor for development of osteoarthritis [131,133,135,136]. Overall, current data indicate that increased BMI, rather than T2DM, is a risk factor for development of osteoarthritis.
Rheumatoid arthritis
Rheumatoid arthritis and T1DM share several genetic associations such as PTPN22, HLA-DR9, the chromosome 4q27 region, the IDDM5 region and the IDDM8 region [137–141]. Furthermore, there is evidence of familial clustering of these disorders; 2.8% of first-degree relatives of probands with rheumatoid arthritis have T1DM, compared with 0.35% of the general population [142]. Despite these observations, there is little evidence that the prevalence of rheumatoid arthritis is increased in patients with T1DM [143].
Skeletal disease in diabetes
Diffuse idiopathic skeletal hyperostosis
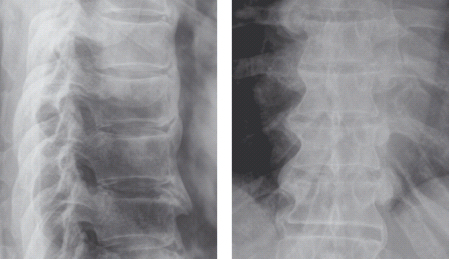
Diffuse idiopathic skeletal hyperostosis (DISH) is a disorder characterized by increased bone formation, particularly at the entheses (the insertions of ligaments and tendons into bone) [144]. Ossification of the anterior longitudinal ligament of the spine occurs, most commonly in the thoracic spine (Figure 48.4). Extraspinal ossification may also be identified. The prevalence has been reported to be as high as 15% in women and 25% in men over the age of 50 [145].
Figure 48.4 Plain radiographs of diffuse idiopathic skeletal hyperostosis (DISH) affecting the thoracic spine. Courtesy of Dr. Anthony Doyle.
Patients may present with back pain and stiffness, although it remains controversial whether DISH is associated with increased back pain, and the disorder is frequently detected as an incidental finding on chest radiographs [146,147]. Rare complications such as dysphagia, vocal cord paralysis, compression of the inferior vena cava and neurologic compression syndromes have been described in patients with florid hyperostosis [148]. Spinal fracture may occur after relatively minor injury and cause significant neurologic compromise [149]. The diagnosis is made radiographically, according to the Resnick criteria, which in brief are:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree