Thomas F. Patterson
Aspergillus Species
Invasive aspergillosis is a major cause of morbidity and mortality in immunosuppressed patients. This infection is caused by Aspergillus, a mold with hyaline hyphae that is the etiologic agent in invasive aspergillosis and a variety of noninvasive or semi-invasive conditions. These syndromes range from colonization, such as fungus ball due to Aspergillus (also known as aspergilloma); allergic responses to Aspergillus, including allergic bronchopulmonary aspergillosis (ABPA); to semi-invasive or invasive infections, from chronic necrotizing pneumonia to invasive pulmonary aspergillosis and other invasive syndromes.
Aspergillus and the resultant aspergillosis are a major focus of clinical mycology because the number of patients with this disease has risen dramatically and because of the morbidity and mortality of this infection.1 The increased number of Aspergillus infections has occurred because more patients are at risk for this infection. Patients with established invasive aspergillosis have poor outcomes.2–5 Successful therapy depends not only on an early diagnosis but also on reversal of underlying immune defects.1 Even when therapy is begun promptly, outcomes are often poor, particularly in patients with disseminated or central nervous system disease and in those who remain profoundly immunosuppressed.1,3 New diagnostic approaches and new management strategies have been established.1 In this chapter, clinical mycology, epidemiology, pathogenesis, clinical presentation, diagnosis, treatment, and prevention of aspergillosis are described.
Mycology
The genus Aspergillus was first recognized in 1729 by Micheli, in Florence, who noted the resemblance between the sporulating head of an Aspergillus species and an aspergillum used to sprinkle holy water.6 In 1856, Virchow published the first complete microscopic descriptions of the organism.7 Aspergillus flavus was formally named by Link in 1809.8 Thom and Church first classified the genus in 1926 with 69 Aspergillus species in 11 groups. The term “group” is now more correctly referred to as “section,” but this reporting is not commonplace in clinical mycology laboratories.9 Because phenotypic methods and internal transcribed spacer sequencing identify Aspergillus isolates within a section and not individual species, it has been recommended that isolates should be reported as members of a “species complex.”10 With the recent use of molecular techniques to characterize pathogenic fungi, aspergilli have now increased dramatically to include more than 250 species in eight subgenera (Aspergillus, Fumigati, Circumdati, Candidi, Terrei, Nidulante, Warcupi, and Ornati), which are subdivided into multiple sections and species complexes.10–13
Most species of Aspergillus reproduce asexually, but a teleomorph (or sexual form with a fruiting body) has been identified for a number of species, including pathogenic species such as Aspergillus nidulans (teleomorph, Emericella nidulans), A. amstelodami (Eurotium amstelodami), A. udagawa (Neosartorya udagawae), and the most common pathogen A. fumigatus (Neosartorya fumigata) and many others.13–16 Even though the correct taxonomic nomenclature would rename these organisms using the sexual form, generally the generic name Aspergillus has been retained to simplify nomenclature regardless of their teleomorphs (sexual forms), rather than separating the organisms into unfamiliar species based on discovery of a sexual state.17 As with other pathogenic fungi, the taxonomy of Aspergillus has undergone extensive reclassification with utilization of molecular studies, such as sequencing of ribosomal, β-tubulin, calmodulin, or rodlet A genes, which has allowed more natural subgroupings of ascomycetous fungi.10,12,18–20 With identity established by means of molecular sequencing, the result of a familiar species may be reported as an unfamiliar telemorph, which has led to assigning one name to one fungus to clarify this potential confusion in clinical mycology.21,22
The genus Aspergillus is an anamorphic member (asexual form) of the family Trichocomaceae. The teleomorphs (sexual forms) of Aspergillus species are classified in eight genera in the order Eurotiales in the phylum Ascomycota.11,13 Identification of the genus and of common pathogenic species is usually not difficult, but species-level identification of less common members can be laborious and misidentification of atypical or “cryptic” members of sections—such as poorly sporulating forms—is common.12,20,23
The most common species causing invasive infection is Aspergillus fumigatus, the most common pathogen in the section Fumigati, which historically has made up a vast majority of invasive isolates: A. flavus; Aspergillus terreus; and, less commonly for invasive infection, Aspergillus niger.3 Recent studies have shown emergence of less common species, including A. terreus and unusual, less pathogenic species as the etiologic agents of invasive infection, including many “cryptic species” that are identifiable only by molecular studies.12,20,23,24 With more prolonged and profound immunosuppression—along with molecular identification of cryptic species within a species complex, the list of rare species causing invasive infection continues to increase, including A. alabamensis, A. alliaceus (teleomorph, Petromyces alliaceus), A. avenaceum, A. caesiellus, A. candidus, A. carneus, A. chevalieri (teleomorph, Eurotium chevalieri), A. clavatus, A. calidoustus, A. flavipes, A. fumigatiaffinis, A. glaucus, A. granulosus, A. insuetus, A. keveii, A. lentulus, A. nidulans (Emericella nidulans), A. novofumigatus, A. ochraceus, A. oryzae, A. puniceus, A. pseudodeflectus, A. restrictus, A. sydowii, Emericella quadrilineata (anamorph, A. tetrazonus), A. tamarii, A. tanneri, A. udagawa (Neosartorya udagawae), A. tubingensis, A. versicolor, A. viridinutans, A. vitus (teleomorph, Eurotium amstelodami), A. wentii, Neosartorya pseudofischeri, and many others, although the authenticity of at least some of these has been questioned and perhaps misidentified prior to molecular studies.9,11,12,20,25–30
Pathogenic Aspergillus species are easily cultured from pathologic samples and grow rapidly (in 24 to 72 hours) on a variety of media. Blood cultures are uncommonly positive and usually reflect contamination rather than invasive disease.31 A distinguishing characteristic of pathogenic Aspergillus species is their ability to grow at 37° C. A. fumigatus is able to grow at 50° C, a feature that, in addition to morphology, can also be used to identify this species and can help distinguish A. fumigatus from cryptic Aspergillus species in the A. fumigatus complex.23,26,27 Most species initially appear as small, fluffy white colonies on culture plates within 48 hours. Presumptive identification of an Aspergillus species complex is usually accomplished by appearance of the fungus on gross and microscopic inspection of the colony, which provides typical sporulation, although specific species-level identification requires molecular confirmation so that laboratories should report isolates identified phenotypically as a “species complex.”12
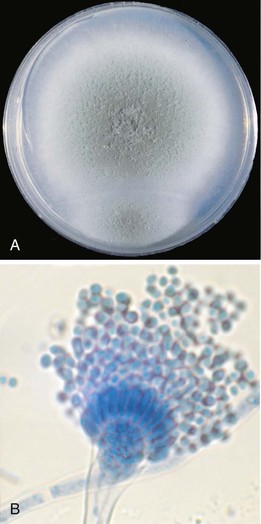
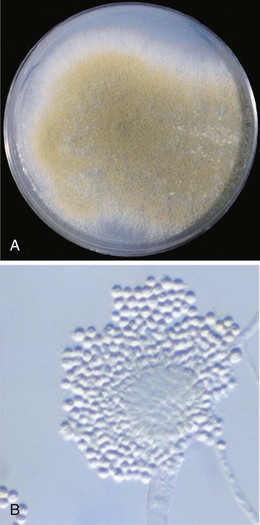
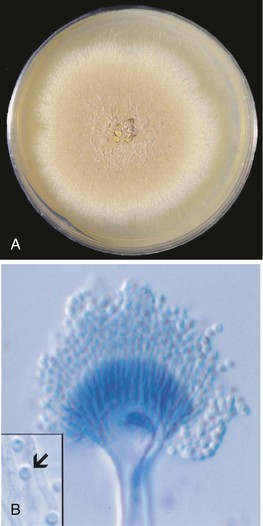
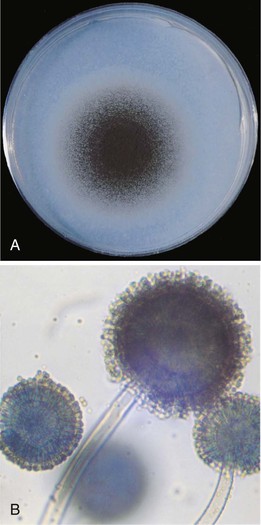
Microscopic features and colony morphology for the most common clinical isolates, A. fumigatus, A. flavus, A. terreus, and A. niger, are described in Table 259-1 and shown in Figures 259-1 to 259-4. Species-level identification of Aspergillus has become increasingly important because of differences in antifungal drug susceptibility.12,32
TABLE 259-1
Characteristics of Aspergillus Species Associated with Invasive Infection
| ASPERGILLUS SPECIES | FREQUENCY OF SPECIES COMPLEX ISOLATED IN CLINICAL INFECTION (%)3,12,20 | COLONY CHARACTERISTICS26 | MICROSCOPIC FEATURES26 | CLINICAL SIGNIFICANCE |
| A. fumigatus (see Fig. 259-1A and B) | 50-67 | Smoky gray-green; may have pale yellow or lavender reverse; grows at 50° C | Columnar; uniseriate; smooth to finely roughened conidia 2-3.5 µm | Most common invasive species; most pathogenic |
| A. flavus (see Fig. 259-2A and B) | 8-14 | Olive to lime green | Radiate to loosely columnar; uniseriate or biseriate; rough conidiophore; conidia 3-6 µm | Sinusitis; skin infection; produces aflatoxin |
| A. terreus (see Fig. 259-3A to C) | 3-5 | Beige to cinnamon buff | Columnar; biseriate; globose; small 2-2.5 µm conidia; globose accessory conidia along hyphae | Increasingly detected; resistant to amphotericin B; more susceptible to newer azoles |
| A. niger. (see Fig. 259-4A and B) | 5-9 | Initially white, rapidly turning black with yellow reverse | Radiate; biseriate; globose, black, very rough conidia 4-5 µm | Uncommon in invasive infections; superficial agent of otic disease; colonization |
A. fumigatus is the most frequent species in invasive infection, constituting more than 90% in some series, although recently a prevalence of 50% to 67% has been described.3,12,20,31 Colonies of A. fumigatus are typically gray-green with a wooly to cottony texture (see Fig. 259-1A).26 Like other species of Aspergillus, hyphae are hyaline (lightly pigmented), have septa, and are usually branched at acute (typically 45 degrees) angles. The conidial head is columnar with conidiophores that are smooth walled and uncolored or are darkened in the upper portion near the vesicle. This species is uniseriate (a term describing phialides that are attached directly to the vesicle), with closely compacted phialides borne only on the upper portion of the vesicle (see Fig. 259-1A). Conidia are smooth to finely roughened and are 2 to 3.5 µm in diameter. The fruiting head (the conidiophore and conidia) is not commonly seen in clinical specimens, although it may be detected in sites exposed to air, such as wounds or lung cavities.26 Like other Aspergillus species, it is widespread in nature—found in soil, on decaying vegetation, in the air, and, more recently, in water supplies.33,34
Other “cryptic” members of the section Fumigati have been described that are human pathogens, including A. lentulus, A. fumigatiaffinis, A. novofumigatus, N. pseudofischeri, A. udagawa (N. udagawae), A. viridinutans, and others.12,20,23,35 These species are frequently poorly sporulating and fail to grow at 50° C.23,27 Importantly, some of these species, such as A. lentulus, A. viridinutans, N. pseudofischeri, and A. fumigatiaffinis, may exhibit decreased antifungal susceptibility and can be associated with a poor clinical outcome.12,32,36 When these species are only identified phenotypically, they should be referred to as members of the A. fumigatus species complex.
A. flavus is a common isolate in sinusitis, skin, and other invasive infections. This species, which produces an aflatoxin when growing on certain culture media, is found in soil and decaying vegetation.37 Colonies are olive to lime green and grow at a rapid rate (see Fig. 259-2A). Some isolates are uniseriate, but most are typically biseriate. In biseriate species, sterile cells known as metulae are attached to the vesicle, and these, in turn, support the phialides. A. flavus has noticeably rough conidiophores and smooth conidia of 3 to 6 µm (see Fig. 259-2B).26 If characterized by morphology alone, A. flavus and other species in the section Flavi should be referred to as members of the A. flavus species complex. Other species, such as A. alliaceus, have been reported to cause clinical infection and may have decreased susceptibility to polyenes.12,38
A. terreus is a common soil-related isolate that has been increasingly reported in invasive infection in immunocompromised hosts.24 A. terreus conidia are small (2.0 to 2.5 µm), and the colony color and fruiting structures are characteristic for this species (see Fig. 259-3A and B). Colonies range in color from buff to beige to cinnamon (see Fig. 259-3A).12,26 Conidiophores are smooth walled and hyaline, and the conidial heads are biseriate and columnar (see Fig. 259-3B). A distinguishing feature of this species is the presence of globose accessory conidia that are produced on hyphae (see Fig. 259-3B, inset). These accessory conidia can be detected in histopathologic samples, which may be used to establish presumptive identification of the species.39 Identification of this species has become increasingly important because of its resistance to antifungal agents, including amphotericin B, although improved susceptibility with newer azoles has been reported.40,41 Molecular characterization has shown A. terreus is also a species complex with species such as A. alabamensis and A. carneus causing infection in immunocompromised hosts and, like A. terreus, is less susceptible to polyene antifungal drugs.12,30,42
A. niger (see Fig. 259-4A and B) is found in soil, on plants, and even in food and condiments (e.g., pepper). Colonies are initially white but quickly become black with the production of the pigmented fruiting structures (see Fig. 259-4A). The organism grows rapidly with a pale yellow reverse. Conidial heads are biseriate and cover the entire vesicle. Conidia are brown to black and are very rough (4 to 5 µm) (see Fig. 259-4B), although the hyphae are hyaline.26 The species may produce oxalate crystals in clinical specimens.43 The role of A. niger in invasive infection is less well established, with its decreased pathogenicity perhaps due in part to the fact that its larger conidia do not readily reach deep into lung tissues. It is a common colonizing isolate and can cause superficial infection, such as otitis externa.31,44 This species complex also contains several related species, including A. tubingensis, which is one of the more common cryptic aspergilli identified, suggesting it may have a role in invasive infection.12,32,45
Other species of Aspergillus are less common in invasive infection but are increasingly reported, perhaps reflecting increased awareness as well as the increased availability of molecular techniques confirming their identity.3,12,20,31 Aspergillus nidulans (section Nidulantes) and A. tanneri (section Circumdati, species complex terreus), for example, have been reported as causes of infection in patients with chronic granulomatous disease and are species that may be resistant to amphotericin B.28,32,46 Aspergillus calidoustus is a species in the section Ustus, which grows at 37° C (formerly called A. ustus, a species that fails to grow at 37° C) and exhibits high minimal inhibitory concentrations to several classes of antifungal agents.47–49 Thus, even previously “nonpathogenic” Aspergillus species must be considered potentially clinically significant in an appropriate clinical setting and host.9,20
Epidemiology
Aspergillus is ubiquitous worldwide, found in soil, water, food, and air, and is particularly common in decaying vegetation.11 The inoculum for infection is not known, but hosts with normal pulmonary host defenses rarely develop disease despite exposure to the organism with normal daily living through airborne conidia, including foodstuffs such as pepper, and so on. Patients with altered host immunity, particularly those with reduced pulmonary host defenses (e.g., those who use corticosteroids) that inhibit the activity of pulmonary macrophages or those who are neutropenic, have increased susceptibility to the organism.50
Patients with prolonged and profound neutropenia are at high risk for invasive aspergillosis, but changing treatment patterns in chemotherapy and transplantation, along with the use of growth factors, have limited the numbers of persistently neutropenic patients.51,52 It is important to recognize that even among high-risk patients with hematologic malignancy there is substantial heterogeneity of risk.53,54 In patients with acute myelogenous leukemia, the incidence of invasive aspergillosis has been reported to range from as low as 1% to 2% to rates as high as 25% to 28% or more, with mean incidence in most series of 4% to 7%.52,55–57 Most cases of invasive aspergillosis occur in the period of neutropenia after primary induction chemotherapy or in patients with refractory or recurrence of malignancy, with the risk for aspergillosis during consolidation chemotherapy very low.53,54
In patients undergoing hematopoietic stem cell transplant (HSCT) or a marrow transplant, a recent increase in the incidence of invasive aspergillosis has been reported and the epidemiology of infection has changed (Table 259-2).52,58 In patients undergoing HSCT, the major periods of risk are bimodal, with peak incidence occurring less than 40 days but more than 100 days after transplant, with mean time to diagnosis more than 180 days in some series.51,52,59–61 In the Transplant-Associated Infection Surveillance Network (TRANSNET) study the median time to onset of invasive aspergillosis was 99 days, with 22% and 53% of 335 invasive aspergillosis cases occurring 1 and 4 months after transplant, respectively.52 One of the factors in the changing epidemiology in this patient population is the use of nonmyeloablative transplant procedures, which has shifted the major risk factor in these patients from neutropenia to that of the use of high doses of corticosteroids for the treatment of acute or chronic graft-versus-host disease.51 Only 31% of the HSCT recipients with invasive aspergillosis reported by Wald and colleagues were neutropenic. Acute or chronic graft-versus-host disease may occur late after HSCT and increases the risk for Aspergillus infection.51,52,59
Other immunosuppressed patients are also at risk for invasive aspergillosis, including patients undergoing organ transplantation, particularly lung transplantation (see Table 259-2).62,63 The increased risk in lung transplantation is because the transplanted organ is constantly exposed to the environment, ciliary clearance is reduced, and many of these patients are colonized with Aspergillus in either the native or transplanted lung.64 In most organ transplant recipients the diagnosis of invasive aspergillosis is a late occurrence: the mean time to diagnosis is typically more than 180 days and may occur a year or more after the organ transplant, although the median onset in liver transplants appears shorter (~100 days).63,65 Other patients at higher risk for invasive aspergillosis include patients receiving corticosteroids or biologic agents, including the tumor necrosis factor-α (TNF-α) antagonists such as infliximab, etanercept, adalimumab, and others.66 Invasive aspergillosis occurs in other immunosuppressed patients, including patients with pulmonary diseases, acquired immunodeficiency syndrome (AIDS), chronic granulomatous disease and other hereditary immunodeficiency syndromes, and others.3,50 Invasive aspergillosis can occur in critically ill patients in intensive care units independent of other risk factors, suggesting additional risk factors for this disease, although the overall impact of invasive aspergillosis in that population is unclear.67,68
Outbreaks of invasive aspergillosis have occurred in patients exposed to aerosols of Aspergillus conidia in association with construction and other environmental risks.69–72 In severely immunosuppressed patients, aspergillosis may occur from other exposures as well, perhaps including aerosols of contaminated water.34,73 Aspergillosis may also occur as endogenous reactivation from prior infection or colonization, so even when it occurs in a hospital setting it may not be possible to prevent all cases by reducing environmental exposures.74 With the prolonged period of risk, these infections become very difficult to prevent with protective environments because much of their health care will occur in a non–hospital-based setting.74
Pathogenicity and Host Defenses
The usual route of infection for invasive aspergillosis is through inhalation of Aspergillus conidia into the lungs or paranasal sinuses. Although less common, invasive infection may also follow local tissue invasion such as through surgical wounds or contaminated intravenous catheters or armboards, leading to cutaneous infection.75
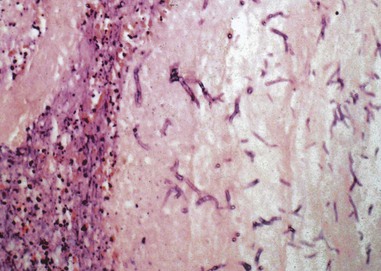
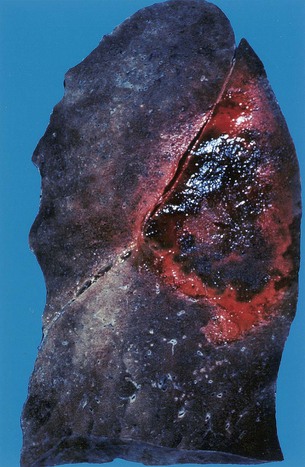
In the absence of effective host defenses after pulmonary exposure, the inhaled small resting conidia enlarge and germinate, resulting in transformation into hyphae with subsequent vascular invasion and eventual disseminated infection.76 The incubation period for conidial germination in pulmonary tissue is variable, estimated to range from 2 days to months, and may even vary by species.39 The growth rate at 37° C may be one determinant of the rate of disease progression and possible pathogenicity of the organism. Hydrocortisone significantly increases the growth rates of Aspergillus, further enhancing the role of corticosteroids as a risk factor for invasive disease.77 The process of hyphal growth and tissue invasion results in a hallmark feature of invasive aspergillosis—vascular invasion (Fig. 259-5) and pulmonary infarction (Fig. 259-6)—which are classic features of invasive pulmonary aspergillosis owing to the angioinvasive nature of the organism.
Although infection in apparently normal hosts can occur, invasive aspergillosis is uncommon in immunocompetent hosts.3 Normal pulmonary defense mechanisms are usually able to contain the organism in a host with intact pulmonary defenses.76 The first line of defense against Aspergillus is ciliary clearance of the organism from the airways and limited access to the alveoli due to conidial size. Smaller conidial size may be one reason for the increased pathogenicity of A. fumigatus as compared with other species of Aspergillus.78 After conidia reach the alveoli, the major line of defense becomes the pulmonary macrophages, which are capable of ingesting and killing Aspergillus conidia.79 After the cells germinate, polymorphonuclear leukocytes act to extracellularly kill both swollen conidia and hyphae.80 Efficacy of host defenses against the organism may be enhanced by opsonization of conidia with complement or other molecules such as mannose-binding protein and surfactant proteins.81 A. fumigatus produces a complement inhibitor, which may increase its pathogenicity.82 Antibodies against Aspergillus are common because of the ubiquitous nature of the organism, although they are not protective. They are also not useful in the diagnosis of infection in high-risk patients, owing to the lack of consistent seroconversion after exposure or infection.83
Many Aspergillus species produce toxins, including aflatoxins, ochratoxin A, fumagillin, and gliotoxin—the last of which may reduce macrophage and neutrophil function; however, the role of these toxins as major virulence factors is not established.84 Aspergillus species possess other potential virulence factors, including production of proteases, phospholipases, and metabolites, although strains deficient in those genes are still capable of producing experimental infection.85
T-helper (Th) cytokines have vital roles in innate and adaptive defense against Aspergillus. Studies in experimental aspergillosis have shown that a Th1 response is associated with a favorable response.86 Pathogen recognition receptors including Toll-like receptors (TLRs) and dectin-1 also mediate defense against A. fumigatus.76,87,88 Recognition of Aspergillus motifs by TLR2 and dectin-1 results in activation of intracellular pathways leading to proinflammatory cytokine production.89,90 These events are needed for an effective initial antifungal defense and bridge the gap between the innate and acquired immunity.91 Polymorphisms in host genes mediating innate immune defenses influence susceptibility to invasive aspergillosis. TLR4 haplotypes and polymorphisms in plasminogen gene alleles have both been shown to be associated with increased risk for invasive aspergillosis in HSCT patients.92,93
In contrast to the deficient host responses that lead to invasive Aspergillus infections, the pathogenesis of ABPA and allergic fungal sinusitis relates to aberrant inflammatory host responses to the organism.94 The immune response to Aspergillus antigens in ABPA as in both asthmatic patients and those with cystic fibrosis is a Th2 response.78 ABPA begins with an allergic inflammatory response that follows after Aspergillus conidia are inhaled into the bronchi, where they germinate and form hyphae.94 These mycelial cells release allergens that are processed by antigen-presenting cells bearing human leukocyte antigen (HLA)-DR2 or HLA-DR5 and presented to T cells within the bronchoalveolar lymphoid tissue. The T-cell response to these allergens favors a Th2 response, with release of cytokines interleukin (IL)-4, IL-5, and IL-13.78 The inflammatory response in the bronchial submucosa leads to excessive mucin production, extravasation of eosinophils into the bronchial mucin, intermittent bronchial obstruction with atelectasis, and, over time, bronchiectasis in some patients. Allergic fungal sinusitis is also characterized by submucosal inflammation and eosinophil-rich mucin in the sinus cavity.
Aspergilloma, called fungus ball of the lung, is a mass of hyphae in a preexisting cavity. Aspergillus causes a brisk immunoglobulin (Ig)G antibody response to the organism even though invasion of the cavity wall is rarely observed. Increased risk for chronic forms of pulmonary aspergillosis and noninvasive forms of pulmonary aspergillosis have been linked to subtle immune defects, including polymorphisms in mannose-binding protein and TLR polymorphisms.95,96
Clinical Manifestations
The spectrum of clinical syndromes associated with aspergillosis is diverse, ranging from allergic responses, asymptomatic colonization, superficial infection, and acute or subacute invasive disease. The clinical presentation reflects the underlying immune defects and risk factors associated with each patient group, with greater immune suppression correlating with increased risk for invasive disease.
Allergic Manifestations of Disease
Allergic Bronchopulmonary Aspergillosis
ABPA is a long-term allergic response to Aspergillus that is characterized by mucoid impaction of the bronchi, eosinophilic pneumonia, and transient pulmonary infiltrates due to atelectasis. Central bronchiectasis occurs in some patients after several years of disease.94 The incidence of ABPA is estimated to range from 1% to 2% in patients with persistent asthma and is approximately 7% (range, 2% to 15%) in patients with cystic fibrosis.78,94,97 Specific criteria are used to establish the diagnosis of ABPA because no single finding except for central bronchiectasis in a patient with asthma is diagnostic for the condition. These classic criteria include (1) asthma, (2) central bronchiectasis on chest computed tomography (CT), (3) immediate skin test reactivity to Aspergillus species (or A. fumigatus), (4) total serum IgE concentration greater than 417 IU/mL (1000 ng/mL), (5) elevated serum IgE and/or IgG antibody to A. fumigatus, (6) fleeting infiltrates on chest radiography, (7) serum precipitating antibodies to A. fumigatus, and (8) peripheral blood eosinophilia.94,97 It has been suggested that the first five are the minimal essential criteria for ABPA in patients with asthma, with the presence of precipitating antibodies further supporting the diagnosis and total IgE levels correlating with exacerbation of disease.94 Other clinical features may be present that can be used to support the diagnosis, including positive sputum cultures for Aspergillus or smears with hyphae consistent with Aspergillus, brown mucus plugs with degenerated eosinophils (Charcot-Leyden crystals) in sputa, and chest radiographic findings suggesting bronchial inflammation.98 These latter chest radiographic findings include the “ring sign,” indicating bronchial thickening without mucus plugs, and “parallel lines” or “tram tracks” suggesting bronchiectasis, which contrasts to tapering seen in the normal bronchus.97
The diagnosis of ABPA in cystic fibrosis may be particularly difficult because many of the diagnostic criteria overlap with common manifestations of cystic fibrosis (Table 259-3).78 In these patients, eosinophilia is not a useful diagnostic tool because the patients may have elevated peripheral blood eosinophils from other causes.
TABLE 259-3
Criteria for Diagnosis and Management of Allergic Bronchopulmonary Aspergillosis (ABPA) in Patients with Cystic Fibrosis
| DIAGNOSTIC CRITERIA | COMMENT |
| Clinical deterioration (increased cough, wheezing, exercise intolerance, exercise-induced asthma, increased sputum, decrease in pulmonary function) | Clinical signs not attributed to another cause |
| Immediate cutaneous reactivity to Aspergillus or presence of IgE to A. fumigatus | Pinprick skin test wheal of >3 mm with surrounding erythema while not receiving antihistamines |
| Total serum IgE concentration >500 IU/mL (>1200 ng/mL) | If ABPA is suspected and level is 200-500, repeat in 1-3 mo; if taking corticosteroids, repeat after their discontinuation. |
| One of the following: (1) precipitins (or IgG) to A. fumigatus or (2) new or recent abnormalities on computed tomography (bronchiectasis) or chest radiography (mucus plugging/infiltrates) | Failure of infiltrates or abnormalities to clear after antibiotic therapy and standard physiotherapy |
| Recommendations for Screening for ABPA | |
| Maintain clinical suspicion for diagnosis. | Especially in patients >6 yr |
| Determine total serum IgE annually. | If total IgE >500 IU/mL, consider skin test or measure IgE to Aspergillus. |
| If total IgE 200-500 IU/mL, repeat if clinical suspicion is high. | Consider retesting with disease exacerbation and perform other diagnostic tests. |
| Therapy for Exacerbations of ABPA | |
| Corticosteroids: 0.5-2 mg/kg/day oral prednisone equivalent (maximum 60 mg/day) for 1-2 wk, tapered over 2-3 mo | Recommended for disease exacerbation in all patients except those with corticosteroid toxicity |
| Antifungal therapy (itraconazole—or other azole with Aspergillus activity—voriconazole, posaconazole) | Slow corticosteroid response or toxicity; itraconazole 5 mg/kg/day up to 400 mg/kg/day102—levels necessary (voriconazole or posaconazole may be effective when itraconazole fails but not extensively studied)103; monitor liver function tests; duration 3-6 mo |
| Adjunctive therapy: inhaled corticosteroids; bronchodilators; environmental manipulation | No evidence for efficacy in ABPA but may be useful in asthma; reasonable to search for source of extensive mold exposure |
ABPA typically progresses through a series of remissions and exacerbations but can eventually lead to pulmonary fibrosis and chronic pulmonary aspergillosis, which is associated with a poor long-term prognosis.97–99 Management of ABPA is directed at reducing acute asthmatic symptoms and avoiding end-stage fibrosis. Corticosteroid therapy is commonly used for treating exacerbations, although few randomized trials have been conducted for their use.100 Guidelines suggest that worsening diagnostic or clinical parameters may warrant a trial of corticosteroid therapy.1 The role for antifungal therapy was evaluated with a randomized double-blind, placebo-controlled trial that showed itraconazole at 200 mg/day for 16 weeks significantly reduced daily corticosteroid use, reduced levels of IgE, and improved exercise tolerance and pulmonary function.101 In patients with severe asthma and fungal sensitization, itraconazole therapy resulted in significant clinical improvement in approximately 60%.102 The efficacy of voriconazole and posaconazole have not been evaluated extensively in this setting but resulted in approximately 70% responses in ABPA or asthma in those who had previously failed therapy with itraconazole.103
Other Allergic Manifestations
Aspergillus is an occasional cause of allergic fungal sinusitis, although most cases in the United States are due to dark-walled molds.104 This entity occurs in patients with a history of chronic allergic rhinitis, often with hyperplastic nasal mucosa forming nasal polyps. A mass of inspissated mucus forms in sinus cavity with Aspergillus hyphae and Charcot-Leyden crystals. The sinus mucosa is hyperplastic but not invaded.105 Management is directed at aerating the sinus and ensuring that tissue invasion is not present. The benefit of treating with either intranasal corticosteroids or systemic antifungal agents has not been shown.1
Saprophytic Colonization and Superficial Aspergillosis
Fungus Balls due to Aspergillus
A pulmonary fungus ball due to Aspergillus—or aspergilloma—is a solid mass of hyphae growing in a previously existing pulmonary cavity. Typically Aspergillus fungus balls of the lung develop in preexisting cavities in the pulmonary apex of patients with chronic lung disease such as bullous emphysema, sarcoidosis, tuberculosis, histoplasmosis, congenital cyst, bacterial lung abscess, or, very rarely, in a pulmonary bleb from Pneumocystis pneumonia in patients with AIDS.106 On a chest radiograph, a pulmonary aspergilloma appears as a solid round mass in a cavity. The detection of Aspergillus in sputum cultures or the detection of high titers of Aspergillus antibodies is further evidence that the radiographic findings are consistent with a diagnosis of fungus ball due to Aspergillus, so that a biopsy is not usually necessary except to diagnose the underlying lung disease.107
In many patients the fungus ball due to Aspergillus remains asymptomatic, but in a significant number of patients hemoptysis occurs and can be fatal.107 Surgical resection is considered the definitive therapy, but the dense pleural adhesions adjacent to the fungus ball and the poor pulmonary reserve of most patients make surgery hazardous. Contamination of the pleural space with Aspergillus and the common complication of bronchopleural fistula in the postoperative period can lead to chronic Aspergillus empyema. Dense adhesions make pleural drainage difficult, often requiring pleural stripping and thus further compromising lung function.107
Aspergillus can also be associated with fungus balls of the sinuses without tissue invasion.104 The maxillary sinus is the most common site for a sinus aspergilloma to occur.108 Clinical presentation is similar to that for any chronic sinusitis. Computed tomography of the sinus can be used to confirm the fungus ball, along with cultures of Aspergillus, usually A. flavus or A. fumigatus. Management is usually directed at surgical removal and a generous maxillary antrostomy for sinus drainage, along with confirmation that invasive disease has not occurred.
Denning and colleagues have described a spectrum of chronic pulmonary aspergillosis ranging from chronic cavitary pulmonary aspergillosis, characterized by the formation and expansion of multiple cavities and the presence of fungal balls, to chronic fibrosing or necrotizing aspergillosis, in which slowly progressive infection occurs, usually in a single thin-walled cavity with demonstration of hyphae invading tissue.109 They estimate the global burden of chronic pulmonary aspergillosis after tuberculosis to be substantial, which is important because of the potential response to antifungal agents in some patients.110 In all of these conditions, the diagnosis is suggested by radiologic and clinical features and the role of therapy remains speculative, although it appears that long-term antifungal therapy may be beneficial in a subset of patients.1,109
Other Superficial or Colonizing Syndromes of Aspergillosis
Otomycosis is a condition of superficial colonization typically due to A. niger.1 The clinical features include findings similar to other causes of external otitis, with the external auditory canal potentially revealing mold growing on cerumen and desquamated epithelial debris. A. fumigatus looks greenish, and A. niger forms a black tuft. With treatment, it is important to focus on the underlying chronic otitis externa rather than the fungus. In immunocompromised patients, invasive otitis externa resembles that due to Pseudomonas aeruginosa clinically and may respond to antifungal therapy.44,111
Onychomycosis due to Aspergillus is another superficial condition that, although rare, can become chronic and respond poorly to antifungal agents.112 Antifungal agents in the setting of nail infection may have a spectrum of activity limited to yeasts; thus, a nail culture can be useful in patients with nonresponsive disease to establish a specific fungal etiology so that appropriate therapy can be initiated.
Aspergillus is an occasional etiology of keratitis, particularly after trauma or corneal surgery (see Chapter 115).113,114 The diagnosis can be established with smears demonstrating hyphae, which may be indistinguishable from other molds such as Fusarium that also cause keratitis, but culture results are usually positive to confirm the diagnosis. Therapy consists of topical antifungal agents, usually topical amphotericin B, natamycin (pimaricin), or voriconazole, although studies demonstrating efficacy of antifungal therapy are limited.1,113,114 Amphotericin B has been injected intracamerally when corneal penetration has occurred.115
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree