Robert W. Bradsher Jr.
Blastomycosis
Blastomycosis is caused by the dimorphic fungus Blastomyces dermatitidis. Infection begins when the organism, which exists in nature in the mold or mycelial phase, is inhaled into the host’s lungs and then converts to the yeast phase at body temperature. It is found mostly in North America but has been isolated in disparate parts of the world. Both pulmonary and extrapulmonary manifestations occur, but there is often a delay in making the diagnosis because the infection can mimic a number of other more common conditions. Treatment of infection has changed in the past 2 decades to include oral azole regimens rather than only amphotericin B.
History
T.C. Gilchrist, a dermatologist, first described blastomycosis in Baltimore in 1896 as a skin infection caused by what he thought was a protozoan organism.1 The illness was known for a time as Gilchrist’s disease. There were some errors in the initial description. First, Baltimore is not in the highly endemic region as it is currently understood. Second, infection of the skin occurs secondarily rather than as a primary infection, and third, the organism is not a protozoan but a fungus. However, Gilchrist was the first to refute portions of his own description when he and Stokes isolated and named the fungus B. dermatitidis.2 The skin manifestations of blastomycosis are often striking, which led to the initial cases being perceived as a dermatologic condition. The concept of primary pulmonary blastomycosis was not recognized until pathologic descriptions allowed the pathophysiologic mechanisms to be delineated.3,4 There are rare cases of cutaneous inoculation of the fungus in laboratory workers and veterinarians, but almost all cases of blastomycosis are considered to originate from a pulmonary portal of entry.3 Blastomycosis was once known as Chicago disease because the epidemiology was initially based on case reports in humans and dogs in that city. The infection was also once known as North American blastomycosis, but that name became obsolete after the infection was found on other continents, and what was once called South American blastomycosis became more accurately named paracoccidioidomycosis.
Organism
The perfect or sexual stage of the fungus is named Ajellomyces dermatitidis, with the imperfect or asexual stage named the familiar B. dermatitidis. Kaufman and others5 performed examinations of the exoantigen of yeast organisms and identified two serotypes of B. dermatitidis, A-antigen positive and A-antigen negative. There have been A-antigen deficient serotypes described from Africa.6 The sexual form, A. dermatitidis, is heterothallic and requires opposite mating types (+ and −) for fertile cultures.7,8 Both exoantigen serotypes are pathogenic, and the frequency of infection with each mating types is similar.9
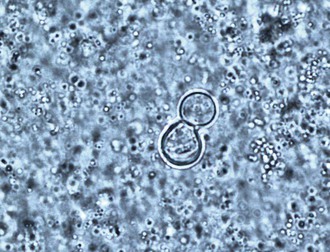
In culture, B. dermatitidis grows at 25° to 28° C as a mold and at 37° C as a yeast. The mechanism for the dimorphism has been shown to be a hybrid histidine kinase–mediated sensing of host signals, which stimulates the conversion from mycelia to yeast.10 The imperfect state grows on Sabouraud agar as a mold at room temperature and as colonies of brown, wrinkled, folded yeast at 37° C. Isolation of B. dermatitidis in the laboratory is the most sensitive method for diagnosis and is most dependable when grown as the mycelial form at 30° C. Mycelial colonies usually grow after 1 to 3 weeks of incubation but generally are apparent on the culture media within 5 to 10 days, beginning growth as a white mold that slowly turns light brown.11 Microscopically, the branching hyphae are 2 to 3 µm in diameter and have right-angled conidiophores resembling lollipops,11 which have a single terminal conidia. The conidia are round or oval in shape and vary from 2 to 10 µm in diameter. The conidia readily become airborne when the mycelia are disturbed and may result in pulmonary infection when inhaled into the lungs. The mycelial form has no morphologically unique characteristics that allow definitive identification and could be confused with a number of other molds, including Pseudallescheria boydii or Chrysosporium spp.11 Commercially available tools can help with early identification of mycelial cultures by fungal specific exoantigen recognition or by unique RNA sequences (see Chapter 16).11,12 Some laboratories, however, confirm the culture identification microbiologically by converting the mycelial form to the yeast form after growth at 37° C. The yeast varies in size from 5 to 15 µm, and the numbers of organisms found histologically range from sparse to numerous (Fig. 266-1). Most are round and have a double cell wall appearance, which actually consists of the interior and exterior components of the thick cell surface, and have 10 to 12 visible nuclei. Budding occurs with a broad base, and the daughter cell becomes as large as the mother cell before detaching. The size of the fungal cells is fairly uniform. The yeast may be found inside or outside of macrophages in the pyogranulomatous tissue response. Unlike Candida or Aspergillus, colonization of humans with Blastomyces does not occur, so a correct diagnosis of infection may be made by detection of the fungus by smear, culture, or histology.
Epidemiology
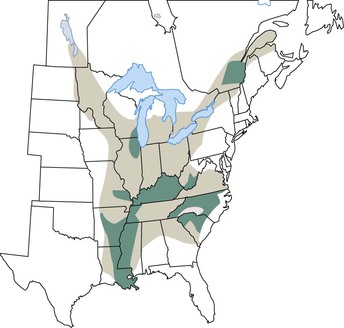
The endemic area for B. dermatitidis is North America and includes the states bordering the Mississippi and Ohio Rivers, the Midwestern states and Canadian provinces that border the Great Lakes, and a small area in New York and Canada along the St. Lawrence River (Fig. 266-2).3 Most patients with blastomycosis reported before the mid-1980s were from a fairly well-defined geographic area of the South Central United States.3 Because reporting of blastomycosis has been voluntary in most states and subclinical cases escape detection because of the lack of sensitive screening tests, the epidemiology of blastomycosis is not as well understood as that of other fungal infections. The number of cases reported varies from one area of the country to the next, dependent on the relative interest of physicians in reporting these infections. From 1896 to 1968 there were 1476 cases reported,13 but prevalence studies by Furcolow and colleagues14 indicate rates in some geographic areas as high as 0.5 to 4 cases per 100,000 population per year. Despite this underreporting, most clinical cases of blastomycosis have been from the states Kentucky, Arkansas, Mississippi, North Carolina, Tennessee, Louisiana, Illinois, and Wisconsin.3 Endemic or sporadic cases account for the majority of cases of blastomycosis, but a few outbreaks of infection from point sources have also been described. A total of nine well-described epidemics from North Carolina, Minnesota, Illinois, Wisconsin, and Virginia have provided information regarding the biology of blastomycosis.11 The association of these cases with outdoor exposure pointed to a common-source outbreak, but the actual site for such a source has been difficult to identify. The likely mechanism for infection is inhalation of spores from the soil. However, B. dermatitidis has not been commonly isolated from soil, in contrast to Histoplasma capsulatum, which is grown easily from soil. B. dermatitidis was recovered from soil and rotted wood in Georgia on three occasions.15 Sarosi16 and Serstock17 isolated the yeast phase of the fungus from bird droppings and from a dirt floor in Canada. The organism was recovered without animal inoculation from a woodpile from a hyperendemic region in Wisconsin after several dogs in a nearby kennel had been diagnosed with blastomycosis.18 Many other investigators have not been successful in recovering the organism from soil, including in some areas with epidemiologic information indicating the location of the common-source exposure. Interestingly, B. dermatitidis was isolated from soil in association with outbreaks in two separate reports.19,20 The isolations were from wet earth containing animal droppings, proving that the fungus exists in microfoci in soil. In both outbreaks, the isolation of the fungus from soil was noted to be associated with either animals or bodies of water. Whether this association simply represents a greater exposure opportunity because of the increased recreational activities in areas with wildlife or water remains to be determined.21
In the past 2 decades, there have been more cases reported from Illinois, Wisconsin, Ontario, and Manitoba.22–26 There have been reports of cases of blastomycosis from Colorado, Hawaii, Israel, India, South America, and several areas of Africa. For the most part, the incidence of blastomycosis depends on the reporting of clinically diagnosed cases of infection because there are no simple and reliable markers of previous mild infection. Mandatory public health reporting of blastomycosis is required in only a few states or provinces, namely Illinois, Wisconsin, Mississippi, Manitoba, and Ontario, and thus, cases are likely underreported in other areas.
When explored in depth, many patients with blastomycosis have a history of recreational or occupational exposure to wooded areas and often to bodies of water such as lakes or rivers. The stereotypical patient is a young to middle-aged man who either works in or visits areas known to be endemic for blastomycosis. In sporadic cases, the male-to-female ratio has been quoted to range from 4 : 1 to 15 : 1 in various series.27 However, some of these reports were conducted in Veterans Affairs institutions, which obviously adds bias to the ratio. In an outbreak, women and children are as likely as men to be infected. Aside from outbreaks, only rarely are children diagnosed with blastomycosis.28,29
Dogs in the same environment as humans can also become infected with B. dermatitidis. Having a pet dog with such a history is a clinical clue to the diagnosis of blastomycosis.30 It is not that the dogs transmit the infection to humans, but rather both are infected from similar exposure in the environment. However, there is one report of a dog with oral lesions transmitting primary skin infection via a bite.31 Other animals such as cats, horses, brown bears, and even exotic pets such as a kinkajou may become infected as well, and pathologists have obtained infection from performing autopsies on dogs.
Investigations of outbreaks of blastomycosis have increased knowledge of the spectrum of disease manifestations of infection with B. dermatitidis and allowed the recognition of subclinical infection due to this organism. The majority of people infected in point-source outbreaks at Big Fork, Minnesota and Eagle River, Wisconsin recovered without antifungal therapy.19,32 In the Eagle River outbreak, only 9 of 44 patients with infection were treated with an antifungal agent, and none of the 35 untreated patients had subsequent manifestation of infection.19
An outbreak of blastomycosis occurred in northern Wisconsin in 2009 and 2010.32a Of the patients, 45% were of the Hmong ethnic group, which is the prevalent Asian group in that area. The incidence was 168 per 100,000 population compared with 13 per 100,000 in the non-Asian population.32a A question was raised about whether there is a genetic propensity for blastomycosis among the Hmong.
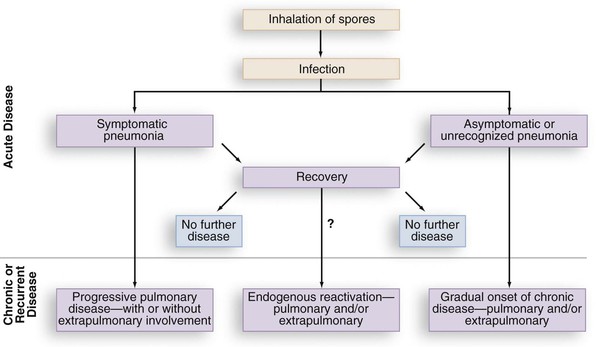
Similar to histoplasmosis and coccidioidomycosis, subclinical infection with B. dermatitidis occurs. In the Eagle River epidemic, many of those with positive skin tests and antibodies or lymphocyte reactivity to Blastomyces antigens had no signs or symptoms characteristic of blastomycosis.19 Lymphocyte studies of persons who had equivalent environmental exposures to patients with clinical blastomycosis have been performed to attempt identification of subclinical blastomycosis. Lymphocyte reactivity assays from forestry workers by Vaalar and colleagues33 were performed in areas endemic for blastomycosis but not histoplasmosis (northern Minnesota and Wisconsin). Thirty percent of the workers had in vitro markers of immunity as evidence of subclinical infection with no question of Histoplasma cross-reactions.33 Therefore, blastomycosis appears to have comparable patterns of subclinical infection as occurs with histoplasmosis and coccidioidomycosis. A patient may totally resolve the infection or may present with relapse of infection years later following the initial blastomycosis infection (Fig. 266-3).
Pathogenesis and Virulence
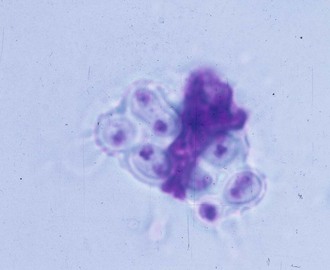
Pulmonary infection occurs with inhalation of conidia of B. dermatitidis into the lungs when natural resistance of polymorphonuclear leukocytes, monocytes, and alveolar macrophages is overcome, although the macrophages can phagocytize (Fig. 266-4) and kill the conidia.34 In addition, macrophages can inhibit the conversion of conidia to the yeast form, which may also be an important host defense.35 This innate resistance may explain how subclinical blastomycosis occurs. Conidia that escape the natural host defenses convert in the tissues to yeast forms that are more resistant to phagocytosis and killing.34 Conversion to the yeast phase most likely results in a survival advantage and contributes to the pathogenicity of B. dermatitidis.
Important factors that contribute to the virulence of the yeast form of B. dermatitidis have been reported. The thick cell wall of the yeast form has been suggested to be antiphagocytic and may contribute to its virulence. Structural components of the cell wall have also been associated with virulence, including the lipid and phospholipid content.36,37 Klein and colleagues have identified a novel 120-kD glycoprotein antigen, originally identified as WI-1 and later as BAD1, localized on the cell wall surface.38 This antigen is the major immunodominant epitope for humoral and cellular immunity38,39 and also functions as an adhesin that can bind to complement receptor 3 (CR3; CD11b/CD18) and CD14 of human macrophages.40 This adhesion activity is mediated by a 24 amino acid tandem repeat that is 90% homologous with the Yersinia adhesin called invasin.41 The carboxy terminal end of BAD1 contains a cysteine-rich domain that is similar to epidermal growth factor and may also mediate binding to extracellular matrix.41 Klein and coworkers42 further compared the quantity and shedding in a wild-type (virulent) strain of BAD1 and two mutant B. dermatitidis isolates, which are hypovirulent in a murine model of infection. Mutant strains expressed more antigen on their cell surface, shed less into the medium, and had twofold to threefold greater binding of the yeast form to macrophages, the latter of which was blocked by specific monoclonal antibody.42 In further studies using the same three strains, Hogan and Klein43 were also able to show that the quantity of alpha-(1,3)-glucan was reduced and its distribution was different in the hypovirulent strains.43 Brandhorst and coworkers44 genetically disrupted the WI-1 gene, which resulted in impaired binding and entry of the organism into macrophages and loss of adherence to lung tissue with subsequent studies showing the mutant to be avirulent in a murine model. When BAD1 was restored to the mutant organism, the fungus was again virulent.44 These studies collectively indicate that BAD1 is an important virulence factor for B. dermatitidis.
Humoral immunity against B. dermatitidis does not appear to provide resistance to or accelerate recovery from disease. The major acquired immune host defense against B. dermatitidis is cellular immunity, which is mediated by antigen-specific T lymphocytes and lymphokine-activated macrophages. Experimental animal models have demonstrated that this arm of the immune system is the critical host defense in preventing progressive blastomycosis. Cox and Larsh45 developed a yeast-phase antigen of B. dermatitidis that has allowed specific cellular immunity testing. Lymphocyte transformation assays and skin tests to this yeast antigen selectively identified Blastomyces-sensitized animals as compared with Histoplasma-sensitized animals.46 This antigen was used in lymphocyte transformation assays to differentiate patients with treated blastomycosis from those with histoplasmosis or those without fungal infection.47 In additional studies, pulmonary blastomycosis patients were shown to develop this marker of specific cellular immunity over a 4-week period. Lymphocyte stimulation with the antigen made by Cox was the most reliable indicator of infection in the Eagle River outbreak of blastomycosis, reported by Klein and coworkers48 as long-term specific cellular immunity. This in vitro correlate of cellular immunity has been found in patients who had been diagnosed and treated for blastomycosis up to 16 years earlier47 and is an indication of long-lasting immunity similar to that seen in other systemic fungal infections, such as histoplasmosis and coccidioidomycosis.
Cellular immunity can be induced in mice by subcutaneous injections of live or heat-killed B. dermatitidis.49 Further, resistance to infection has been shown to parallel the development of cellular immunity, and resistance to infection in mice has been transferred by T lymphocytes.50 The development of cellular immunity has also been documented clinically by antigen-induced lymphocyte proliferation against a variety of Blastomyces antigens.39,48,51 More recent studies from the laboratory of Klein have utilized a genetically engineered strain of B. dermatitidis that lacked BAD1 and was not pathogenic as a live attenuated vaccine in a murine model.52 Administration of the vaccine strain not only induced delayed hypersensitivity but also protected animals against lethal pulmonary infection.52
Clinical Manifestations
Blastomycosis is often not diagnosed on initial presentation because the clinical presentations are similar to other, much more common conditions. Nonspecific patient complaints, including weight loss, fever, malaise, and fatigue, are common but not helpful diagnostically. The pulmonary manifestations may be diagnosed as acute bacterial community-acquired pneumonia on one end of the spectrum or as chronic lung diseases such as tuberculosis or lung cancer on the other. The skin lesions may be diagnosed as pyoderma gangrenosum or keratoacanthoma, whereas lesions in the larynx, central nervous system (CNS), skin, breast, lymph nodes, or essentially any organ are sometimes mistakenly diagnosed as cancer. Careful epidemiologic and histologic investigation allows the correct diagnosis of blastomycosis in such cases. In an observational review of referrals of 135 patients over a 13-year period in Arkansas, 78 were male and 57 female.53 Extrapulmonary manifestations were found in 47%, and 53% had only lung involvement. Women accounted for only 30% of the extrapulmonary cases, whereas 47% of the pneumonia cases were in women.53 Greater numbers are required to have confirmation, but men could be more likely than women to have extrapulmonary disease, as is the case with paracoccidioidomycosis.
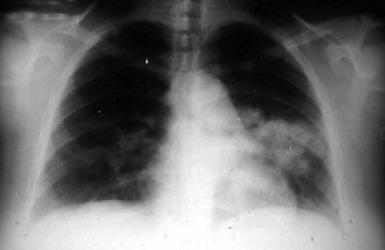
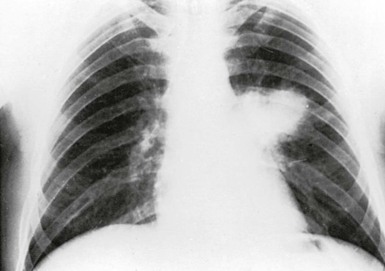
Pneumonia is the most common presentation of blastomycosis. A pulmonary infiltrate is found with the majority of patients having either an alveolar (Fig. 266-5) or masslike infiltrate (Fig. 266-6). This type of radiographic appearance was seen in 16 of 17 patients with abnormal radiographs in one report.54 In one series of 46 patients, 32% had a mass and 48% had an alveolar infiltrate on chest radiograph.53 The lobar location of the infiltrate of blastomycosis is not helpful diagnostically, although upper lobe locations have been reported slightly more commonly. Miliary or reticulonodular patterns on radiographs are the next most frequent pattern.
Kinasewitz and associates55 reported 11 of 26 patients with blastomycosis to have major pleural disease, a condition commonly reported by some authors but considered by others to be distinctly unusual in this infection. Although 26% of patients in one series had pleural disease, only two had massive effusions and only one required chest tube drainage.53 Of interest, in that patient, the chest tube site developed a local cutaneous infection similar to a patient with a complication of transthoracic needle aspiration described by Carter and colleagues.56 Although cavitary disease may occur, this pattern is not found as commonly as in chronic pulmonary histoplasmosis or tuberculosis. Because of the mass lesions on chest roentgenograms, many blastomycosis patients are initially thought to have lung cancer. In summary, none of the radiographic patterns are diagnostic for this fungal infection.
The different types of clinical presentations in pulmonary blastomycosis include acute pneumonia, chronic pneumonia, and those with no pulmonary symptoms at all.53 Patients with acute pneumonia resemble those with bacterial pneumonia with fever and chills and a productive cough with purulent sputum; a significant number also have hemoptysis. There is no response to antibacterial antibiotics, but some patients with either epidemic or endemic disease have had spontaneous resolution of their acute pneumonia, making it appear that antibiotics have worked. Some patients with this acute process have developed erythema nodosum during the resolution phase of the blastomycosis.57 The symptoms that accompany the more chronic pneumonia presentation of blastomycosis usually last 2 to 6 months and include weight loss, night sweats, fever, cough with sputum production, and hemoptysis mimicking tuberculosis. Many of these patients were tobacco smokers and thought to have lung cancer until bronchoscopy showed B. dermatitidis on cytology, which dispelled the misdiagnosis (see Fig. 266-5). In the series of Arkansas patients, 2 were asymptomatic, 16 had a chronic pneumonia picture, and 8 initially had acute pneumonia.53
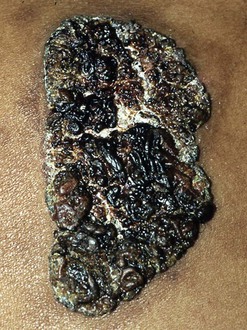
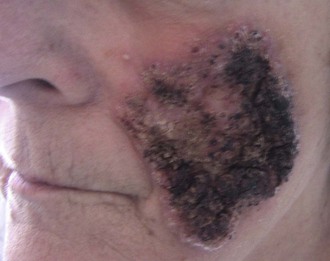
Skin lesions are the next most common manifestation of blastomycosis. The cutaneous and subcutaneous lesions are of two major types, verrucous and ulcerative. The verrucous form has an elevated, sharp border in an irregular manner with crusting above an abscess in the subcutaneous tissue (Figs. 266-7 through 266-11). These lesions show histologic evidence of papillomatosis, downward proliferation of the epidermis with intraepidermal abscesses, and inflammatory cells in the dermis.58 The organism may be seen with hematoxylin and eosin staining (Figs. 266-12 and 266-13), but hyperplasia and acanthosis may suggest other diagnoses such as skin cancer unless fungi are sought with specific histologic stains, such as Gomori methenamine silver (GMS) stain (Figs. 266-14 and 266-15) or periodic acid–Schiff (PAS) stain (Fig. 266-16). The ulcerative form of blastomycosis has histologic changes like the verrucous form. The borders are heaped up, and the base usually contains exudate (Figs. 266-17 and 266-18). Although many of these ulcers of blastomycosis originate from subcutaneous pustular lesions that drain, some patients do not have appreciable inflammation and will have a clean base in the ulcer. Polymorphonuclear leukocytes are typically present on biopsy, even in those patients with little inflammation clinically apparent in the ulcer. Subcutaneous localization lacking either ulceration or the verrucous appearance may be found. These lesions are typically tender and may be confused with panniculitis or Weber-Christian disease.59 Aspiration of the subcutaneous mass or biopsy will reveal B. dermatitidis on microscopy and culture. Only one fairly minimal lesion may be found (Fig. 266-19), or the infection can be progressive and destructive (Fig. 266-20).