ACUTE VIRAL MYELITIS
J. DAVID BECKHAM AND KENNETH L. TYLER
Viral infections of the spinal cord occur as part of more extensive infection of the central nervous system (CNS) or peripheral nervous system. When myelitis occurs in association with encephalitis or meningitis, the resulting syndromes are referred to as encephalomyelitis or meningoencephalomyelitis. Myelitis associated with involvement of spinal nerve roots or peripheral nerves is referred to as myeloradiculitis or myeloradiculoneuritis. This chapter focuses on acute viral infections in which spinal cord involvement is the dominant feature. Acute myelitis associated with rabies virus and HIV infection is discussed elsewhere (see Chapters 17 and 19), as are cases of chronic viral myelitis due to infection with retroviruses including HIV and human T lymphotropic viruses (HTLVs). In addition to directly infecting and injuring the spinal cord, viruses can trigger postinfectious immune-mediated tissue injury. Spinal cord involvement is a common but rarely dominant feature of acute disseminated encephalomyelitis (ADEM) (see Chapter 22). Transverse myelitis (TVM) is an acute syndrome defined by the nature and extent of the anatomic injury to the spinal cord, often associated with antecedent viral infections. Specific causes of TVM are discussed under the individual viruses involved, and the idiopathic syndrome is briefly reviewed at the end of this chapter.
The term myelitis means “inflammation of the spinal cord” and refers to disease of the spinal cord caused by a direct infectious process, a postinfectious process, or another indirect mechanism of injury. The clinical features are determined to a large degree by the location and extent of the process both in the craniocaudal and the transverse axes of the spinal cord rather than by the inciting agent.
The clinical features of myelitis provide important clues to the anatomic location of the lesion but do not enable myelitis to be separated from other causes of intramedullary spinal cord injury. The clinical features of myelitis caused by different viruses overlap substantially, and identification of a specific viral etiology typically depends on the results of laboratory tests. The characteristic features of myelitis include variable combinations of weakness; sensory loss; and bowel, bladder, and sexual dysfunction, typically evolving over days. Apoplectic or hyperacute (hours) evolution of symptoms is occasionally seen in viral myelitis but is more typical of vascular spinal cord disease (1) (e.g., infarction resulting from atherosclerosis, arteritis, emboli or hemorrhage, or even venous thrombosis [Foix-Alajouanine syndrome]). Viral causes of chronic myelitis or myelopathy in which symptoms evolving over weeks or months are largely limited to HIV and HTLV.
Weakness in viral myelitis may be either of the upper motor neuron type with associated spasticity, hyperreflexia, and extensor-plantar reflexes or of the lower motor neuron type with flaccid weakness and decreased or absent deep tendon reflexes. Lower motor neuron involvement in the absence of significant sensory signs or symptoms is often referred to as acute flaccid paralysis or poliomyelitis-like illness, although the latter term is best reserved for cases in which pathology is limited to the anterior horns of the spinal cord. Involvement of motor neurons in the anterior horns or involvement of the anterior roots can result in prominent clinical and electrophysiologic evidence of denervation, including the presence of fasciculations and fibrillations. Sensory loss in myelitis can be radicular, dermatomal, or both. Depending on the transverse localization of the lesion(s), either loss of position and vibration sense or loss of pain and temperature may occur. Finding a “sensory level” below which sensory functions are lost is a classic hallmark of spinal cord disease. Either relative or absolute sparing of sensation in sacral dermatomes (“sacral sparing”) may occur when an intramedullary process such as viral myelitis leaves the most peripheral fibers in the spinothalamic tract relatively unharmed.
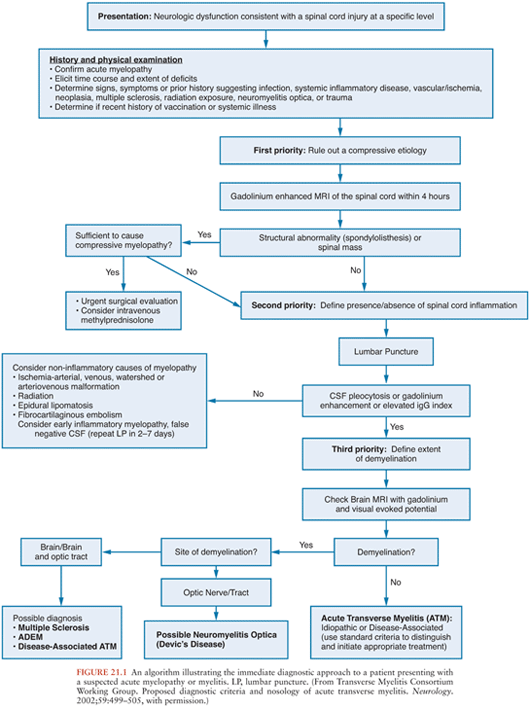
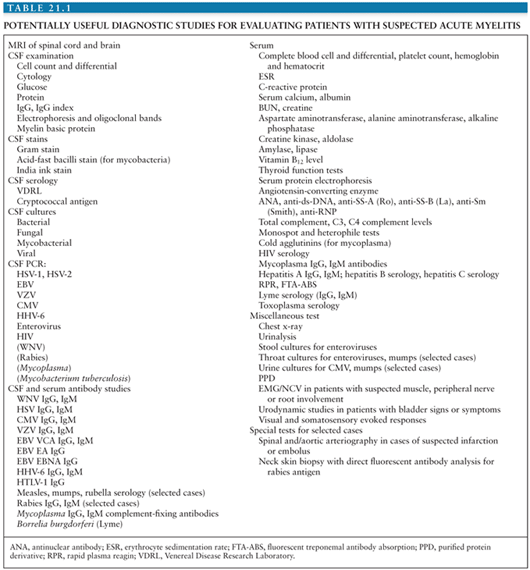
Patients with acute onset of signs and symptoms suggestive of spinal cord dysfunction are a medical emergency. Initial clinical and laboratory studies should be directed at trying to identify whether a compressive lesion is present and whether it is intramedullary or extramedullary in location. An algorithm for the immediate diagnostic approach to patients with acute myelopathy is shown in Figure 21.1(2). Table 21.1 summarizes key diagnostic tests that may be useful in evaluating a patient with suspected acute viral myelitis. In the following sections, viral etiologies of acute myelitis are discussed individually.
HERPESVIRUSES
Herpes Simplex Virus
Both herpes simplex virus type 1 (HSV-1) and type 2 (HSV-2) can cause myelitis. HSV-2 most commonly causes myelitis in adults and HSV-1 most commonly in children (1,3). The clinical presentation ranges from mild forms of disease with full recovery to severe necrotizing myelitis with permanent sequelae. Most cases are monophasic, although about 20% of patients experience recurrent episodes of myelitis, a feature common to infection with several herpesviruses (4–7). In patients with recurrent disease, the interval between recurrences may vary between 1 week and several months, with three or more discrete recurrences being noted (5,7). Up to two thirds of patients with HSV myelitis have an ascending pattern of spinal cord involvement, with the remainder having TVM (5).
Most cases of monophasic HSV myelitis are due to HSV-2 (5). Patients with HSV-2 myelitis often have a history of genital herpes. However, in one series, only two of seven patients with HSV-2 myelitis had known genital herpes, and lesions, when present, often preceded spinal involvement (5). Clinical features of HSV myelitis include paresis or paralysis, more commonly involving the legs than the arms. Patients may have either reduced and/or absent tendon reflexes or hyperreflexia with extensor-plantar responses. Decreased sensation to pain, temperature, and touch is common and tends to be more severe in sacral dermatomes. Patients can have decreased anal tone and urinary incontinence with overflow. HSV-2 can also cause a lumbosacral radiculomyelitis characterized by urinary retention associated with constipation, dull pain in the anogenital region, paresthesias, loss of sensation, or flaccid paresis of the leg muscles (8).
The most severe form of HSV myelitis is an acute necrotizing myelopathy, which occurs predominantly in patients with underlying diseases including HIV infection (9), malignancy (10), and diabetes (11,12), although rare cases occur in the absence of associated disease (13). All cases to date have been associated with HSV-2. At autopsy, these patients often show areas of necrosis in the gray and white matter of the spinal cord associated with perivascular lymphocytic cuffing. Cowdry type A inclusions are found in neurons, viral antigen can be demonstrated by immunocytochemistry, and herpesvirus-like particles have been seen by electron microscopy (9–13). Some cases have a prominent necrotizing arteritis associated with myelomalacia with Cowdry A inclusion-bearing cells seen in the wall of the anterior spinal artery (9).
In HSV myelitis, the cerebrospinal fluid (CSF) typically shows a lymphocytic pleocytosis and normal glucose concentration. Patients with recurrent attacks may have a progressive reduction in the degree of pleocytosis with succeeding attacks (7). Typical cell counts range between 10 and 200 cells/mm3, although rare cases with normal cell counts have been reported (5,14). The CSF profile in patients with acute necrotizing myelopathy may show striking pleocytosis with up to 5,750 cells and a predominance of polymorphonuclear (PMN) leukocytes rather than lymphocytes (12). Surprisingly, some reported patients have had no or minimal pleocytosis (9,11). The CSF protein concentration is almost invariably elevated (range, 50 to 430 mg/dL). Oligoclonal bands were found in one of nine patients in one series (5) and have been noted in other case reports (7,15).
HSV is only rarely cultured from CSF in patients with myelitis (15), and diagnosis depends on demonstration of HSV DNA in CSF by polymerase chain reaction (PCR) (4–6,15–17). In one series of nine patients, all had PCR amplifiable HSV DNA in CSF, with six cases due to HSV-2, two cases due to HSV-1, and one case indeterminate. In this series, HSV-2 DNA was found in six of six cases with ascending myelitis, whereas HSV-1 DNA was found in two of the three patients with nonascending TVM (5). In an HSV CSF PCR-negative patient, a presumptive diagnosis can be made by demonstrating intrathecal synthesis of HSV-specific antibodies (7). Antibody studies may be particularly useful in cases in which CSF is only available late in infection (e.g., >14 days), when HSV DNA is likely to have disappeared. Evidence of intrathecal synthesis of HSV-specific antibodies can be based on (a) the presence in CSF of immunoglobulin M (IgM) anti-HSV antibodies (these antibodies cross the blood–brain barrier poorly and their detection in CSF is generally indicative of intrathecal synthesis); (b) the detection of HSV-specific oligoclonal bands present in CSF but not in serum; (c) the comparison of HSV-specific immunoglobulin G (IgG) levels in CSF and serum with correction for blood–brain barrier leak using either the CSF/serum albumin ratio or the ratio of antibody titers in CSF to that in serum for an “irrelevant” virus (5,14,16,18). Basic laboratory studies usually add little to the diagnosis, although some patients have elevations in erythrocyte sedimentation rate and C-reactive protein (6,18).
Magnetic resonance imaging (MRI) is exceedingly important for its role in both excluding other potential diagnoses and in establishing the presence of an intramedullary process. The typical appearance of HSV myelitis is of an intramedullary fusiform or spindle-shaped area of an increased T2-weighted signal (5,7,14,16). The spinal cord is often enlarged or swollen in the area of the lesion. Areas of an increased T2-weighted signal may also have T1-weighted signal hypointensity. In rare cases, areas are both T1 and T2 hyperintense, a finding suggestive of hemorrhagic necrosis (5). Contrast (gadolinium) enhancement may be seen in the area of the lesion and the adjacent meninges and nerve roots (6,7,16,17). Lesions are most commonly in the upper thoracic and cervical cord (5,6) but can also involve the lower cord including the conus medullaris and cauda equina (7,15–17).
No controlled clinical trials of antiviral therapy for HSV myelitis are available. Based on anecdotal reports, patients should be treated with intravenous acyclovir for at least 14 days (10 mg/kg three times per day). This can be followed by oral antiviral drugs (e.g., valacyclovir at 1 g three times per day) until symptoms resolve. The utility of adding steroids is unproven. When given, steroids should be used only in combination with antiviral therapy. A typical regimen involves intravenous methylprednisolone (500 to 1,000 mg per day for 3 to 5 days) followed by oral prednisone (100 mg per day) with doses tapered over 2 weeks (5,7,17).
The prognosis of HSV myelitis is extremely variable. In one series of nine patients, one third (n = 3) made a complete recovery, and the remaining two thirds (n = 6) had residual sequelae including paraplegia and tetraplegia (5).
Varicella-Zoster Virus
Myelitis is an unusual complication of varicella-zoster virus (VZV) infection, with most cases occurring in immunocompromised individuals (19–21). Myelitis can also occur as a complication of primary varicella infection or chickenpox (20,22,23). In immunocompromised patients, common underlying diseases include HIV infection (19,20,21,24,25), Hodgkin and non-Hodgkin lymphoma (19), and immunosuppressive therapy (26,27). In a recent review of 31 cases of VZV myelitis, 55% of patients with VZV myelitis were immunocompromised, and the majority of the remaining patients had an underlying comorbidity such as malignancy or autoimmune disease (28). Cases of VZV myelitis in immunocompetent patients are reported but uncommon (29). Many patients have antecedent zoster, although several cases without rash (myelitis sine herpete) have been reported (30–32). When zoster is present, it can involve virtually any dermatome, but myelitis is often associated with disseminated zoster in immunocompromised patients (19). Cord dysfunction typically follows the onset of zoster with a median of 12 days (range, 5 to 21 days), but symptoms of myelitis may follow rash by up to 3 months or myelitis symptoms may precede the appearance of rash (19,24). Cases of TVM following zoster have also occurred in patients who have previously received zoster vaccine (33).
Patients with zoster myelitis present with subacute onset of asymmetric leg weakness which progresses to bilateral leg weakness with paraparesis in up to 85% of patients (19). Sensory loss is common (75%), with involvement of pain and temperature more common than position and vibration sense. A Brown-Séquard syndrome may occur, with posterior column signs (loss of position and vibration sense) ipsilateral to the rash and spinothalamic tract signs (loss of pain and temperature sense) contralateral (19). Approximately one third of patients will have a level to all sensory modalities, urinary incontinence occurs in about 50%, and bowel incontinence can also occur. In a few cases, VZV myelitis may show a relapsing and remitting pattern (20,25,34), a feature also seen in some cases of HSV-2 myelitis (see earlier discussion). Atypical presentations of VZV myelitis including cases not associated with rash (zoster sine herpete), skin lesions developing after myelopathy, or lack of correspondence of zoster lesions to spinal cord injury level occur more frequently in immunocompromised patients (28).
In VZV myelitis, CSF shows a mononuclear pleocytosis in about 75% of patients with an increase in PMN predominance in patients with rapid onset and severe disease (19). Recurrent episodes of CSF PMN pleocytosis with recurrent episodes of VZV myelitis have been reported (30). CSF protein concentration is elevated in 70%, but glucose concentration is almost always normal. It is important to recognize that at least 50% of immunocompetent patients with a zoster rash in the absence of myelitis have a CSF lymphocytic pleocytosis with cell counts from 5 to 1,440 cells/mm3 and exceeding 100 cells/mm3 in 30% of those with pleocytosis (35).
Approximately 25% of patients with a zoster rash will also have evidence of CSF anti-VZV IgG and detectable VZV DNA by PCR, but CSF anti-VZV IgM is usually not detectable and the CSF VZV IgG index is usually normal, suggesting that intrathecal synthesis of VZV-specific antibody does not occur in uncomplicated herpes zoster reactivation (35). By contrast, patients with zoster myelitis often have a positive CSF VZV PCR and intrathecal VZV-specific IgG synthesis, even VZV specific CSF oligoclonal bands (36).
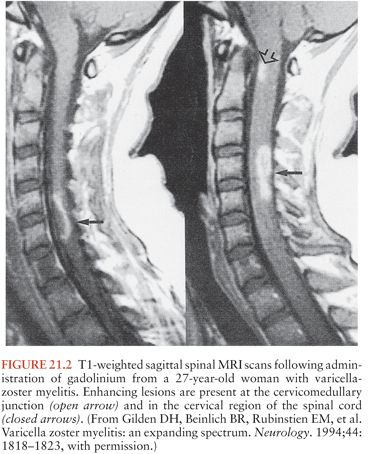
Patients with VZV myelitis frequently have abnormalities on spinal MRI (Fig. 21.2). These can include diffuse swelling of the cord and areas of high T2-weighted signal with or without associated T1 hypointensity and contrast enhancement (20,22,27,32,37–39). MRI lesions in the brainstem and cervical cord may also occur in immunocompetent patients with zoster myelitis (35).
The diagnosis of VZV myelitis is generally suspected when signs of myelitis develop following a typical zoster eruption in an immunocompromised patient. Definitive diagnosis depends on isolation of virus from CSF (24,31), demonstration of intrathecal synthesis of VZV-specific antibody (20,36,37,40), or PCR amplification of VZV DNA from CSF (20,27,37,40–42). Comparative studies of the sensitivity and specificity of these tests are lacking, although clinical experience suggests that PCR is likely to be the most sensitive, and culture the least. Antibody and PCR test results should be viewed as complementary rather than mutually exclusive; as in other viral infections, antibodies typically develop later than detectable nucleic acid. The duration for which VZV DNA remains detectable in CSF following varicella myelitis is unknown. In patients with relapsing-remitting myelitis, CSF VZV DNA may persist, as two patients had positive CSF PCR results 8 and 11 months after onset of disease (20).
No controlled clinical trials of treatment for VZV myelitis are available. Treatment typically involves intravenous acyclovir given alone or in combination with steroids (19,31,37,39,42–44), although the paucity of cases and the variability in treatment regimens make efficacy difficult to access (20). In several patients who received intravenous acyclovir after myelopathy was established, treatment seemed to be without effect (24,39,44). By contrast, there are also reports of complete recovery in AIDS patients with VZV myelitis following acyclovir therapy (10 mg/kg every 8 hours) for 21 to 35 days (42) and improvement in patients treated with either acyclovir alone (20,37) or a combination of acyclovir and high-dose steroids (31). One patient apparently responded to oral famciclovir (500 mg every 8 hours), who had not previously responded to steroids, acyclovir, or foscarnet (37).
Pathologic studies of VZV myelitis are limited (19,25,32). In a comprehensive review of nine fatal cases, there was extensive hemorrhagic necrosis with necrotizing vasculitis and thrombosis in the dorsal root ganglia associated with Cowdry type A intranuclear inclusions in both ganglion and satellite cells (19). Almost all cases had abnormalities in the posterior roots and the posterior horns of the spinal cord, although severity varied. Inclusion bodies were seen in about 50%, and patients could have demyelination and signs of necrotizing vasculitis and hemorrhagic spinal cord necrosis (19,32). A similar necrotizing process with demyelination and Cowdry type A inclusions was described in an AIDS patient with chronic zoster myelitis (25). In a recent review of five postmortem cases of VZV myelitis, tissue necrosis and inflammatory cell infiltration were the primary findings, but other pathologic changes were noted as well, including vasculitis, thrombosis, hemorrhagic transformation, and Cowdry type A inclusions (28).
Cytomegalovirus
Cytomegalovirus (CMV) involvement of the spinal cord either can result in a pure TVM or can produce myeloradiculitis, or radiculomyelopathy. Most cases of CMV-associated myeloradiculopathy occur in HIV-infected patients (45–48), and this can be the initial manifestation of AIDS (49). Myelitis can be a complication of both adult and pediatric HIV infection (50). Although CMV myelitis is predominantly a disease of immunocompromised individuals, there have been isolated reports of both TVM and myeloradiculopathy in immunocompetent individuals (51–57). Isolated cases of dual infection of the cord with both HSV and CMV have been reported in patients with AIDS (58). The incidence of CMV-associated myeloradiculopathy has declined dramatically since the introduction of highly active antiretroviral therapy (HAART). Myeloradiculopathy occurs with approximately equal frequency as an isolated manifestation of CMV infection and in systemic CMV disease (59). Patients present with rapidly progressive flaccid paralysis of the legs with hyporeflexia or areflexia (60). Urinary retention occurs in almost all patients (60). Pain, often involving the perianal region and low back, is a common initial symptom. Sensory loss usually involves small-fiber modalities (pain, temperature) more than vibration or proprioception, although both can occur. The symptoms are typically progressive, although some patients have a more indolent course.
One of the unusual features of CMV myeloradiculitis is the common occurrence of a PMN rather than a lymphocytic CSF pleocytosis (61,62). This feature is distinctive enough that its presence in an HIV-infected patient with myeloradiculopathy should always suggest the possibility of CMV infection (63). Cell counts exceeding 1,000/mm3 can occur (46,64–66), although median values of 150 to 650 cells/mm3 are more typical (60,61). In addition to pleocytosis, the CSF typically shows an elevated protein concentration. Severe hypoglycorrhachia may occur (46), although a CSF glucose concentration of less than 50% of the coincident plasma value has been reported in only about 30% of patients (62).
Imaging studies are useful in demonstrating the location and extent of lesions. Typical findings on spinal MRI include areas of increased T2-weighted signal within the spinal cord, associated with enhancement of the pial lining of the cord, conus, cauda equine, and lumbosacral nerve roots on contrast-enhanced T1-weighted images (66,67). The presence of prominent radicular enhancement in association with myelitis occurs more often with CMV infection than in other forms of viral myelitis and may provide a clue to the diagnosis.
Clinical electrophysiologic studies may be helpful in demonstrating the presence of radicular and peripheral nerve involvement in association with myelitis, especially because this component of the disease may be clinically obscured when the myelitis is severe. Both slowed conduction velocities consistent with demyelination and reduced amplitude of motor and sensory action potentials consistent with axonal injury occur (59,68).
PCR tests to amplify CMV DNA from CSF are the diagnostic procedure of choice (59,69–72). The diagnostic sensitivity of CSF PCR in CMV myelitis is more than 80%, with a specificity of more than 90% (59). It is important to emphasize that CSF PCR is often positive when cultures are negative (69,72). Quantitative PCR studies of CMV DNA in CSF suggest that extremely high DNA levels, exceeding 10 million copies of CMV DNA per milliliter of CSF can be found in patients with myeloradiculopathy (59,69,73).
CMV may be cultured from CSF in cases of radiculomyelitis (59). The high seroprevalence rate of anti-CMV antibodies in serum generally renders serologic studies of limited value. However, the demonstration of specific intrathecal synthesis of anti-CMV antibodies or the presence of detectable CSF anti-CMV IgM antibodies can be considered presumptive evidence for CNS infection.
Controlled clinical trials of antiviral therapy in CMV-associated neurologic disease are lacking, and most reports of treatment of CMV-associated neurologic disease involve isolated cases (59,69,74,75). Response to treatment of myeloradiculopathy is variable. Improvement or stabilization of symptoms has followed treatment with ganciclovir, foscarnet, or a combination of the two agents (60,62,64,68,76–80). Disease has been associated with ganciclovir-resistant strains (81,82), and this risk may be enhanced in patients whose disease developed during ganciclovir treatment for CMV infection elsewhere. As a general rule, the presence of myelitis is an extremely poor prognostic indicator, with one review citing mean survival times of 5.4 ± 1.8 weeks in patients not receiving ganciclovir and 14.6 ± 9.4 weeks in those receiving ganciclovir therapy (76).
Successful treatment is usually associated with disappearance of CMV DNA from CSF, whereas viral DNA persists in nonresponders (74). A typical induction regimen for ganciclovir involves 14 to 21 days of intravenous therapy with doses of 5 mg/kg every 12 hours. Intravenous foscarnet at a dose of 90 mg/kg every 12 hours provides an acceptable alternative. Patients who fail to respond to either foscarnet or ganciclovir alone may respond to combined therapy with the two drugs (79). A third agent, cidofovir, has been shown to be efficacious in treatment of CMV retinitis, but experience with this agent in CMV-associated neurologic disease is limited (83). A typical dosing regimen is 5 mg/kg intravenously every week for 2 weeks followed by infusions of 5 mg/kg every 2 weeks. Fourteen to twenty-one days of ganciclovir or foscarnet therapy is likely to be sufficient for immunocompetent patients. In most organ transplant recipients, 14 to 21 days of therapy is also likely to be adequate assuming clinical and virologic response has occurred. Maintenance therapy is usually not required for CMV myelitis in organ transplant recipients, although maintained vigilance for recurrent disease is essential. In patients with HIV infection, it is critical that HAART be initiated or optimized concomitantly with anti-CMV therapy. HIV-infected patients invariably require maintenance therapy with an orally bioavailable valine ester of ganciclovir (valganciclovir). Induction and maintenance doses of intravenous and oral ganciclovir, intravenous foscarnet, and intravenous cidofovir require adjustment in patients with renal insufficiency. Neutropenia is the major dose-limiting side effect with ganciclovir, and nephrotoxicity with foscarnet and cidofovir. Nephrotoxicity can occur with ganciclovir but is less common. Neurotoxicity can occur with ganciclovir and foscarnet and in the case of foscarnet is often related to electrolyte alterations (especially hypocalcemia). Patients whose HIV infection responds to HAART and who consistently (>6 months) demonstrate nondetectable HIV viral loads and CD4 cell counts of more than 100 cells/mm3 may be able to discontinue maintenance therapy.
Few detailed pathologic studies of CMV myeloradiculitis have been performed. There is often a prominent PMN and mononuclear cell infiltrate involving the sacral spinal cord, cauda equina, and lumbosacral nerve roots associated with both demyelination and axonal destruction. CMV antigen is detectable in the spinal cord and in involved roots and endothelial cell (47,59).
Human Herpesvirus-6 and Human Herpesvirus-7
Human herpesvirus type 6 (HHV-6) causes exanthema subitum (roseola infantum). Most individuals are infected in early childhood (age 6 to 12 months). Although primary HHV-6 infection is usually benign, there have been isolated reports of meningoencephalitis. Myelitis appears to be extremely rare in patients with HHV-6 infection. HHV-6 neuroinvasive disease including encephalitis and/or myelitis are most commonly reported as a complication in bone marrow transplant recipients (84,85) and occur with a frequency of about 3% in allogeneic hematopoietic stem cell transplantation and up to 16% in patients receiving cord blood transplantation from an unrelated donor (86). Median onset of clinical symptoms is 23 days after transplant, and limbic encephalitis is the most common presenting syndrome with or without associated myelitis. Very rare cases of HHV-6 myelitis in immunocompetent individuals have been reported (87). HHV-6 may also be responsible for rare cases of chronic myelitis presenting as spastic paraparesis (88).
There is one reported case of combined encephalitis and acute flaccid paralysis in an immunocompetent 19-year-old man infected with HHV-7 (89). The CSF had a lymphocytic pleocytosis, an elevated protein concentration, and a normal glucose. HHV-7 DNA was amplified from CSF by PCR. Serologic studies were also consistent with acute HHV-7 infection. Virus was not cultured from either blood or CSF, and serum PCR studies, in contrast to those in CSF, were negative.
Epstein-Barr Virus
Neurologic complications of Epstein-Barr virus (EBV) infection have been estimated to occur in 1% to 5% of patients with severe infectious mononucleosis (90). EBV-associated neurologic disease can also occur in the absence of, or even precede, symptoms of infectious mononucleosis (91,92). CNS and peripheral nervous system manifestations of EBV infection include meningoencephalitis, cerebellitis, Guillain-Barré syndrome, and TVM. The spinal cord manifestations of EBV infection are generally considered among the more unusual neurologic complications of EBV infection, although exact data about their frequency are not available (91–95). Many cases of EBV myelitis have occurred in apparently immunocompetent individuals and often present as a meningoencephalomyeloradiculopathy (96,97). Isolated reports of myelitis in immunocompromised patients, including a case in a bone marrow transplant recipient, have appeared (98).
TVM typically develops 1 to 2 weeks after the onset of infectious mononucleosis. It is important to recognize that the symptoms of mononucleosis may be mild (e.g., pharyngitis alone). Patients present with flaccid weakness with absent or decreased reflexes (91,92,98–101). Most patients have a sensory level, often associated with some radicular sensory signs and symptoms. Urinary retention is common (101). Less typically, the acute onset of paraparesis or tetraparesis is associated with spasticity, hyperreflexia, and extensor-plantar reflexes (91). A more indolent course in which weakness is preceded by back pain and radicular sensory symptoms has also been reported (91). A lower motor neuron pattern of asymmetric flaccid weakness resembling poliomyelitis can occur with absence of associated sensory or bladder symptoms (102).
In some patients, even though myelitis is the dominant feature, associated radicular and encephalitic symptoms coexist, and the syndrome has been referred to as encephalomyeloradiculopathy (103), encephalomyelitis (101), meningoencephalomyelitis, meningomyeloradiculitis, and encephaloradiculitis (96). When signs and symptoms suggesting involvement of multiple areas of the CNS occur, they can appear concomitantly (98) or sequentially (104). One reported patient had associated bilateral optic neuritis, suggestive of Devic disease (neuromyelitis optica [NMO]) (99).
Spinal MRI in EBV myelitis typically shows an area of increased intramedullary T2-weighted and decreased T1-weighted signal with enhancement of the lesion and adjacent meninges after administration of gadolinium (91,96,98,99,101). The affected area of the spinal cord may appear widened or swollen (91,98). Nerve root enhancement has also been noted in patients with myeloradiculitis (96). A patient who presented with a poliomyelitis-like syndrome had no abnormal intramedullary signal but did show meningeal enhancement around the cauda equina (102). Myelitis can occur with a normal MRI scan (93,103).
In patients with a prominent radicular component, results of clinical electrophysiologic tests may be abnormal with prolonged F-wave latencies on electromyography (EMG) and increased spontaneous activity consistent with denervation (100–102). Laboratory studies can provide clues to the diagnosis. Complete blood cell count may show lymphocytosis and atypical lymphocytes (93). The presence of significant numbers of atypical lymphocytes in blood or CSF should prompt consideration of EBV infection but can occur with other infections (101). Older serologic tests, including the heterophil antibody test (the Paul-Bunnell test), have been largely replaced by commercial spot and slides tests (e.g., Monospot test) to detect heterophil antigens. It is important to recognize that heterophil tests are often negative in patients with EBV-associated TVM (91,92). Serologic tests measuring antibodies against distinct virus-specific antigens, including the viral capsid antigens (VCAs), early antigens (EAs), and Epstein-Barr nuclear antigen (EBNA), provide more sensitive and specific confirmation of diagnosis. Serodiagnosis of EBV infection is made by demonstrating serum IgM VCA, which is generally present acutely then declines over 1 to 3 months (99). Detection of IgM antibodies to VCA is both sensitive and specific for diagnosis of recent EBV infection and can be found in about 90% of patients. The presence of IgG antibodies to VCA, IgG antibodies to EA, and no antibodies to EBNA in convalescent sera provides evidence of recent EBV infection (91,92,99). A fourfold increase in titer of anti-VCA IgG antibody between acute and convalescent sera is also presumptive evidence of acute infection. However, IgG VCA antibody titers are often elevated in the acute sera, and seroconversion is demonstrable only in a few patients (about 10% to 20%). IgG VCA and EBNA antibodies can persist for life, and their presence, in the absence of other serologic signs of acute infection, reflect past rather than active infection. In rare cases, seroconversion may be delayed for up to 2 months after onset of illness (92), further complicating diagnosis.
In patients with EBV myelitis, the CSF typically shows a mild lymphocytic pleocytosis (25 to 500 cells), mildly elevated protein concentration, and normal or mildly depressed glucose concentration (91,93,98,99,101–104). EBV can be cultured from oropharyngeal washings and circulating lymphocytes in patients with infectious mononucleosis. However, virus is only rarely isolated from CSF in patients with neurologic disease (105
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree