Fred Y. Aoki
Antiviral Drugs for Influenza and Other Respiratory Virus Infections
In this chapter, antiviral agents against influenza viruses and certain other respiratory viruses such as parainfluenza and respiratory syncytial virus are reviewed (Table 44-1). The antiviral agents are presented in alphabetical order and include licensed (approved) as well as investigational agents. Agents that have been investigated in rhinovirus infections but have been utilized primarily in non–respiratory tract infections, such as interferons and pleconaril, are discussed in Chapter 47.
TABLE 44-1
Antiviral Agents of Established Therapeutic Effectiveness for Respiratory Virus Infection
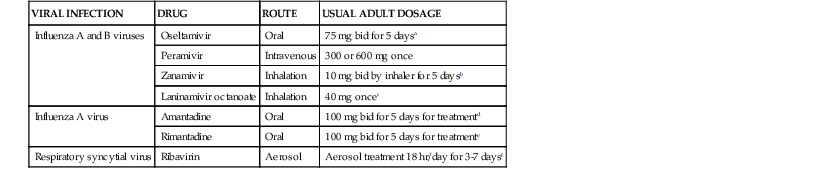
| VIRAL INFECTION | DRUG | ROUTE | USUAL ADULT DOSAGE |
| Influenza A and B viruses | Oseltamivir | Oral | 75 mg bid for 5 daysa |
| Peramivir | Intravenous | 300 or 600 mg once | |
| Zanamivir | Inhalation | 10 mg bid by inhaler for 5 daysb | |
| Laninamivir octanoate | Inhalation | 40 mg oncec | |
| Influenza A virus | Amantadine | Oral | 100 mg bid for 5 days for treatmentd |
| Rimantadine | Oral | 100 mg bid for 5 days for treatmente | |
| Respiratory syncytial virus | Ribavirin | Aerosol | Aerosol treatment 18 hr/day for 3-7 daysf |
a Pediatric dosages: For infants 2 wk to <1 yr of age dose is 3 mg/kg twice daily. For children ≥1 yr of age, doses are weight adjusted: 30 mg bid for <15 kg, 45 mg bid for 16-23 kg, 60 mg bid for 24-40 kg, and 75 mg bid for >40 kg. Prophylactic dosage is given once daily (one half of total daily treatment dosage). Not FDA approved currently for prophylaxis in children <1 yr old or treatment in children <2 wk old.
b FDA approved at same dosage for treatment of children ≥7 yr of age. Prophylactic dosage is 10 mg inhaled once daily for adults and children ≥5 yr of age.
c Adult dose and for children ≥10 yr of age. Pediatric dose: 20 mg once for children <10 yr of age.
d Maximum recommended dosage for older adults (≥65 yr) is 100 mg/day. Recommended pediatric dosage is 5 mg/kg/day up to a maximum of 150 mg/day in divided doses. For prophylaxis, the same daily dosage should be given for period at risk.
e Pediatric dosage is 5 mg/kg up to a maximum of 150 mg/day in divided doses. Not approved by FDA for treatment in children <13 yr of age. For prophylaxis, same daily dosage should be given for period at risk.
f Reservoir concentration of 20 mg/mL. Special aerosol-generating device (available from manufacturer) and expert respiratory therapy monitoring for administration are required. Higher reservoir concentration (60 mg/mL) given for 2 hr tid is an alternative.
Note: Please consult text and manufacturer’s product prescribing information for dosage adjustments in renal or hepatic insufficiency and in other circumstances.
Amantadine and Rimantadine
Spectrum
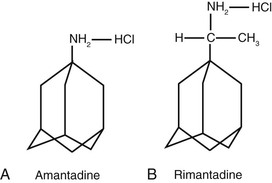
Amantadine (1-adamantanamine hydrochloride; Symmetrel) and rimantadine (α-methyl-1-adamantane methylamine hydrochloride; Flumadine) are symmetrical tricyclic amines (Fig. 44-1A and B) that specifically inhibit the replication of influenza A viruses at low concentrations (<1 µg/mL). Influenza B and C viruses are resistant.1 In the past, epidemic human and avian strains of influenza viruses have generally been susceptible to amantadine.2 However, since 2008-2009, isolates of influenza A/H1N1 and H3N2, highly pathogenic avian H5N1, and A (H1N1)pdm09 are resistant to amantadine and rimantadine (see later discussion).3 By plaque assay, inhibitory concentrations of the drugs range from 0.1 to 0.4 µg/mL or less for sensitive human influenza A viruses. Rimantadine is 4 to 10 times more active than amantadine in some assay systems. Both drugs are inhibitory for virus containing the M protein from the 1918 pandemic strain.4
Higher concentrations (10 to 50 µg/mL) inhibit other enveloped viruses in vitro, including parainfluenza, influenza B, rubella, dengue, several arenaviruses (Junin, Lassa, Pichinde), rabies, and African swine fever virus, but these concentrations are not achievable clinically and can be cytotoxic in vitro.5 Rimantadine has pH-dependent trypanocidal activity at concentrations of approximately 1 µg/mL6; amantadine at the same concentration in combination with doxycycline inhibits Coxiella burnetii.7 Amantadine may transiently inhibit hepatitis C virus (HCV) replication in humans.8
These agents have prophylactic and therapeutic activity in experimental influenza A virus infection of animals after oral or parenteral dosing. Combinations of M2 inhibitors and neuraminidase inhibitors and ribavirin show enhanced antiviral and therapeutic effects in vitro or in animal models of influenza.9–12
Mechanism of Action
Amantadine and rimantadine share two concentration-dependent mechanisms of anti-influenza action. Low concentrations inhibit the ion channel function of the M2 protein of influenza A viruses, which affects two different stages in virus replication.13–15 The primary effect involves inhibition of viral uncoating or disassembly of the virion during endocytosis. For subtype H5 and H7 viruses, a late effect on hemagglutinin maturation and viral assembly is presumably mediated through altered pH regulation of the trans-Golgi network. Amantadine and rimantadine block proton permeation and prevent M2-mediated changes in pH. This action probably accounts for inhibition of the acid-mediated dissociation of the matrix protein from the ribonucleoprotein complex within endosomes early in replication and potentiation of acidic pH–induced alterations in the hemagglutinin during its transport late in infection.
Amantadine and rimantadine are also concentrated in the lysosomal fraction of mammalian cells. Drug-mediated increases in lysosomal pH may inhibit virus-induced membrane fusion events and account for the broader antiviral spectrum at higher concentrations. In contrast, the selective anti–influenza A virus effects are quickly lost after removal of the drug from the surrounding medium, which suggests that drug must be present in extracellular fluid early in the replicative cycle.
Amantadine inhibits the ion channel activity of expressed HCV p7 protein at low concentrations,16 an effect that might account for its reported anti-HCV effects in vivo. Neither agent inhibits HCV enzyme functions or internal ribosome entry in biochemical assays.17
Resistance
Amantadine-resistant virus is readily selected by virus passage in the presence of drug. Resistance with more than 100-fold increases in inhibitory concentrations has been associated with single amino-acid substitutions at critical sites (positions 26, 27, 30, 31, 34) in the trans-membrane region of the M2 protein.13 Amantadine and rimantadine share cross-resistance. In avian models, resistant viruses are virulent, genetically stable, and able to compete with wild-type virus so that transmission of drug-resistant virus may occur after cessation of drug use.
Before 2003, a small percentage of untreated patients (<1%) had infection with resistant influenza A virus.18 Approximately 30% of drug-treated ambulatory children and adults and 80% of hospitalized children or immunocompromised patients shed resistant virus.19–21 Immunocompetent individuals shedding resistant virus resolve their illness promptly,22 whereas immunocompromised hosts may experience prolonged illness associated with persistent virus shedding.20 Transmission of M2 inhibitor–resistant virus, associated with failure of drug prophylaxis, occurs in household contacts of treated index cases23 and in nursing home residents.24 Resistant variants can cause typical influenza illness. It is prudent to avoid contact between treated patients and susceptible high-risk contacts and to avoid use of treatment (specifically of young children) and postexposure prophylaxis in the same household.
Globally, up to 2003, epidemic influenza A H1N1 and H3N2 strains were M2 inhibitor sensitive. Since 2003, the prevalence of amantadine resistance has increased progressively, although rates vary by virus type and geography.25,26 Among H3N2 isolates, amantadine resistance increased from 12% worldwide in 200325 to 91% by 2005 and greater than 95% in 2008-2009.26 In the United States prior to March 2009, nearly all of the A/H1N1 isolates tested were sensitive to the adamantanes and, subsequently, virtually all A/H1N1 isolates have been resistant up to the present, including the A (H1N1)pdm09 virus.27 Among nonpandemic H1N1 isolates, the prevalence of amantadine resistance was 4% in 2004-2005 worldwide and 16% in isolates from 2005-2006, with rates ranging from 2% in South Korea to 72% in China.26,28,29 The reason for the emergence and global spread of amantadine-resistant strains is unclear. Widespread inappropriate use of amantadine30 and acquisition of undefined advantageous mutations combined with lack of fitness impairment may have been contributing factors. Ribavirin and the neuraminidase inhibitors zanamivir and oseltamivir carboxylate are active in vitro against M2 inhibitor–resistant strains.
The triple combination of amantadine, oseltamivir, and ribavirin impedes the selection of drug-resistant influenza A virus in vitro at clinically achievable concentrations31 compared with double combinations and the agents used singly in vitro.32 The same combination of drugs was also synergistic in vitro in inhibiting the growth of both amantadine- and oseltamivir-resistant influenza A virus strains at concentrations that had no activity as single agents.32
Pharmacokinetics
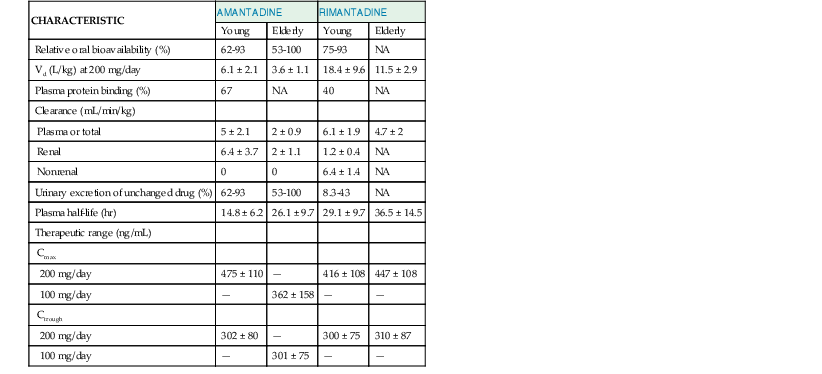
The clinical pharmacokinetic characteristics of amantadine and rimantadine are shown in Table 44-2.
Amantadine
Amantadine is well absorbed after oral administration of capsule, tablet, or syrup forms.5 Steady-state peak plasma concentrations average 0.5 to 0.8 µg/mL with a 100-mg twice-daily regimen in healthy young adults. Older adults require only one half of the weight-adjusted dosage needed for young adults to achieve equivalent trough plasma levels of 0.3 µg/mL. Plasma protein binding of amantadine is about 67%, and amantadine’s volume of distribution (Vd) is large (4 to 5 L/kg). Nasal secretion and salivary levels of amantadine approximate those found in the serum. Cerebrospinal fluid levels are 52% to 96% of those in plasma, and amantadine is excreted in breast milk.
Amantadine is eliminated largely unchanged in the urine by glomerular filtration and probably by tubular secretion by a bicarbonate-dependent organic cation transporter.33 The plasma elimination half-life ( ) is 12 to 18 hours, ranges widely, and correlates with the creatinine clearance (CrCl). Because of age-related declines in renal function,
) is 12 to 18 hours, ranges widely, and correlates with the creatinine clearance (CrCl). Because of age-related declines in renal function,  increases twofold in older adults and even more in patients with impaired renal function. Dosage reductions are required in renal insufficiency (Table 44-3). Amantadine is inefficiently cleared in patients receiving hemodialysis or continuous ambulatory peritoneal dialysis, and additional doses are not required. Monitoring of plasma concentrations in such patients is desirable but impractical.
increases twofold in older adults and even more in patients with impaired renal function. Dosage reductions are required in renal insufficiency (Table 44-3). Amantadine is inefficiently cleared in patients receiving hemodialysis or continuous ambulatory peritoneal dialysis, and additional doses are not required. Monitoring of plasma concentrations in such patients is desirable but impractical.
TABLE 44-3
Amantadine Dosage Regimens for Prophylaxis and Alterations in Renal Failure
| CONDITION | SUGGESTED DOSAGE |
| No Renal Insufficiency | |
| Children 1-9 yr | 5 mg/kg/day in two divided doses, ≤150 mg/day |
| Ages 10-64 yr | 100 mg twice daily |
| Ages ≥65 yr | 100 mg once daily* |
| Creatinine Clearance (mL/min/1.73 m2)† | |
| ≥80 | 100 mg (1.4 mg/kg) twice daily |
| 79-35 | 100 mg once daily |
| 34-25 | 100 mg every 2 days |
| 24-15 | 100 mg every 3 days |
| <15 | 100 mg every 7 days |
| Older Adults and Creatinine Clearance (mL/min/1.73 m2)‡ | |
| ≥80 | 100 mg daily |
| 60-79 | 100 mg and 50 mg on alternate days |
| 40-59 | 100 mg every 2 days |
| 30-39 | 100 mg twice weekly |
| 20-29 | 50 mg twice weekly |
| 10-19 | 100 mg and 50 mg on alternate weeks |
* Use weight-adjusted dosing for smaller patients (<50 kg). Dosages of 1.4 mg/kg/day have been suggested.5
† Based on adult dosage of 200 mg/day. Proportionate reductions should be made for older adults receiving lower dosages and for children.
‡ This dosing schedule for older adults with renal insufficiency is taken from the Canadian guidelines and has been found to be reasonably well tolerated.44
Modified from Wu MJ, Ing TS, Soung LS, et al. Amantadine hydrochloride pharmacokinetics in patients with impaired renal function. Clin Nephrol. 1982;17:19-23.
Amantadine pharmacokinetics remained unaffected by concurrent administration of oseltamivir and ribavirin in healthy adult volunteers or stable immunocompromised patients.34
Rimantadine
Rimantadine is well but slowly absorbed, with the time to peak plasma concentration averaging 2 to 6 hours. Absorption does not seem to be decreased by food. With multiple doses of 100 mg twice daily, the steady-state peak and trough plasma concentrations in healthy adults are 0.4 to 0.5 µg/mL and 0.2 to 0.4 µg/mL. In infants receiving dosages of 3 mg/kg each day, peak serum levels range from 0.1 to 0.6 µg/mL. No important age-related changes in pharmacokinetics have been found in healthy older adults or in children. However, steady-state plasma concentrations in older nursing home residents receiving 100 mg twice daily average more than twofold higher (mean, 1.2 µg/mL) than concentrations observed in healthy adults, which indicates the need for lower dosages in these patients. Plasma protein binding is about 40%. Rimantadine has a very large Vd (~12 L/kg), and concentrations in nasal mucus average 50% higher than those in plasma.
In contrast to amantadine, rimantadine undergoes extensive metabolism by hydroxylation, conjugation, and glucuronidation before renal excretion.5 The plasma  of rimantadine averages 24 to 36 hours. No clinically important differences in pharmacokinetics are found in patients with chronic liver disease without significant hepatocellular dysfunction. In hemodialysis patients with severe renal failure, the clearance of rimantadine is decreased by 40% and the
of rimantadine averages 24 to 36 hours. No clinically important differences in pharmacokinetics are found in patients with chronic liver disease without significant hepatocellular dysfunction. In hemodialysis patients with severe renal failure, the clearance of rimantadine is decreased by 40% and the  is about 55% longer. Reducing dosages by one half (e.g., to 100 mg/day) is recommended for marked hepatic or renal insufficiency (CrCl <10 mL/min). Hemodialysis removes only a small amount of rimantadine, so supplemental doses are not required.
is about 55% longer. Reducing dosages by one half (e.g., to 100 mg/day) is recommended for marked hepatic or renal insufficiency (CrCl <10 mL/min). Hemodialysis removes only a small amount of rimantadine, so supplemental doses are not required.
Interactions
The risks for central nervous system (CNS) adverse effects with amantadine and possibly with rimantadine are increased by concomitant ingestion of antihistamines, antidepressants, anticholinergic drugs, and other drugs affecting CNS function. Concurrent use of trimethoprim-sulfamethoxazole or triamterene-hydrochlorothiazide has been associated with CNS toxicity resulting from decreased renal clearance of amantadine. Cimetidine is associated with 15% to 20% increases, and aspirin or acetaminophen is associated with 10% decreases in plasma rimantadine concentrations, but such changes are unlikely to be significant. Neither adverse clinical nor adverse pharmacokinetic effects are observed when amantadine and oseltamivir are co-administered.35
Concurrent administration of recommended doses of amantadine, oseltamivir, and ribavirin for 10 days was well tolerated.34
Toxicity
Amantadine or rimantadine given in treatment courses of 5 days is generally well tolerated in young healthy adults.36 Longer periods of administration, such as 6 weeks for seasonal prophylaxis in young adults,36 and administration to fragile, elderly nursing home residents, such as octogenarians, for 10 days for outbreak control are associated with a significant frequency of adverse reactions and drug withdrawals.37
A case-control study demonstrated that in children younger than 12 months of age, amantadine and rimantadine were well tolerated, as was oseltamivir.38 No evidence of adverse maternal or neonatal outcomes were observed after antepartum influenza treatment with adamantane antiviral agents.39
The most common side effects related to amantadine ingestion are minor, dose-related gastrointestinal and CNS complaints, including nervousness, lightheadedness, difficulty concentrating, confusion, insomnia, and loss of appetite or nausea.40 Complaints typically develop within the first week of administration, often resolve despite continued ingestion, and are reversible on drug discontinuation. CNS side effects occur in 5% to 33% of amantadine recipients at dosages of 200 mg/day but are significantly less frequent with rimantadine. When used for influenza prophylaxis in ambulatory adults, dosages of 200 mg/day are associated with excess withdrawals in 6% to 11% of recipients because of drug side effects. Dosages of 100 mg/day are better tolerated and may be protective against influenza illness. Amantadine dosage reductions are required in older adults (100 mg/day), but 20% to 40% of nursing home residents experience significant adverse effects on this lower dosage despite some adjustment for renal insufficiency.41–43 Consequently, further dosage reductions based on CrCl are warranted in this population.44
In the setting of renal insufficiency or high dosages, serious neurotoxic reactions, including delirium, hostility, hallucinations, tremor, myoclonus, seizures, or coma; cardiac arrhythmias; and death can occur in association with elevated amantadine plasma concentrations (1 to 5 µg/mL).45 Neurotoxic reactions may be transiently reversed by physostigmine administration, and lidocaine has been used to treat ventricular arrhythmias. Long-term amantadine ingestion has been associated with livedo reticularis, peripheral edema, orthostatic hypotension, and, rarely, congestive heart failure, vision loss, or urinary retention. Peripheral edema and livedo reticularis may improve if treatment is switched from amantadine to rimantadine.46 Patients with preexisting seizure disorders have an increased frequency of major motor seizures during amantadine use, and dosage reductions are advised. Psychiatric side effects in patients with Parkinson’s disease and psychotic exacerbations in patients with schizophrenia may occur with addition of amantadine. Rash and leukopenia have been described rarely.
Rimantadine administration is associated with dose-related side effects similar to side effects observed with amantadine, although the risk for CNS side effects is lower with rimantadine at dosages of 200 mg/day or 300 mg/day in ambulatory adults.5 During prophylaxis, excess withdrawal rates are usually less than 5%. In older nursing home residents, dosages of 200 mg/day are associated with higher side effect rates, whereas dosages of 100 mg/day seem to be better tolerated.41,47 Rimantadine may uncommonly cause exacerbations of seizures in patients not receiving anticonvulsants and was associated with an unexplained excess mortality in one nursing home study.47
The clinical observations of dry mouth, pupillary dilation, toxic psychosis, and urinary retention in acute amantadine overdose suggest that anticholinergic activity is present in humans. Amantadine shows activity on the adrenergic nervous system by affecting accumulation, release, and reuptake of catecholamines in the CNS and in the peripheral nervous system. Malignant ventricular arrhythmia after amantadine overdose has been described in humans.
Amantadine and rimantadine lack mutagenicity in vitro; carcinogenicity studies have not been reported for either. Amantadine is teratogenic and embryotoxic in rats, and rimantadine may cause teratogenic effects in rabbits and maternal toxicity and embryotoxicity at high dosages in rodents. Both drugs are classified in pregnancy category C. Birth defects have been reported after amantadine exposure during pregnancy.48 The safety of neither amantadine nor rimantadine has been established in pregnancy. Because of excretion in breast milk, use is not recommended in nursing mothers.
Clinical Studies
Influenza A
Amantadine and rimantadine have been efficacious for the prevention and treatment of influenza A virus infections in young healthy adults.5,40,49 A systematic review of published studies in children and the elderly concluded that available data only demonstrate that amantadine has prophylactic efficacy and a modest therapeutic effect in children.50 In the elderly, no data were available to support a conclusion of prophylactic or therapeutic efficacy of either adamantane. The emergence of widespread and nearly complete amantadine resistance among influenza A/H3N2 isolates,26 as well as the amantadine resistance of the pandemic A (H1N1)pdm09 strains, precludes the empirical use of adamantanes for management of an untyped influenza A outbreak. Amantadine and rimantadine, both at a dosage of 200 mg/day in adults, are about 70% to 90% protective against clinical illness caused by various susceptible influenza A subtypes, including susceptible pandemic strains.51 Prophylaxis is effective in preventing nosocomial influenza and possibly in curtailing nosocomial outbreaks caused by such strains. Protection seems to be additive to that provided by vaccine.52
Rimantadine was less effective than zanamivir in reducing cases of influenza A illness in adults in a long-term care facility.53 The difference in protective efficacy was largely due to the emergence of rimantadine-resistant viruses that caused rimantadine prophylactic failure; no zanamivir-resistant viruses were isolated. Rimantadine administration to school-aged children (5 mg/kg/day) decreased the risk for influenza A illness in recipients and possibly in their family contacts. Postexposure prophylaxis with these drugs provided inconsistent protection to family contacts, however, in part, depending on whether ill index children were treated.19 Dosages of 100 mg/day seem to be protective against influenza A illness and are well tolerated in adults.54
Amantadine and rimantadine are also effective therapies for uncomplicated adamantane-susceptible influenza A illness in healthy adults,5,22 but it is uncertain whether treatment reduces the risk for complications in high-risk patients or is useful in patients with established pulmonary complications. Early treatment in ambulatory adults (200 mg/day for 5 days) reduces the duration of fever and systemic complaints by 1 to 2 days, decreases virus shedding, and shortens time to resumption of usual activities.22 In illness caused by H3N2-subtype influenza viruses, certain abnormalities of peripheral airways function, but not of airway hyperreactivity, resolve more quickly in amantadine-treated patients. Amantadine or rimantadine treatment in adults with leukemia or stem cell transplantation may reduce the risk for pneumonia,55 but more recent data suggest that in stem cell transplant recipients, early neuraminidase inhibitor therapy may be preferred to adamantanes, because it may prevent progression to pneumonia and decrease viral shedding, thereby possibly preventing both influenza-related death in index patients and nosocomial transmission to others.56 In children, rimantadine treatment is associated with lower symptom burden, fever, and viral titers during the first 2 days of treatment compared with acetaminophen administration, but rimantadine-treated children have more prolonged shedding of virus. Treatment generally does not seem to affect humoral immune responses to infection, but may blunt secretory antibody levels.57
Intermittent aerosol administration of amantadine or rimantadine seems to be therapeutically useful in uncomplicated influenza. No injectable formulation of either drug is available in the United States.
Other Viruses
Amantadine has been used in multiple trials for treatment of chronic hepatitis C with inconsistent evidence for increases in sustained viral response (SVR). In treatment-naïve patients, the addition of amantadine (200 mg daily in single or divided doses) to interferon58,59 or to interferon plus ribavirin60 may modestly increase biochemical responses and the likelihood of SVR. In re-treatment of interferon nonresponders, the combination of interferon plus amantadine is ineffective61 but the addition of amantadine to the combination of interferon plus ribavirin may be associated with SVR in 10% to 25%.62 Amantadine plus combined pegylated interferon and ribavirin may increase SVR modestly in treatment-experienced patients compared with pegylated interferon plus ribavirin.63 Reports of possible activity in bornavirus infections and associated neuropsychiatric symptoms require confirmation.
DAS181 (Fludase)
DAS181 is an investigational antiviral agent with activity against influenza A and B viruses and parainfluenza viruses types 1-3.64–69 It has a novel mechanism of action in that it is a sialidase from Actinomyces viscosus (Fig. 44-2) linked to a respiratory epithelium-anchoring domain.70 It cleaves the terminal sialic acid residues on the surface of human respiratory cells, thus reducing the binding of respiratory viruses, which use those as receptors. Desialylation is rapid and results in an antiviral effect, which lasts for at least 2 days.64 The effective concentration (EC) for 50% of all isolates (EC50) against influenza A and B viruses ranges from 0.04 to 0.9 µM.65 DAS181 is active against influenza viruses that are resistant to neuraminidase inhibitors.71 Low-level resistance to DAS181 can be induced, but resistant variants appear to be reduced in fitness.72
DAS181 is administered by oral inhalation and appears to be generally well tolerated. A phase II placebo-controlled study was recently conducted in 177 subjects with influenza A and B virus infections.73 DAS181 was administered either as a single 10-mg dose or as a daily 10-mg dose for 3 days. Compared with placebo recipients, DAS181 recipients had a statistically significant decrease in virus load determined by polymerase chain reaction (PCR) assay between days 1 and 3 and days 1 and 5. However, there were no differences in resolution of clinical illness among the groups. Administration of DAS181 appeared to be generally well tolerated, although transient elevations in alkaline phosphatase level were reported.73
DAS181 has also been utilized to treat parainfluenza virus type 3 infections in lung transplant and stem cell transplant patients.74,75 These case reports described clinical improvement, increased pulmonary function, and decreased virus loads. Additional clinical studies of DAS181 are being planned.
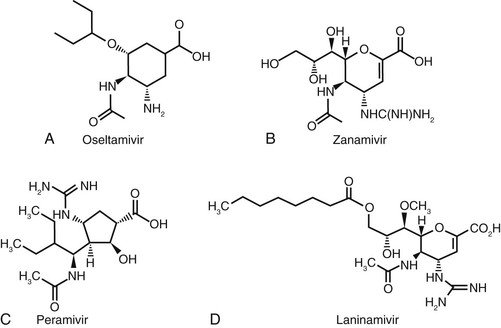
Laninamivir Octanoate
Laninamivir octanoate (Inavir) is an investigational drug except for its approval in Japan. It is the prodrug of laninamivir, an inhibitor of influenza A and B neuraminidases.76 Laninamivir is (2R,3R,4S)-3-acetamido-2-[CIR,2R-2,3-dihydroxy-1-methoxypropyl]-4-quanidino-3,4-dihydro-2-H-pyram-6-carboxylic acid (Fig. 44-3D). Laninamivir octanoate consists of an octanoic acid ester side chain attached at the C3 position of laninamivir. Laninamivir octanoate, like polymeric zanamivir conjugates, shares the pharmacokinetic characteristic of persisting for a prolonged period in the respiratory tract after administration intranasally or intratracheally in animals or by oral inhalation in humans. These observations have presaged therapeutic effects of a single dose in animals with experimentally induced influenza in patients as well.
Spectrum
Laninamivir octanoate exhibits little or no influenza virus neuraminidase inhibitory activity in vitro.77 However, its hydrolysis product is a potent inhibitor of neuraminidases of N1 to N9 influenza A viruses plus influenza B and their replication in cell culture at nanomolar concentrations.78 These include seasonal and pandemic influenza A/H1N1, highly pathogenic avian influenza (HPAI) H5N1 viruses, and clinical isolates of oseltamivir-resistant H1N1, H3N2, H5N1, and A (H1N1)pdm09. Median inhibitory concentrations in cell culture vary over a wide range and in general appear to be intermediate between those of oseltamivir carboxylate (lower) and zanamivir (higher), but the clinical importance of these differences is not yet known.
In preclinical studies, laninamivir octanoate reduced fever in ferrets, mortality in mice, and virus concentrations in lung in ferrets and mice and brain in mice after induced influenza with a variety of viruses: A/PR/8/34, HPAI H5N1, A (H1N1)pdm09, B/Malaysia/2506/2004, as well as oseltamivir-resistant A H1N1 and HPAI H5N1 clinical isolates possessing the H274Y mutation, as reviewed by Yamashita and associates.78 In these studies, laninamivir octanoate was administered as a single intranasal dose after intranasal inoculation of virus and was either as or more efficacious than multiple doses of oral oseltamivir or intranasal zanamivir. The results of these studies in animals with experimental influenza have been replicated in part in therapeutic trials of a single laninamivir octanoate dose in the clinic (see later).
Single doses of laninamivir octanoate are also efficacious prophylactically in mice. One dose prevents mortality and reduces virus concentration in lungs and brain when administered as much as 7 days before virus challenge.79
Mechanism of Action
See subsequent discussion of mechanism of action under “Oseltamivir.”
The basis for the prolonged persistence of laninamivir in the respiratory tract after intranasal or intratracheal administration of laninamivir octanoate in animals or oral inhalation in humans is not completely understood. In human volunteers, bronchoalveolar lavage samples obtained serially over 24 hours after oral inhalation of a single 40-mg dose of laninamivir octanoate reveal concentrations that exceed influenza virus neuraminidase inhibitory concentrations at all test times.80 In mice, intranasal administration of carbon-14 (14C)–labeled laninamivir octanoate demonstrates prolonged retention of laninamivir in lung tissues. Microautoradiography indicates that laninamivir octanoate is taken into airway epithelial cells, seemingly hydrolyzed to the antiviral molecule laninamivir by intracellular esterases, and then released slowly extracellularly, perhaps as a result of its hydrophobic poor membrane permeability.81 The cellular and molecular processes underlying these observations are not yet determined.
Resistance
No extensive studies have been reported on the emergence of laninamivir-resistant strains after laninamivir exposure in vitro or laninamivir octanoate treatment in animals or patients. However, in one study in mice infected with an A H1N1 virus, no viruses with reduced susceptibility to laninamivir were recovered.82
Pharmacokinetics
Epithelial lining fluid concentrations of laninamivir octanoate and laninamivir calculated from analysis of bronchoalveolar lavage washings after a single oral inhalation of 40 mg laninamivir octanoate were 102.4 and 8.6 µg/mL, respectively, at 4 hours in healthy adult volunteers.76 The disappearance half-times in bronchoalveolar lavage fluid were 41 and 141 to 241 hours, respectively. The plasma  values were 2.6 and 45.7 hours, respectively. Laninamivir concentrations in epithelial lining fluid exceeded the median inhibitory concentrations for influenza neuraminidases at all time points for 240 hours after dose inhalation. In other healthy adult volunteers, evaluation of the pharmacokinetics of laninamivir octanoate and laninamivir was done after oral inhalation of single doses from 5 to 120 mg.83 Laninamivir octanoate appeared rapidly in plasma with a Cmax at 0.5 to 1.0 hour compared with 4.0 hours for laninamivir. Plasma
values were 2.6 and 45.7 hours, respectively. Laninamivir concentrations in epithelial lining fluid exceeded the median inhibitory concentrations for influenza neuraminidases at all time points for 240 hours after dose inhalation. In other healthy adult volunteers, evaluation of the pharmacokinetics of laninamivir octanoate and laninamivir was done after oral inhalation of single doses from 5 to 120 mg.83 Laninamivir octanoate appeared rapidly in plasma with a Cmax at 0.5 to 1.0 hour compared with 4.0 hours for laninamivir. Plasma  values were 1.8 and 71.6 to 80.8 hours, respectively. The plasma area under the concentration-time curve (AUC) of laninamivir octanoate was linearly related to dose, while that of laninamivir increased disproproportionately. The mean cumulative excretion in urine over 144 hours was 2.3% to 3.6% and 10.7% to 14.6%, respectively.
values were 1.8 and 71.6 to 80.8 hours, respectively. The plasma area under the concentration-time curve (AUC) of laninamivir octanoate was linearly related to dose, while that of laninamivir increased disproproportionately. The mean cumulative excretion in urine over 144 hours was 2.3% to 3.6% and 10.7% to 14.6%, respectively.
After intravenous administration of 14C-laninamivir in rats, almost 90% of the radioactivity was recovered in urine.84 In human volunteers, the clearance of both laninamivir octanoate and laninamivir is linearly related to CrCl.85 In subjects with none, mild, moderate, or severe renal impairment given a single orally inhaled dose of 20 mg laninamivir octanoate, the renal clearance of laninamivir octanoate and laninamivir is directly related to CrCl, whereas  values are not. Geometric mean laninamivir octanoate clearance values declined from 26.0 mL/min in normal control subjects to 6.5 mL/min in patients with severe renal impairment. However,
values are not. Geometric mean laninamivir octanoate clearance values declined from 26.0 mL/min in normal control subjects to 6.5 mL/min in patients with severe renal impairment. However,  values were 2.3 to 3.5 hours and not different among the four groups. Laninamivir renal clearance declined from 65.0 to 12.7 mL/min across the four groups, whereas
values were 2.3 to 3.5 hours and not different among the four groups. Laninamivir renal clearance declined from 65.0 to 12.7 mL/min across the four groups, whereas  was not different among the groups, ranging from 53.2 to 57.0 hours. The likely explanation is that the elimination of both laninamivir octanoate and laninamivir reflect slow release of these compounds from tissues into plasma, rather than renal elimination, a pharmacokinetic concept called “flip-flop.”86 These pharmacokinetic data indicate that reduction of laninamivir octanoate doses may be appropriate for patients with renal impairment for pharmacokinetic reasons, but the lack of clear dose-related toxicity (see later) and the minimal absorption of orally inhaled drugs suggest that no dose adjustment will be needed.
was not different among the groups, ranging from 53.2 to 57.0 hours. The likely explanation is that the elimination of both laninamivir octanoate and laninamivir reflect slow release of these compounds from tissues into plasma, rather than renal elimination, a pharmacokinetic concept called “flip-flop.”86 These pharmacokinetic data indicate that reduction of laninamivir octanoate doses may be appropriate for patients with renal impairment for pharmacokinetic reasons, but the lack of clear dose-related toxicity (see later) and the minimal absorption of orally inhaled drugs suggest that no dose adjustment will be needed.
Toxicity
Like orally inhaled zanamivir, orally inhaled laninamivir octanoate powder is well tolerated. In a double-blind study in healthy adult volunteers, single doses from 5 to 120 mg or multiple doses of 20 or 40 mg twice daily for 5 days were as well tolerated as placebo.85
In clinical trials, patients with influenza were randomized to single laninamivir octanoate doses of 20 or 40 mg in adults or children 10 years old or older, 20 mg in children younger than 10 years old, or inhaled zanamivir as the control neuraminidase inhibitor treatments. Laninamivir octanoate inhaled once was as well tolerated as inhaled zanamivir 20 mg twice daily for 5 days.87 In a double-blind trial in children 9 years of age or younger with influenza, a single dose of inhaled laninamivir octanoate of 20 or 40 mg was as well tolerated as oseltamivir at 2 mg/kg body weight twice daily for 5 days.88 In a phase III double-blind trial in adults 20 years of age or older with influenza, a single dose of inhaled laninamivir octanoate of 20 or 40 mg was as well tolerated as oral oseltamivir at 75 mg twice daily for 5 days.89 Notwithstanding the lack of data from large, randomized, placebo-controlled, double-blind trials to establish the tolerability of laninamivir octanoate across the range of persons in healthy and high-risk groups, these published data on laninamivir octanoate tolerance plus those from studies of orally inhaled zanamivir collectively suggest that orally inhaled laninamivir octanoate will likely prove to be well tolerated and safe in the clinic.
Postmarketing studies of laninamivir octanoate in Japan concluded that the safety profile of laninamivir octanoate for abnormal behavior/delirium and syncope is similar to that of other neuraminidase inhibitors.90 In Japan, it is recommended in the product labeling that teenage patients inhaling laninamivir octanoate should remain under constant parental supervision for at least 2 days to monitor for behavioral changes to prevent associated self-injury. To avoid syncope, patients should inhale laninamivir octanoate in a relaxed sitting position. In another postmarketing survey for laninamivir octanoate tolerance, 50 patients of 3542 (1.4%) reported an adverse event.91 Commonly reported adverse events included psychiatric disorders (abnormal behavior), gastrointestinal symptoms, and nervous system disorders such as dizziness, with frequencies of 0.48%, 0.45%, and 0.17%, respectively. These usually appeared on the day of laninamivir octanoate treatment and resolved in 3 days. These adverse reactions and their frequency were considered comparable to those previously observed during clinical trials, and thus were believed to confirm no noticeable problem with safety.
Clinical Studies
Limited data from controlled trials are available on the efficacy of orally inhaled laninamivir octanoate for influenza treatment, although three randomized, controlled trials on the efficacy and tolerance of laninamivir octanoate and one observational study comparing it with other neuraminidase inhibitors have been reported. In these trials, laninamivir octanoate has been administered as an orally inhaled powder with a proprietary device that has two containers of 10-mg dry laninamivir octanoate powder. The manufacturer’s instructions recommend two inhalations from each 10-mg changer. For children, four inhalations are necessary, whereas eight inhalations from two devices are required for adults. Occasionally, young children do not inhale the medication completely owing to technical difficulty with the device.87
Of 87 pediatric patients with influenza of less than 48 hours in duration, 44 were randomized to treatment with a single inhaled dose of laninamivir octanoate (N = 55), 20 or 40 mg, according to age, or inhaled zanamivir, 10 mg twice daily for 5 days (N = 41).87 Median times to fever resolution were 36 hours in the laninamivir octanoate groups and 37 hours in the zanamivir-treated group. This relatively small study suggested that a single dose of inhaled laninamivir octanoate was as efficacious as the recommended 5-day treatment with zanamivir. In another study, 180 children 9 years or younger with influenza of less than 36 hours in duration were randomized to a single oral inhalation of 40 (N = 61) or 20 mg (N = 61) laninamivir octanoate or oseltamivir 2 mg/kg (N = 62) ingested twice daily for 5 days.88 Of the 180 children, 62% (112) were infected with influenza A H1N1 virus, of which all but 4 possessed the H274Y mutation, mediating oseltamivir resistance. Oseltamivir therapy was likely not to have been different from placebo. The median times to alleviation of influenza illness in children were significantly less (49.6 and 44.3 hours) in the 40- and 20-mg laninamivir groups, respectively, than in the oseltamivir-treated group (110.5 hours). Treatment effects on virus concentration and persistence in upper airway secretions were inconsistent, although on day 3, 10%, none, and 25% of subjects in the three groups, respectively, were still excreting virus. There were no clinical therapeutic or virologic differences among children infected with influenza A H3N2 or B viruses, but the numbers of cases were small.
In a double-blind, randomized noninferiority trial, 1003 young healthy adults with febrile influenza for no more than 36 hours were randomized to receive either 40 mg or 20 mg of laninamivir octanoate by oral inhalation once or oseltamivir, 75 mg twice daily orally, for 5 days.89 The primary end point was time to influenza illness alleviation. Unfortunately, as in the pediatric study of Sugaya and Ohashi,88 66% of the subjects were infected with oseltamivir-resistant influenza A H1N1 virus. The median times to resolution of illness in patients infected with this virus were 74.0, 85.8, and 77.8 hours, respectively, which were not different. Virus was detected by culture significantly less often at day 3 in the laninamivir octanoate 40-mg (28%) and 20-mg (32%) groups than in the oseltamivir group, which might be considered analogous to a placebo-treated cohort. Among individuals infected with influenza A H3N2 virus, median times to illness alleviation were not different between those treated with laninamivir octanoate 40 mg (73.5 hours) and oseltamivir (67.5 hours) but significantly longer in the group treated with laninamivir octanoate 20 mg (91.2 hours). There were no differences among the groups in H3N2 virus concentration in upper airway secretions or persistence. The 95% confidence intervals of the pooled analysis of all data were less than the prescribed noninferiority margin. It was concluded that a single inhalation of laninamivir octanoate is effective for treatment of seasonal influenza including that caused by oseltamivir-resistant virus in adults.
In an observational study, 211 children with febrile influenza of less than 48 hours due to influenza A H3N2 infection and 45 with A (H1N1)pdm09 infection were treated according to the recommendations of clinicians and the preference of patients or their guardians.92 Of the 256 children, 119 were treated with oseltamivir in weight-appropriate doses, zanamivir (124 cases), one dose of intravenous peramivir (4 children),79 or a single dose of orally inhaled laninamivir octanoate of 40 mg for children 10 years or older or 20 mg for those younger than 10 years (9 children). The primary end point was duration of fever from the first dose of neuraminidase inhibitor. There were no differences in the duration of fever among the oseltamivir, zanamivir, or laninamivir octanoate groups. The median time to resolution of fever in the peramivir group (17.0 hours) was significantly less than in the other three groups.
Available data suggest that a single inhaled dose of laninamivir octanoate is efficacious in children with influenza of less than 48 hours, but efficacy in other populations, especially those with high-risk conditions, remains to be evaluated, as does the impact on complications of influenza.
Oseltamivir
Spectrum
Oseltamivir phosphate (Tamiflu) is the ethyl ester prodrug of oseltamivir carboxylate, a sialic acid analogue (see Fig. 44-3A) that is a potent, specific inhibitor of the neuraminidases of influenza A and B viruses.93,94 The metabolite, oseltamivir carboxylate, is approximately 50-fold more potent than the phosphate prodrug.95 Oseltamivir carboxylate competitively and reversibly interacts with the active enzyme site to inhibit neuraminidase activity at low nanomolar concentrations.96 Inhibitory concentrations for neuraminidase inhibitors in cell culture have a broad range (≥1000-fold), depending on the assay method, and may not correlate with in vivo activity.97,98 Oseltamivir carboxylate is active against viruses containing all nine influenza A neuraminidase subtypes recognized in nature, including more recent pathogenic avian viruses (H5N1, H7N7, H9N2), reassortant virus containing neuraminidase from the 1918 pandemic strain, M2 inhibitor–resistant strains,4,99 and the recently circulating (2009) pandemic A/H1N1 viruses (S-OIV).27 Resistance to oseltamivir has been recently reported in an H7N9 isolate.100
Influenza B viruses are 10-fold to 20-fold less susceptible to oseltamivir carboxylate than influenza A viruses, and influenza B virus illness responds less well clinically and virologically to oseltamivir than influenza A illness.101,102,103 The carboxylate is not cytotoxic and inhibits neuraminidases from mammalian sources or other pathogens only at 106-fold higher concentrations. Oral oseltamivir is active in murine and ferret models of influenza.94,97 A prophylactic regimen given orally twice daily for 10 days completely protected ferrets against morbidity and mortality caused by H5N1 infection and did not interfere with development of a protective immunity against subsequent H5N1 infection.104 Neuraminidase inhibitors combined with M2 inhibitors or ribavirin show enhanced antiviral activity in vitro and in animal models of influenza A virus infection,105 including H5N1 virus.106,107 Amantadine combined with oseltamivir prevented the emergence of amantadine resistance in cell culture.108
Mechanism of Action
The neuraminidase inhibitor drugs oseltamivir, zanamivir, peramivir, and laninamivir share a common mechanism of action. Influenza neuraminidase cleaves terminal sialic acid residues on glycoconjugates and destroys the receptors recognized by viral hemagglutinin on cells, on newly released virions, and on respiratory tract mucins. This action is essential for release of virus from infected cells and for spread within the respiratory tract.109 Inhibition of neuraminidase action causes newly formed virions to adhere to the cell surface and to form viral aggregates. Inhibitors limit spread of virus within the respiratory tract and may prevent virus penetration of respiratory secretions to initiate replication.
Resistance
Resistant variants selected by in vitro passage with oseltamivir carboxylate or zanamivir have point mutations in the viral hemagglutinin or neuraminidase genes.98,110 Hemagglutinin variants generally have mutations in or near the receptor binding site that make them less dependent on neuraminidase action for release from cells in vitro and that confer cross-resistance among neuraminidase inhibitors. Most of these variants retain full susceptibility in vivo.98 Neuraminidase variants contain single amino-acid substitutions in the framework or catalytic residues of the active enzyme site that alter drug binding and cause approximately 30-fold to more than 1000-fold reduced susceptibility in enzyme inhibition assays.96 Influenza A variants selected by oseltamivir carboxylate are subtype specific, most commonly Arg292Lys in N2 and H275Y in N1, without cross-resistance to zanamivir. The altered neuraminidases have reduced activity or stability in vitro, and early studies of these variants usually demonstrated decreased infectivity and transmissibility in animals.111
Oseltamivir therapy has been associated with recovery of viruses with reduced susceptibility in about 1% of immunocompetent adult and 18% of pediatric recipients.112,113 Generally, emergence of resistant variants has not been associated with clinical worsening, although prolonged recovery of resistant variants, sometimes in combination with M2 inhibitor resistance, has been observed in highly immunocompromised hosts.114 Transmission of oseltamivir-resistant virus has been documented.115,116
Although isolation of oseltamivir-resistant strains from treated immunocompetent patients was uncommon, in 2007-2008, oseltamivir-resistant seasonal H1N1 virus appeared widely in immunocompetent individuals in Norway in the absence of antiviral pressure.117 This mutant virus became the transmissible, pathogenic prevalent global H1N1 virus strain. Similarly, during the 2009 A (H1N1)pdm09 pandemic, there was no linkage between prevalent use of oseltamivir in immunocompetent patients and the appearance of oseltamivir-resistant A (H1N1)pdm09 strains, which was uncommon. The prevalence of oseltamivir-resistance ranged from 0.6% (5/804 strains) tested in Ontario, Canada,118 to 1.0% in the United States119 and 1.1% (16/1488 isolates) in Southeast Asia.120 The prevalence was 8.11% in children whose immunocompetence was not specified.121
On the other hand, oseltamivir-resistant isolates are not uncommonly recovered from immunocompromised patients being treated with the drug. Reports indicated that some of the A (H1N1)pdm09 oseltamivir-resistant strains retained replicative fitness,116 transmissibility,122 and pathogenicity comparable with wild-type oseltamivir strains in murine and ferret models of influenza infection.123 Clinical illness caused by oseltamivir-resistant H1N1 strains in immunocompetent children responded less well to oseltamivir,124 as evidenced by higher fever at day 4 or 5 of treatment, although some found no evidence of prolonged illness in children infected with drug-resistant virus.125 Others reported a significantly longer time to achieve nondetectable virus load in patients with oseltamivir-resistant H1N1 compared with oseltamivir-sensitive strains.126
Pharmacokinetics
Oral oseltamivir is rapidly absorbed and metabolized by esterases in the gastrointestinal tract, liver, and blood to the active carboxylate. The estimated bioavailability of the carboxylate is approximately 80%,127 and its time to maximal plasma concentrations averages 2 to 4 hours. Dose proportionality of oseltamivir has been reported over the dose range from 75 to 675 mg. Only low blood levels of the prodrug are detectable. Rarely, possession of a constitutive variant of carboxylesterase 1, the enzyme that normally catalyzes the conversion of oseltamivir phosphate to carboxylate, can markedly impair the hydrolysis of the parent compound, resulting in the potential for a compromised antiviral effect after oseltamivir administration.128,129 Ingestion with food delays absorption slightly but does not decrease overall bioavailability. Oseltamivir administered via a nasogastric tube to patients with respiratory failure requiring mechanical ventilation was well absorbed and converted to oseltamivir carboxylate.130,131 In healthy adults, peak and trough plasma concentrations average 0.35 µg/mL and 0.14 µg/mL after 75-mg doses.132 In infants up to 1 year of age, systemic exposure (AUC0-12 hr) to the carboxylate exhibits decreasing variability while clearance increases.121 Recommended doses of oseltamivir are 3.0 mg/kg twice daily for infants from birth to 8 months of age and 3.5 mg/kg twice daily for those 9 to 11 months of age. In children older than 1 year, carboxylate exposure increases gradually with increasing age132 so that weight-based dosing is recommended.133 In healthy elderly adults, overall drug exposure is about 25% greater than in younger adults, most likely owing to differences in renal elimination. Morbid obesity (body mass index ≥40 kg/m2) does not alter oseltamivir pharmacokinetics.134 The effects of pregnancy on the pharmacokinetics of oseltamivir are unclear. One study reported no differences among women in the third trimester of pregnancy and historical controls,135 whereas another reported a 25% to 30% reduction in systemic (AUC0-12 hr) oseltamivir-carboxylate exposure in pregnant women compared with concurrent nonpregnant controls, perhaps suggesting a need for 75 mg three times a day of oseltamivir for treatment.136
Plasma protein binding of the prodrug (42%) and the carboxylate (<3%) is low.127 The Vd is moderate (23 to 26 L). In animals, lower respiratory tract levels are similar to or exceed the levels in blood137; and in humans, the carboxylate is detectable in middle ear and maxillary sinus fluid at concentrations similar to those in plasma.138
Oseltamivir concentrations occur in breast milk.139 In the ex vivo human placenta model, oseltamivir was extensively metabolized to the carboxylate moiety, but transplacental passage of oseltamivir carboxylate occurred at a low rate, inferring that fetal exposure during maternal treatment with oseltamivir may be minimal.140 No carboxylate was detected in cerebrospinal fluid in one child,141 whereas Cmax values in cerebrospinal fluid were 2.1% and 3.5% for corresponding plasma concentrations for oseltamivir and oseltamivir carboxylate in eight healthy adults after ingestion of 150 mg of oseltamivir.142 After oral oseltamivir, the plasma  of the carboxylate averages 6 to 10 hours in healthy adults. The prodrug and carboxylate are excreted primarily unchanged through the kidney; the carboxylate is eliminated by glomerular filtration and tubular secretion via a probenecid-sensitive anionic transporter. Clearance varies linearly with CrCl, such that
of the carboxylate averages 6 to 10 hours in healthy adults. The prodrug and carboxylate are excreted primarily unchanged through the kidney; the carboxylate is eliminated by glomerular filtration and tubular secretion via a probenecid-sensitive anionic transporter. Clearance varies linearly with CrCl, such that  increases to 22 hours in patients with CrCl less than 30 mL/min, and dosage reductions are needed.127 Oseltamivir carboxylate is removed with different degrees of efficiency by different renal replacement therapies (peritoneal, hemodialysis, and continuous renal replacement therapies). Doses of oseltamivir for patients with renal impairment receiving renal replacement therapy have been published.143
increases to 22 hours in patients with CrCl less than 30 mL/min, and dosage reductions are needed.127 Oseltamivir carboxylate is removed with different degrees of efficiency by different renal replacement therapies (peritoneal, hemodialysis, and continuous renal replacement therapies). Doses of oseltamivir for patients with renal impairment receiving renal replacement therapy have been published.143
Uncomplicated influenza illness does not seem to alter the pharmacokinetics of oseltamivir.127 Cystic fibrosis patients appear to clear oseltamivir carboxylate more rapidly than patients who do not have the disease.144
Interactions
Probenecid reduces renal clearance of oseltamivir by about 50%.145 Few other clinically important drug interactions have been recognized. Sotalol appeared to induce a torsades de pointes cardiac arrhythmia during oseltamivir therapy for influenza.146 Specific studies have found no interactions with antacids, acetaminophen, or aspirin or known inhibitors of selected renal tubular secretion pathways, amoxicillin, cimetidine, cyclosporine, mycophenolate, tacrolimus,127,147 warfarin,148 or rimantadine.149
Toxicity
Preclinical studies have found no evidence of mutagenic, teratogenic, or oncogenic effects. High-dose oseltamivir causes renal tubular mineralization in mice and maternal toxicity in rabbits. It is classified as pregnancy category C.
Oseltamivir is generally well tolerated in patients of all ages, including pregnant women and fetuses,150,151 and no serious end-organ toxicity has been recognized.97,152–154 Oral administration is associated with nausea, epigastric distress, or emesis in 10% to 15% of adults receiving 75 to 150 mg twice daily. These gastrointestinal complaints are usually mild to moderate in intensity, resolve despite continued dosing, and are ameliorated by administration with food. Nausea and vomiting (and possibly, dizziness) are dose related in adults.155 Discontinuation rates of 1% to 2% were observed in controlled treatment studies. The mechanism of nausea and vomiting is uncertain, but the risk seems to be lower in older adults. Long-term prophylaxis has not been associated with an increased risk for adverse events,97,156 although headache may occur in older recipients. Self-injury, delirium, and psychiatric illness have been reported in patients, primarily pediatric or adolescent, with influenza treated with oseltamivir, mostly in Japan.157 Analyses of neuropsychiatric reactions among patients with influenza treated with oseltamivir in three large U.S. administrative databases did not demonstrate such an association.158–160 The decline in cases in Japan after a regulatory recommendation to restrict oseltamivir use in children 10 to 19 years of age has been associated with a decline in oseltamivir-related cases but a corresponding rise in cases associated with zanamivir, the inhaled, minimally systemically bioavailable neuraminidase inhibitor. The latter fact raises further doubts about a causal association between oseltamivir therapy and neuropsychiatric and behavioral adverse reactions in patients with influenza.161 Erythematous rashes and rare instances of severe eruptions or Stevens-Johnson syndrome, hepatic inflammation, hemorrhagic colitis, anaphylaxis, and thrombocytopenia have been reported, but their relationship to oseltamivir is uncertain.
Clinical Studies
Oseltamivir is efficacious for the prevention and treatment of influenza A and B virus infection. In the United States, it is approved for the prevention of influenza in patients 1 year and older and the treatment of acute uncomplicated influenza in patients 2 weeks of age and older who have been symptomatic for no more than 2 days.133
In early clinical experiments in volunteers with induced influenza it was demonstrated that oral oseltamivir is highly protective against experimental human influenza, and early treatment is associated with reductions in viral titers, symptoms, nasal cytokines, and middle ear pressure abnormalities.97 Subsequent controlled trials in patients—mostly healthy adults and children with naturally acquired seasonal influenza A infection—demonstrated that early oseltamivir treatment of acute influenza reduces the time to illness alleviation by 1 to  days, fever duration, and viral titers in the upper respiratory tract.112,162–164 Earlier treatment maximizes the speed of resolution of illness.165 Treatment of children reduces the risk for otitis media and decreases overall antibiotic use.112 In healthy and high-risk adults, early treatment has been reported to decrease the risk for lower respiratory tract complications leading to antibiotic administration and to hospitalization,166 but this has been questioned.167 A meta-analysis of observational studies of high-risk patients with seasonal influenza concluded that oseltamivir treatment may reduce hospitalization, whereas treatment of hospitalized patients reduces respiratory failure, intensive care unit admission, and mortality.168,169 A recent meta-analysis based on a large number of observational data from individual cases suggested that oseltamivir treatment may be associated with a reduction in mortality risk.169a However, a Cochrane analysis did not conclude that the evidence indicated that oseltamivir treatment reduced complications or hospitalizations.169b In hospitalized patients with infection with influenza A (H1N1)pdm09, oseltamivir provides similar benefits even if treatment is started more than 48 hours after clinical illness has begun.170–172
days, fever duration, and viral titers in the upper respiratory tract.112,162–164 Earlier treatment maximizes the speed of resolution of illness.165 Treatment of children reduces the risk for otitis media and decreases overall antibiotic use.112 In healthy and high-risk adults, early treatment has been reported to decrease the risk for lower respiratory tract complications leading to antibiotic administration and to hospitalization,166 but this has been questioned.167 A meta-analysis of observational studies of high-risk patients with seasonal influenza concluded that oseltamivir treatment may reduce hospitalization, whereas treatment of hospitalized patients reduces respiratory failure, intensive care unit admission, and mortality.168,169 A recent meta-analysis based on a large number of observational data from individual cases suggested that oseltamivir treatment may be associated with a reduction in mortality risk.169a However, a Cochrane analysis did not conclude that the evidence indicated that oseltamivir treatment reduced complications or hospitalizations.169b In hospitalized patients with infection with influenza A (H1N1)pdm09, oseltamivir provides similar benefits even if treatment is started more than 48 hours after clinical illness has begun.170–172
It is uncertain to what extent oseltamivir treatment may reduce transmission, although a review of four trials of prophylaxis suggests that oseltamivir may have reduced transmission.173,174
Oseltamivir is less efficacious for the treatment of influenza B than for influenza A virus infection in children175,176 and adults.176 An analysis of 284 cumulated cases of influenza A (H5N1) infections in a global registry demonstrated that crude mortality was significantly less in those treated with oseltamivir (40%) than in those not treated (76%) when started up to 6 to 8 days after symptoms onset.177
Oseltamivir treatment of hematopoietic stem cell transplant recipients with influenza may prevent the development of pneumonia and virus shedding, thereby both preventing influenza-related death in index patients and nosocomial transmission to others.178 Of 21 patients with leukemia who developed influenza and were treated with oseltamivir, none died, compared with 3 of 8 who were not treated.179
Prophylactic administration of once-daily oral oseltamivir (75 mg) is highly effective in reducing the risk for developing febrile illness during influenza season in unimmunized adults (efficacy 84%),180 immunized nursing home residents (efficacy 92%),181 and transplant recipients (efficacy 80%).156 Prevention of influenza may reduce secondary complications in institutionalized older adults.181 Once-daily oseltamivir for 7 to 10 days is also effective for postexposure prophylaxis in household contacts, including children, and when ill index cases receive concurrent treatment.182,183 Oseltamivir chemoprophylaxis has been used to control institutional outbreaks of influenza A continuing despite M2 inhibitor use and of influenza B.184
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree