BARTONELLA INFECTIONS, INCLUDING CAT-SCRATCH DISEASE
MICHAEL GILADI, MOSHE EPHROS, AND DAVID F. WELCH
The bacterial genus Bartonella is named for Dr. A. L. Barton, who described the erythrocyte-adherent Bartonella bacilliformis in 1909. It is the etiologic agent of Oroya fever, an acute bacteremic infection characterized by sepsis and hemolysis, and of verruga peruana, principally a cutaneous nodular vascular eruption representing chronic infection. The previously suspected link between the two conditions was tragically confirmed in 1885 by Daniel Carrión, a medical student who injected himself with bloody material from a verruga and subsequently died of Oroya fever. This form of bartonellosis is thus known as Carrión disease and South American bartonellosis because it is limited to the Andean mountain regions of Peru, Ecuador, and Colombia. It affects the local population and, rarely, travelers to these countries. Bartonellosis garnered little attention outside its endemic zone in recent years until related bacteria, then named Rochalimaea species, were found to be important pathogens, primarily in patients with acquired immunodeficiency syndrome (AIDS).
TAXONOMY
The now supplanted genus Rochalimaea was formerly classified with Bartonella in the order Rickettsiales and consisted of only two species, Rochalimaea (Rickettsia) vinsonii, the “Canadian vole agent,” and Rochalimaea (Rickettsia) quintana, the agent of trench fever. The latter is a debilitating but self-limited human illness so named after it affected many military personnel in World War I. Except for sporadic outbreaks, trench fever had all but disappeared from the clinical scene in recent decades. However, the 1990s saw the reemergence of R. quintana as a pathogen of considerable interest (1–5), coincident with the discovery of two related species that also cause human disease, originally named Rochalimaea henselae and Rochalimaea elizabethae (6–9).
Bartonella species are alphaproteobacteria, which also contains Afipia, Agrobacterium, and Brucella. Unlike members of the order Rickettsiales, Bartonella species have been cultured on cell-free media. Sequencing of 16S ribosomal RNA (rRNA) genes to determine phylogenetic relationships among these organisms revealed high levels of relatedness between B. bacilliformis and the former Rochalimaea species (10) and confirmed that all of them are more closely related to Brucella and Agrobacterium than to members of the rickettsiae. Based on DNA hybridization and 16S rRNA similarity, the former Rochalimaea species were combined with Bartonella in 1993 (11), and the members of the family Bartonellaceae were removed from the order Rickettsiales. A further proposal was made in 1995 to merge into the genus Bartonella a number of species of the genus Grahamella, which are intraerythrocytic pathogens of rodents, birds, fish, and other animals (12). Recently, an increasing number of Bartonella species have been identified and characterized. Currently, the genus Bartonella consists of at least 27 recognized species or subspecies, of which at least 13 have been recognized as confirmed or potential human pathogens. Others have been isolated from nonhuman wild and domestic mammals, including rodents, cervids, and cattle, without associated identifiable human illness (13). Comparison of phylogenetic data, inferred mainly from 16S rDNA, 16S-23S rRNA intergenic spacer, citrate synthase and 60-kd heat shock protein gene sequences have identified six evolutionary clusters within the genus Bartonella (14). Cat-scratch disease (CSD)–causing B. henselae are classified into two serotypes, Houston-1 and Marseille, which correspond to two genotypes based on 16S rRNA gene sequences, genotype I and genotype II. The significance of this and other classifications with respect to pathogenesis and clinical manifestations has not been established (15,16).
EPIDEMIOLOGY
Infections with B. bacilliformis are geographically limited to middle altitudes of the Andes mountains, probably because of the distribution of species of the genus Lutzomyia (formerly Phlebotomus), its sandfly vectors. B. quintana is globally distributed; there have been reports of focal, but widely separated, outbreaks of trench fever, also known as quintan or 5-day fever. Outbreaks commonly have been associated with conditions of poor sanitation and personal hygiene that predispose to exposure to the human body louse Pediculus humanus, the only identified vector of B. quintana. Although B. quintana has also been identified in the human head louse P. humanus capitis, there is no strong evidence that head lice are vectors of this organism between human hosts (17). Nonhuman vertebrate reservoirs have not yet been identified for either B. bacilliformis or B. quintana.
Cats bacteremic with B. henselae constitute the major reservoir of this pathogen. B. henselae has been documented to cause bacteremia (18,19) in seemingly healthy domestic cats, including some that have been specifically associated with bacillary angiomatosis (19) or typical CSD (18) in their human contacts. B. henselae bacteremia has been globally reported among pet, impounded, or stray cats. Rates of bacteremia can vary and may be as high as 89% (18–25). Other animals, particularly dogs, have been implicated as a possible reservoir for B. henselae, but reports are anecdotal and evidence essentially circumstantial. Fleas and ticks are arthropod vectors of B. henselae, based on epidemiologic associations (26,27) and reports of identification of B. henselae by both culture and DNA amplification from cat-associated fleas (18,19). Cat-to-cat transmission by infected fleas has been shown to occur (21), although evidence of cat-to-human transmission by fleas is lacking. Like B. quintana, B. henselae infection is globally endemic. However, regional variations in the prevalence of either B. henselae or B. quintana may occur. Transmission to humans has been linked to cats by serologic and epidemiologic studies (27,28), its recovery from cases of human lymphadenitis consistent with CSD (18,29), and the identification of B. henselae DNA by polymerase chain reaction (PCR) in CSD lymphadenitis and other affected tissues (30–33).
OVERVIEW OF CLINICAL/PATHOLOGIC MANIFESTATIONS
Oroya Fever and Verruga Peruana
Bartonella bacilliformis
Oroya fever, the bacteremic illness of primary B. bacilliformis infection, develops 2 to 14 weeks (mean 3 weeks) after inoculation by the sandfly Lutzomyia verrucarum (34). Bacteria invade blood vessel endothelium, proliferate, and upon reentry into blood vessels replicate and destroy erythrocytes. Microvascular thrombosis results in end-organ ischemia. In its milder form, the febrile illness often remits in a week. When abrupt in onset, high fever, chills, diaphoresis, headache, and mental status changes are associated with a rapidly developing severe hemolytic anemia (35–38). Lymphadenopathy, thrombocytopenia, severe myalgia and arthralgia, and complications such as delirium, coma, dyspnea, and angina can occur during this stage. Without antimicrobial treatment, mortality rates up to 40% to 80% have been reported (39,40); however, a disease milder in severity and with a low (0.7%) case-fatality rate may occur (41). Convalescence is associated with a decline of fever and disappearance of bacteria on blood smears as well as increased susceptibility to intercurrent opportunistic infections such as salmonellosis (42) or toxoplasmosis (43). Usually within months of acute infection, verruga peruana may become evident. This late-stage manifestation of infection is characterized by crops of nodular skin lesions; mucosal and internal lesions can also occur. Their histology typically contains neovascular proliferation with occasional bacteria evident in interstitial spaces. B. bacilliformis invasion of endothelial cells, which was described by Rocha-Lima and believed to be the etiology of cytoplasmic inclusions, is rare (44). Verruga peruana lesions may develop at one site while receding at another. They may persist for months to years and eventually become fibrotic with involution. Asymptomatic persistent bacteremia with B. bacilliformis infection can occur in up to 15% of survivors of acute infection (45) who may serve as the organism’s reservoir.
Bacteremic Illness and Endocarditis
Bartonella quintana, Bartonella henselae, and Other Bartonella Species
The natural course of trench fever includes a spectrum of self-limited clinical patterns (46). Incubation may span days to weeks before the typical sudden onset of fever. The febrile illness may be brief (lasting 4 to 5 days), prolonged (uninterrupted for 2 to 6 weeks), or most commonly, paroxysmal (three to five episodes, each of about 5 days). Fever may be accompanied by other nonspecific symptoms and signs such as headache, vertigo, retroorbital pain, conjunctival injection, nystagmus, myalgia, arthralgia, hepatosplenomegaly, rash, leukocytosis, and albuminuria. B. quintana has reemerged as a cause of bacteremic illness (designated also as urban trench fever) in human immunodeficiency virus (HIV)–uninfected homeless patients with chronic alcoholism (5,47,48). B. quintana was also identified in body lice from these patients. Clinical characteristics include headache, sweats, severe leg pain, and low platelet counts. Many bacteremic patients were afebrile and some were asymptomatic. Chronic bacteremia, as indicated by positive blood cultures up to 78 weeks, and intermittent bacteremia were found to occur.
B. quintana or B. henselae bacteremia in HIV-infected persons is often characterized by insidious development of fatigue, malaise, body aches, weight loss, progressively higher and longer recurring fevers, and sometimes headache. Hepatomegaly may occur. Although there is evidence implicating Bartonella species in some cases of HIV-associated encephalopathy, meningoencephalitis, and neuropsychiatric disease, lumbar puncture during acute bacteremia in HIV-infected persons can be unrevealing (6,9). Rarely, fever without localizing symptoms or signs in association with B. henselae bacteremia has been reported in immunocompetent patients (6,9). Aseptic meningitis concurrent with bacteremia has been documented in an immunocompetent host (26). B. henselae bacteremia can evolve into long-term asymptomatic persistence (26).
Both B. quintana and B. henselae are considered important pathogens of endocarditis, accounting for approximately 3% of all patients with infective endocarditis, and a much larger proportion, up to 28%, of patients with culture-negative endocarditis (1,4,49–51). B. elizabethae, B. vinsonii subspecies berkhoffii, B. alsatica, and B. koehlerae have rarely been isolated from patients with endocarditis (8,52–54). Patients with B. quintana endocarditis often have been homeless and alcoholic, whereas patients with B. henselae endocarditis have commonly reported being in contact with a cat. The typical clinical presentation is that of subacute bacterial endocarditis, including neurologic manifestations such as stroke. In a retrospective study of 101 patients with Bartonella endocarditis, embolic phenomena were reported in 43% of patients. A significant number of patients were afebrile at presentation, 12 of the 101 patients died, 2 relapsed, and 76 underwent valvular surgery (55).
Bacillary Angiomatosis and Peliosis
Bartonella quintana and Bartonella henselae
Bacillary angiomatosis (BA), also termed epithelioid angiomatosis or bacillary epithelioid angiomatosis, is a disorder of neovascular proliferation originally described involving skin and regional lymph nodes of HIV-infected persons (56–58). It has since been demonstrated to involve a variety of internal organs (59–61), including the brain (62), and to occur in other immunocompromised (59,63) and immunocompetent hosts (64–66). B. quintana infections have a predilection for causing subcutaneous and deep soft tissue disease and lytic bone lesions, whereas B. henselae infections are associated with lymph node disease and parenchymal peliosis of the liver and/or spleen. B. henselae and B. quintana (2,9,67) equally cause BA of the skin, the most common manifestation of this illness. Risk factors for B. quintana infection are low income, homelessness, and body louse infestation, whereas B. henselae infection is associated with cat or cat fleas contact (67).
Skin lesions often arise in crops, but their timing and gross appearance can vary. They can be remarkably similar to the lesions of verruga peruana. However, most cases of BA have been identified outside the region of endemic B. bacilliformis. Thus, the most important differential diagnoses are Kaposi sarcoma and pyogenic granuloma. Studies addressing the potential association between pyogenic granuloma and Bartonella infection have resulted in conflicting results (68,69). The histologic distinction of BA from other neovascular tumors has been clearly described (70,71).
Bacillary peliosis (BP), originally described involving the liver and sometimes spleen in HIV-infected persons (72), has since been identified in other immunosuppressed persons and found to involve lymph nodes as well (59,73). Involved organs contain numerous blood-filled, partially endothelial cell–lined cystic structures and surrounding clumps of bacilli (identified by Warthin-Starry silver staining) in the midst of inflammatory cells.
Cat-Scratch Disease
Bartonella henselae (Possibly Bartonella clarridgeiae, Bartonella quintana, and Afipia felis)
B. henselae is the major etiologic agent of CSD (18,19,28–32). B. clarridgeiae, B. quintana, and Afipia felis have rarely been associated with CSD in humans (74–76). The various manifestations that comprise CSD have been recognized over the past 100 years, but the syndrome per se was not really defined until 1950 (77).
In typical CSD (about 90% of cases), a cutaneous papule or pustule usually develops within a week after an animal contact (more commonly a kitten) at a site of inoculation (usually a scratch or bite) (78–80). Regional adenopathy (mostly involving head, neck, or upper extremity) develops in 1 to 7 weeks (Fig. 26.1). About one third to one half of patients have fever, and about one sixth develop lymph node suppuration. The histopathology of nodes includes a mixture of nonspecific inflammatory reactions including granulomas and stellate necrosis. Bacilli may be demonstrable by Warthin-Starry staining. Atypical CSD (about 10%) occurs as extranodal or complicated disease in the absence or presence of lymphadenopathy and includes Parinaud oculoglandular syndrome, encephalopathy, neuroretinitis and other neurologic syndromes, fever of unknown origin, hepatic and splenic abscesses, granulomatous hepatitis, debilitating myalgia, arthritis or arthralgia (affecting mostly females older than age 20 years), osteomyelitis and other musculoskeletal manifestations, and erythema nodosum (79,81–84). Other manifestations and syndromes (e.g., pneumonitis, myocarditis, and thrombocytopenia) have also been associated with CSD (85–88).
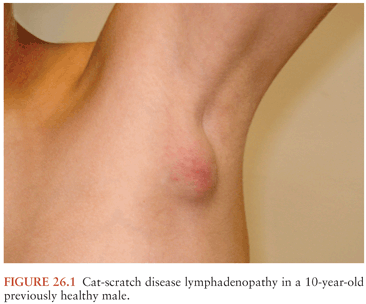
In most cases, whether typical or atypical, spontaneous resolution occurs in 2 to 4 months. The prolonged course of CSD lymphadenopathy, which is often accompanied by fever, night sweats, weight loss, and liver or spleen involvement, may resemble lymphoma or other malignant processes. Consequently, this may lead to unnecessary, extensive, costly, and sometimes invasive diagnostic procedures (89–91).
NEUROLOGIC MANIFESTATIONS OF BARTONELLA INFECTIONS
Associated with Oroya Fever
Acute B. bacilliformis infection (Oroya fever) has long been recognized to have associated neurologic manifestations (34). Acute onset of severe headache is usually coincident with onset of fever and development of hemolysis. Characteristics of the meningoencephalitis that occurs in about 1 in 10 cases include diffuse neurologic impairment that may result in seizures, hallucinations, delirium, and/or reduced consciousness, which can progress to obtundation and coma. Diminished level of consciousness is associated with a poorer prognosis. Meningeal findings without encephalitis also can occur and vice versa. Less commonly, localized findings in the form of cranial or spinal nerve palsies occur, with the latter sometimes causing a meningomyelitis with flaccid or spastic paralysis. Cerebrospinal fluid (CSF) protein elevation and mild mononuclear leukocytosis can occur, and bacteria may be identified within these leukocytes. Peripheral nerve palsies that occur during the later eruptive verruga stage are usually due to granulomatous inflammatory lesions within peripheral nerves; such impediments are usually chronic but associated with gradual resolution.
The often fatal course of Oroya fever has allowed histopathologic correlation with clinically evident neurologic manifestations (92,93). Most of the neurologic manifestations appear to be the result of the vascular endothelial cell damage that develops. In the leptomeninges, capillary and venous congestion and thrombosis are common, associated with microhemorrhages, adventitial proliferation, perivascular edema, and rarely, formation of new microvasculature. As a consequence of profound hemolytic anemia (hemoglobin concentration ≤4 g/L has been reported in Oroya fever) and microvascular thrombosis, resulting in ischemia, there can be subacute neuronal degeneration. Reactive glial proliferation is usually diffuse but in some cases may be nodular. Occasionally, granuloma-like nodules composed of microglial cells and histiocytes, named verrucomas, are found in the choroid plexus, ependyma, and brain parenchyma. Ultimately, the meningoencephalitis associated with Carrión disease appears to be more a consequence of the damage done to the host’s microvasculature, complicated by the associated profound anemia, than the result of a primary neurotropic affinity on the part of B. bacilliformis.
Associated with Trench Fever and Other Manifestations of Bartonella quintana Infection
Although trench fever is often associated with headache, specific neurologic manifestations of this form of B. quintana infection are uncommon. Few cases of B. quintana infection with distinct central nervous system (CNS) pathology have been described. A 19-year-old HIV-uninfected patient with hypogammaglobulinemia presented with fever, left hemiparesis, slurred speech, urinary incontinence, blurred vision, and behavioral changes, which developed over 2 months due to a necrotizing granulomatous process involving the right thalamus and surrounding tissues. B. quintana was identified in brain tissue, bone marrow, and serum specimens from this patient by PCR and nucleotide sequencing. B. quintana DNA was also amplified from the CSF of an 8-year-old immunocompetent child with encephalitis and axillary adenitis (94). A previously healthy 16-month-old girl was admitted to the intensive care unit with encephalopathy complicated by Guillain-Barré syndrome and hydrocephalus, which necessitated placement of a ventriculoperitoneal shunt. She had serologic and molecular evidence of central nervous system infection by B. quintana (95).
Associated with Cat-Scratch Disease
The most commonly recognized neurologic manifestations associated with B. henselae infection are those of CSD, predominantly encephalopathy and neuroretinitis. Isolated cranial and peripheral neuropathies (e.g., facial palsy), polyneuropathy, transverse myelitis, and other manifestations uncommonly occur (96–145). CSD vertebral osteomyelitis may rarely present with neurologic complications, including intraspinal extension (146–150). Encephalopathy probably occurs in 2% to 4% of all recognized CSD cases, although estimates range from 1% to 7% (115). Extrapolating from an estimated U.S. CSD case rate of 9.3 per year per 100,000 population, 2% to 4% would represent between 500 and 1,000 annual CSD encephalopathy cases in the United States. The California Encephalitis Project reported Bartonella species as the causative agent in 7 (2%) of 334 patients with encephalitis, making it the most common bacteria associated with encephalitis (151). In contrast, Bartonella cases were found neither among 203 patients with encephalitis in a multicenter prospective study from England nor among 253 patients in a national prospective study conducted in France (152,153). However, the diagnosis of Bartonella encephalitis in the latter study may have been underrepresented because the authors excluded survivors hospitalized for more than 5 days because these patients were assumed to have aseptic meningitis rather than encephalitis. Patients with encephalitis due to Bartonella species may have a fulminant presentation but often recover fully within several days after onset and thus could have been excluded from the study (154). Such diagnoses can easily be overlooked if the clinician fails to obtain an adequate history. With domestic cats representing the single largest category of companion animals in the United States, the importance of an accurate history regarding animal exposure cannot be overemphasized when evaluating a patient with findings consistent with one of these syndromes. A common pitfall in history taking is to inquire about a cat scratch or a cat bite rather than cat contact, as a significant proportion of CSD patients report cat contact without injury. Though less established, one must also keep an open mind to the possibility of transmission of B. henselae from other animals such as dogs.
CSD encephalopathy remains predominantly a clinical diagnosis, now subject to laboratory confirmation by techniques described later in this chapter (predominantly antibody testing). Adolescents and adults may represent a greater proportion of cases of CSD encephalopathy than they do of CSD overall (81). Encephalitis was also reported to be more common in elderly patients (older than 60 years of age) with CSD than in younger patients (155). The pathogenesis of CSD encephalopathy and other CNS manifestations associated with CSD remains unclear. Whether these rare complications are attributable to direct invasion of the CNS by B. henselae or to other mechanisms such as vasculitis or immune response is unknown. B. henselae has been shown to infect feline microglial cells in vitro and survive intracellularly for up to 4 weeks; however, no ultrastructural abnormalities were identified within infected brain cells by electron microscopy (156).
In most patients, encephalopathy usually follows lymphadenopathy, by a period of days up to 2 months, although it has also been reported to precede lymph node involvement or to occur in its absence. Persistent generalized headache is a common part of the history, but fever is an inconsistent finding. Patients may become restless and combative. Nearly half of patients develop seizures, which may range from focal to generalized and from brief and self-limited to status epilepticus. Short-term anticonvulsant therapy may be required, as may be supportive therapy in the face of obtundation or coma. Nuchal rigidity, pathologic reflexes, or pupillary dilation may be present transiently. Neurologic deficits such as aphasia, cranial nerve palsy, paresis, hemiplegia, and ataxia are usually self-limited, although time to resolution may span weeks to months to as long as a year. Persistence of intellectual impairment, ataxia, and seizures have been reported (81,112,118,125,128), as well as rare cases of death due to CSD meningoencephalitis in two previously healthy children, aged 4 and 6 years (157,158).
Laboratory studies in the setting of CSD encephalopathy do not add specific positive diagnostic findings to the clinical picture, but they serve to exclude other processes. CSF measurements fit no consistent pattern, except that hypoglycorrhachia is rare. Elevation of CSF protein concentration and pleocytosis with lymphocytic predominance occur in only about one third of patients (but not necessarily in the same patients) (151). Peripheral blood leukocytosis occurs as well in only about one third of patients. CSF cultures have been consistently negative.
Studies of the brain with computed tomography (CT) and/or magnetic resonance imaging (MRI) usually show no abnormalities. Transient nonspecific abnormalities are occasionally identified, but a few cases of persistent structural abnormalities have been reported (128,159). Electroencephalography during the acute phase of CSD encephalopathy commonly reveals diffuse slowing, yet another nonspecific feature that resolves with clinical recovery. Brain biopsy is usually not indicated because of the self-limited nature of CSD encephalopathy, and thus little is known about the histologic correlates of the clinical manifestations. At autopsy of a fatality due to CSD encephalomeningitis, there was marked cerebral edema with no gross evidence of acute meningitis. Microscopic examination revealed multiple granulomatous lesions, meningitis, and encephalitis. Warthin-Starry silver stain of the brain and liver revealed pleomorphic rod-shaped bacilli consistent with B. henselae infection. Analysis of brain tissue with PCR confirmed the presence of B. henselae DNA (157). Histologic examination of the second fatality showed extensive diffuse perivascular lymphocytic infiltrates with microglial nodules scattered throughout the frontal, parietal, and occipital lobes and the pons. In some foci, the nodules appeared vaguely granulomatous (158). Biopsy of concurrent lymphadenopathy, when done, reveals features typical of CSD.
The neuroretinitis associated with CSD (96,108,116,117, 120,121,124,129,133,160–164) has been confirmed by serology and culture to be related to B. henselae infection. Neuroretinitis in association with B. henselae bacteremia (96), aseptic meningitis (165), and encephalopathy (166) have been reported in patients with CSD. Chorioretinitis and multiple hypodense areas within the spleen and liver parenchyma have been described in a 10-year-old previously healthy boy several weeks after a cat scratch (167). Although long-term prognosis is usually good, some individuals may develop mild postinfectious optic neuropathy, and few may develop permanent visual disturbances. Vitrectomy is only rarely indicated. With the refinement of techniques for identification of Bartonella infection, diagnostic accuracy has improved, broadening the spectrum of CSD-associated retinal manifestations and identifying new Bartonella species as possible pathogens in neuroretinitis. B. grahamii was identified by PCR amplification and sequence analysis in the intraocular fluid of an HIV-seronegative patient with bilateral neuroretinitis and behavioral changes, and B. elizabethae infection was diagnosed serologically in another patient with neuroretinitis (168,169).
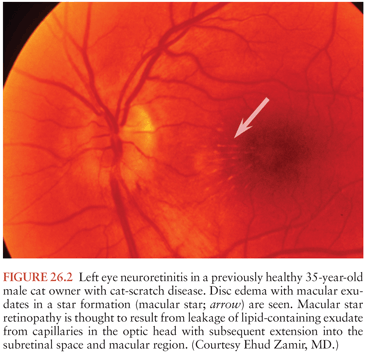
The typical clinical scenario of CSD neuroretinitis, a process first described as Leber idiopathic stellate retinopathy (116,170), is that of painless, fairly sudden loss of visual acuity, usually unilaterally, and sometimes preceded by an influenza-like syndrome or development of regional lymphadenopathy. Neuroretinitis is characterized by papilledema often associated with macular exudates in a star formation (Fig. 26.2). In a retrospective study among 24 patients with CSD with 35 affected eyes, isolated foci of retinitis or choroiditis were the most common ocular manifestation identified in 83% of eyes and 83% of patients. Optic disk swelling was the second most common finding (46% of eyes, 63% of patients), followed by a macular star (43% of eyes, 63% of patients) and vascular-occlusive events (14% of eyes, 21% of patients). Final visual acuity was 20/25 or better in 26 (74%) of 35 eyes and was similar in both treated and untreated patients (161). Optic disk edema associated with peripapillary serous retinal detachment has been described as an early sign of ocular CSD. The typical macular star may or may not follow these early manifestations (171). Other types of ocular CSD manifestations include optic neuritis, anterior uveitis, panuveitis, vitreitis, pars planitis, focal retinal vasculitis, retinal white spot syndrome, branch retinal arteriolar or venular occlusions, central retinal artery and vein occlusion, focal choroiditis, vitreous and retinal hemorrhages, and a process associated with peripapillary angiomatosis (172–181). The pathophysiology of neuroretinitis is thought to be leakage of lipid-containing exudate from capillaries in the optic head with subsequent extension into the subretinal space and macular region. This type of process has been recognized as a secondary phenomenon in most circumstances, occurring in association with traumatic injuries of the eye or brain, ocular vascular disturbances, toxins, autoimmune states (e.g., Behçet syndrome), or a variety of infections (e.g., influenza-like syndromes, syphilis, leptospirosis, tularemia, tuberculosis, psittacosis, endemic mycoses, and parasites). Thus, although this process can be considered characteristic of CSD neuroretinitis, it is not pathognomonic and many other causes must be included in the differential diagnosis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree