B VIRUS
RICHARD J. WHITLEY
HISTORY
Of the nearly 35 herpesviruses that have been isolated from nonhuman primates, only one—B virus of Old World monkeys—is highly pathogenic for humans. In 1932, a young physician (W.B.) bitten by a monkey developed localized erythema at the site of the animal bite. This apparently localized infection was followed by lymphangitis, lymphadenitis, and ultimately transverse myelitis. The demise of W.B. was ascribed to respiratory failure. Tissue specimens from W.B. were obtained by two independent research groups. In 1933, Gay and Holden (1) reported the identification of an ultrafilterable agent that recreated in rabbits a disease similar to that observed in W.B. This virus was recovered from the neurologic tissues of W.B. The inoculation of this virus by either intradermal or intracranial routes was lethal in the animal model. These investigators thought that the virus was similar to herpes simplex virus (HSV) and referred to the isolate as W. While attempts at transmission of this virus to rhesus monkeys failed, infection of Cebus monkeys was successful. In 1934, Sabin and Wright (2) reported the identification of a similar filterable agent, which they identified as B virus, named W.B. They isolated virus from several neurologic tissues as well as from peripheral organs (spleen but not lymph nodes). As with the isolate named W, B-virus infection resulted in lethal disease when inoculated by either intradermal or intracerebral routes into rabbits. However, the virus was avirulent when inoculated by the same routes into a variety of other species, including mice, dogs, and guinea pigs. Furthermore, Sabin (3) recognized by immunologic characterization of the isolate that it was related to both HSV and pseudorabies virus (3,4). At the time when latinization of viral names was fashionable, B virus acquired the name herpes simiae. The name is a misnomer because nonhuman primates have yielded numerous unrelated herpesviruses. According to the present nomenclature, it is designated as the cercopithecine herpesvirus type 1. In this chapter, the virus is designated by its common name, B virus.
B virus is indigenous to Old World monkeys (5,6), being enzootic in rhesus (Macaca mulatta) and cynomolgus (Macaca fascicularis) monkeys, as well as in other Asiatic species of the genus Macaca. Most reported human cases result from bites by rhesus monkeys. Since the original reports of human herpes B-virus infections, advances in our knowledge about human disease caused by this virus have proceeded slowly. Although B-virus infection of humans is not a common public health problem, the diagnosis of B virus in four individuals living in Pensacola, Florida; two in Kalamazoo, Michigan; and an animal handler at Yerkes Primate Center has refocused medical and public health attention on the neuropathogenicity of infection (7). Approximately 50 cases have occurred, although only 26 are well documented (7). Other cases are pending verification. Person-to-person transmission of infection from one of the fatal cases to a relative was documented for the first time. Additionally, a case has been identified that led to infection, but without evidence of life-threatening clinical disease (8). Lastly, ocular inoculation of virus proved lethal (7).
INFECTIOUS AGENT
B virus was propagated on the chorioallantoic membrane of embryonated eggs in 1939 (8). Tissue culture isolation of B virus did not occur until 1954, when it was recovered from rhesus kidney tissue used for preparation of poliomyelitis vaccines (9). During vaccine production, suspended cell culture systems from six kidneys elicited cytopathic effects similar to those of HSV.
Notwithstanding numerous attempts at isolation of virus from macaques, the first report of virus isolation is that of Reissig and Melnick (10), who recovered B virus from tissue cultures of purportedly normal rhesus monkeys. Further studies led to the realization that this virus was easily propagated in monkey kidney and chick embryo cell lines (10, 11). B virus also multiplies well in rabbit kidney cells and other established cell lines such as BS-C-1 (12) and LLC-RK (13). Virus is stable in tissue culture media when stored at 4°C and viral infectivity is maintained if the culture is stored at −72°C.
Replication characteristics of B virus have been reported by several laboratories (4,10,11,14–16). The reproductive cycle is relatively short. The virus inhibits host cell DNA and protein synthesis during the first 4 hours after infection. Infectious virus is detectable approximately 6 hours after infection, and both extracellular and intracellular virus levels reach a plateau approximately 24 to 36 hours after infection and decline thereafter (10,16).
PATHOLOGY AND PATHOGENESIS
Pathology
The characteristic cytopathic effect is similar to that of HSV. Cells balloon, fuse into polykaryocytes, and form clusters as focal areas of infection spread through the entire cell sheet. On fixation and staining, Cowdry type A eosinophilic intranuclear inclusions are demonstrable.
Pathologic findings in humans are indicative of the target organs involved. As described later in this chapter, there is little difference in the histopathologic findings encountered following either infection of humans or simians.
Pathogenesis of Human Infection
Disease in humans usually results from an animal bite or scratch, although disease that is the consequence of respiratory spread and reactivation has been reported (see later discussion). Following exposure, replication of virus at the local site of inoculation occurs. If the skin is the primary site of inoculation, virus replicates in the skin; this leads to erythema and calor at the initial site of involvement. A vesicular rash may appear in association with tingling and numbness. Usually, there is evidence of local inflammation at the site of infection with mononuclear infiltrate followed by evidence of lymphangitis and subsequent lymphadenopathy. Although viremia has been documented in both rabbits and monkeys, this route of pathogenesis has not been documented for humans. With lymphatic involvement, it is possible for the virus to spread by lymphangial routes. Visceral organs that are involved, including the heart, liver, spleen, lungs, kidneys, and adrenals, demonstrate evidence of congestion and focal hemorrhagic necrosis; however, the degree of involvement varies from one patient to the next. A striking characteristic of human B-virus infection is the propensity to involve the central nervous system (CNS) and likely reflects spread via neuronal routes. Transverse myelitis is a prominent neurologic finding, with ultimate progression of infection to the brain. B-virus infection can involve all regions of the brain without evidence of localization to any particular region, in contrast to HSV infection of the CNS, which tends to localize in the temporal lobe. Histopathologic findings of the brain include hemorrhagic foci, necrosis, and inflammatory changes, as evidenced by perivascular cuffing with mononuclear infiltrates. Edema and degeneration of motor neurons are prominent. Even with advanced disease, Cowdry type A eosinophilic intranuclear inclusions can be found in only a few cases. In addition, gliosis and astrocytosis are late histopathologic findings. Thus, there can be evidence of myelitis, encephalomyelitis, encephalitis, or combinations thereof (17).
Pathogenesis of Latency in Macaques
Like other herpesviruses, B-virus infection in macaques becomes latent and can recur. As is discussed later in this chapter, prevalence of antibodies to B virus is widespread in Old World rhesus monkeys. Rhesus monkeys captured in the wild and shipped to primate centers develop vesicular lesions of the oropharynx, suggesting a pattern of virus reactivation and recrudescence of lesions similar to that encountered with HSV. Unequivocal evidence of latent B-virus infection in rhesus monkeys evolved with the studies of the recovery of B virus in monkey kidney cell culture systems. Thus, Wood and Shimada (9) obtained six isolates from 650 pools of monkey kidneys, suggesting that at least 1% of rhesus monkeys contain latent virus that can be reactivated by culturing kidney cells. Virus was isolated from rhesus tissues also by Boulter (18). B virus was recovered by cocultivation from a variety of neuronal tissues (including gasserian ganglia, trigeminal ganglia, and dorsal root ganglia) and the spinal cord (19). Latent virus was also isolated by cocultivation of tissues from experimentally infected rabbits (9).
As in the case of HSV infections in humans, the most prominent factor associated with reactivation of B virus in rhesus monkeys is stress, particularly stress associated with the capture and shipping of animals from the wild to captivity. To date, the state of viral DNA during latency or on the molecular or biochemical events associated with the establishment and reactivation of latent virus are unknown.
EPIDEMIOLOGY OF HUMAN INFECTION
B-virus infections of humans must be considered in the context of the epidemiology of any infectious disease, including the susceptibility of the host and quantity of virus to which they are exposed. Importantly, the frequency of excretion of B virus in infected animals averages 2% to 3% at most; thus, if a human is bitten by an infected animal, the probability of infection is low, albeit existent. Direct contact with a source of virus—namely, an actively or latently infected animal (or cells obtained from an infected animal)—is the means by which an infection is transmitted. Virus cannot penetrate intact skin; thus, a break in the skin is required for acquisition of infection. Sites that have been documented as initially infected in humans include wounds, the eyes, and perhaps the respiratory tract. Because it is well established that infected animals can excrete virus in saliva, from the eyes, in vesicular fluid, and perhaps in the stool, these must all be considered potential sources for human infection. Furthermore, as has been well documented in the history of the development of poliomyelitis vaccine, infected cell culture tissues can also be a source of viral infections for humans.
Person-to-person transmission of B virus has been documented (17). In this instance, the wife of an animal worker, who subsequently died of B-virus infection of the CNS, acquired vesicular lesions of her ring finger. She provided direct care of her husband’s vesicular lesions. Although she survived infection without evidence of neurologic disease, the extent of disease may have been limited by early intervention with acyclovir. Notably, individuals who developed B-virus infection had either skin-to-skin or face-to-face contact with at least 40 other individuals during a cluster of cases in Pensacola, Florida. Although these other individuals were under close surveillance, none developed evidence of infection. Thus, casual contact can be excluded as a method of transmission of B-virus infection. This is particularly important from a public health standpoint.
Nonfatal infection has not been well documented in reports of human disease. One recent animal bite resulted in localized viral replication without systemic involvement (20). Serologic assessment of humans for evidence of B virus as a marker of subclinical infection is confounded by cross reactivity to HSV.
MANIFESTATIONS OF CENTRAL NERVOUS SYSTEM DISEASE
Human B-virus disease is characterized by an ascending myelitis or encephalomyelitis. To date, there are a limited number of cases of human disease caused by B virus in the literature, depending on the criteria for case definition (7). Several cases have been cited in the literature but have not been documented. Regardless of the exact number of cases, the total number of patients who have experienced disease is limited. Probably thousands of animal workers have been exposed to animals excreting virus, but very few have developed disease. As noted earlier, casual transmission is unlikely. Nevertheless, the lessons learned regarding the clinical manifestations of disease in individuals exposed to B virus, as well as the necessity for methods of intervention and prevention, are reinforced by the findings in these particular patients.
Infection can occur in one of four fashions. First, infection may be asymptomatic. An animal worker exposed to a rhesus monkey with conjunctivitis was stuck by a needle that had been used for inoculation of this animal (20). Subsequent biopsy of the needlestick site revealed evidence of multinucleated giant cells. B-virus DNA was detected in the biopsy specimen, but there was no evidence of clinical disease. This form of infection may be an underreported event for individuals exposed to B virus. A second, and more common, route of infection is by animal bite. Following a bite by a monkey excreting B virus, a localized vesicular lesion associated with erythema and edema develops at the site of viral inoculation. Subsequently, lymphangitis with spread to regional lymph nodes occurs, along with the development of secondary lymphadenopathy. These early stages of disease are generally accompanied by fever, myalgia, vomiting, abdominal cramping, meningeal irritation, and such cranial nerve signs as nystagmus and diplopia. Neurologic symptoms develop very rapidly. Altered sensation, hyperesthesia, or paresthesia of the limbs usually precede evidence of weakness, areflexia, and flaccid paralysis. Transverse myelitis with urinary retention is likely to occur. With progressive involvement of the CNS, decreased levels of consciousness, altered mentation, respiratory depression, seizures, and ultimately neurologic death ensue.
Although the incubation period for B virus has been debated, most cases have a relatively short incubation period of approximately 3 to 5 days; however, some cases have been reported to occur as late as 24 days after an animal bite. The onset of neurologic symptoms generally occurs 3 to 7 days after the appearance of a vesicular rash. Time to death varies and can occur as early as within 10 to 14 days or much later. Improvements in intensive care for critically ill patients probably have influenced these survival data. The progression of infection is likely related to a variety of factors, including host immunologic status quantity of neutralizing antibodies to HSV, age of the patient, site of the bite, and quantity of virus inoculated. Prolonged incubation periods, as well as the mechanism for these prolonged incubation periods, have not been well characterized (7).
Third, cases have been documented following exposure to infected secretions by an ocular or respiratory route. An animal care provider was exposed to direct inoculation of saliva into the eye. In spite of rigorous rinsing, she became infected and subsequently died (7). Two other cases illustrate the potential for respiratory spread. Symptoms in these individuals were localized to the respiratory tract, including coryza, cough, laryngitis, and pharyngitis. While associated with fever, these symptoms progressed to respiratory distress, as evidenced by a radiographic evidence of interstitial pneumonitis. One of these two patients had virus isolated from a vesicle. Subsequently, neurologic symptoms developed, ultimately leading to death of these patients (5).
The fourth manifestation of human disease is recurrent infection. At least two patients illustrate that a recurrent vesicular rash can occur as a consequence of B-virus infection. In one case, infection was acquired as a consequence of person-to-person transmission. In another case, an individual developed lesions compatible with the diagnosis of herpes zoster; however, B virus was isolated from these lesions. Of the well-described cases of herpes B-virus infection, the majority developed encephalitis (>90%), with a documented mortality rate of 75%. Survivors of B-virus infection of the CNS have a broad spectrum of neurologic impairment. Of those documented in the literature before the availability of antiviral therapy, two had mild or minimal impairment, one had moderate neurologic impairment, and the remainder had severe impairment. The literature also documents three patients who were infected with B virus and survived infection but did not develop encephalitis. One had severe neurologic impairment (presentation of herpes zoster), and two appeared normal on follow-up. With the use of antiherpetic drugs, survivors who were treated early, even if they had CNS disease, tended to survive without significant impairment (21–23). Thus, taken together, these cases serve to emphasize the severity of B-virus disease.
DIAGNOSIS
Clinical
The total number of cases of B-virus infection is small. The possibility of B-virus infection must be considered in any individual having contact with Old World monkeys, particularly Asiatic Macaca species. Because of the recent identification of person-to-person transmission, individuals having intimate contact with workers exposed to these animals should be considered as having B-virus infection if compatible clinical findings occur. The suspicion of B-virus infection should be reinforced in the presence of historical evidence of direct contact with a rhesus monkey, either by bite, scratch, or laboratory accident. In addition, some cases have been acquired by exposure to monkey tissues; consequently, this route of transmission should not be excluded as a possibility for human infection.
Clinical evidence of disease has been defined earlier. Of particular interest to the clinician is the presence of a vesicular rash at a bite site with ipsilateral regional lymphadenopathy. With a compatible incubation period, the progressive appearance of neurologic symptomatology—particularly altered sensation in the extremities, weakness, hyporeflexia or areflexia, and altered mentation—in the presence of a wound should suggest the possibility of B-virus infection.
Virus Isolation
Recovery of virus from humans suspected of having B-virus infection must be attempted. Sources for retrieval of virus are those indicative of the pathogenesis of disease. These include vesicles, conjunctivae, pharyngeal swabs, and tissue biopsy material. Furthermore, retrieval of virus from the cerebrospinal fluid (CSF) should be attempted, although the yield is very low. Specimens for virus isolation should be processed in cell lines that are susceptible to B virus. As noted earlier in this chapter, these include primary vervet monkey kidney, rabbit kidney cells, or established strains such as BS-C-1 or LLC-RK-1. B virus replicates in all these cell lines, in contrast to other simian viruses, which have a more limited spectrum of susceptible cell lines (24).
Once viral isolation has been achieved, identification of virus can be accomplished using either molecular methods (25,26) or neutralization assays, although the latter assay is cumbersome and tedious (5,27,28). Rapid identification of isolates is essential for purposes of intervention with appropriate therapeutic agents (29,30).
Serologic Evaluation
Serologic determination of B-virus infection is exceedingly difficult because of its cross reactivity with HSV. Attempts at antibody absorption are not satisfactory. The extensive cross reactions of sera to simian viruses in the presence of HSV antibodies have made diagnosis difficult (5,31,32). Animal workers providing care for Macaca monkeys should be serially bled for antibody determinations. Sera should be banked for future reference. The standard serologic assays that have been employed generally use either neutralization assays or complement fixation (33). In general, complement-fixing antibody titers to HSV are higher in humans than in monkeys, whereas titers against B virus are higher in Macaca monkeys than in humans. As previously mentioned, human sera contain higher levels of neutralizing antibodies to HSV than to B virus. Furthermore, sera derived from monkeys with B-virus infection have been reported to neutralize HSV better than they neutralize the endogenous virus. No simple test exists to distinguish antibodies to HSV from antibodies to B virus. Nevertheless, a variety of assays for the rapid evaluation of human antibody response are under development (29,34). The advent of these newer diagnostic assays will be of value for prospectively evaluating patients exposed to B virus.
Polymerase Chain Reaction Detection of B-Virus DNA
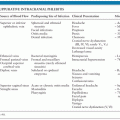
Recently, a single-step polymerase chain reaction (PCR) assay has been reported to be of value for diagnostic purposes (35). Because of the rarity of disease, field performance characteristics need to be determined. Primate workers should carry a card that indicates symptoms of B virus and contact information for clinical and laboratory consultation regarding B virus (Table 14.1).

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree