ACUTE BACTERIAL MENINGITIS
KAREN L. ROOS, ALLAN R. TUNKEL, DIEDERIK VAN DE BEEK, AND W. MICHAEL SCHELD
The meningitis syndrome has been recognized for centuries. Hippocrates realized the important intracranial consequences of otitic infection, and clear clinical descriptions of meningitis have been found dating from the sixteenth century. However, the syndrome of epidemic meningitis with a purpuric rash was not identified until 1805, when Viesseux wrote about an epidemic of “malignant purpuric fever” surrounding Geneva, Switzerland, the first clinical description of meningococcemia with meningitis. The pathologic hallmark of the condition, inflammation within the subarachnoid space (SAS), was described in autopsy reports in the French literature the following year. Danielson and Mann (1) recorded the first observations of meningococcemia and meningitis in the United States in 1806. Many of these early descriptions were collated in a treatise by Elisha North of Connecticut in 1811 and summarized in references 1 and 2. Then, as now, the disease could present dramatically in a fulminant form. The epidemic nature of meningococcemia was frightening to physicians and lay persons alike. For example, Dr. Samuel Woodward, of Torrington, Connecticut, wrote the following in The American Mercury, Hartford, in 1807:
The violent symptoms were great lassitude, with universal pains in the muscles, chills; heats, if any, were of short duration; unusual prostration of strength; delirium, with severe pain in the head; vomiting, with indescribable anxiety of stomach; eyes red and watery, and rolled up, and the head drawn back with spasm; pulse quick, weak, and irregular; petechiae and vibices all over the body, and a cadaverous countenance and smell; death often closed the scene in ten or fifteen hours after the first attack . . . the body, near the fatal period, and soon after, became as spotted as an adder. . . .
Similarly, the following was written by the Reverend Festus Foster of Petersham, Massachusetts, as a letter to the editor of The Worcester Spy, dated March 6, 1810:
I hasten to give you a sketch of the spotted fever in this place. It made its first appearance about the beginning of January last; but the instances were few and distant from each other, until last week. Although it had proved fatal in most instances, seven only had died belonging to this town, previous to the 25th of February. Since that time the disorder has come upon us like a flood of mighty waters. We have buried eight persons within the last eight days. About twelve or fifteen new cases appeared on Thursday last; many of them very sudden and violent. This was the most melancholy and alarming day ever witnessed in this place. Seven or eight physicians were continually engaged in the neighborhood north of the meeting house, and I believe not one half hour passed in the forenoon without presenting a new case. Pale fear and extreme anxiety were visible in every countenance. . . .
It is inconceivable that this fulminant form of meningococcemia had been previously unrecognized, especially given the excellent clinical descriptions of rashes in the literature from the period. One must speculate that the virulence of meningococci for humans changed in the early nineteenth century.
Meningococci were first isolated in 1887 by Anton Weichselbaum in Vienna; they were obtained from the cerebrospinal fluid (CSF) of six patients with meningitis and were initially named Diplococcus intracellularis meningitidis. All three of the major meningeal pathogens (Neisseria meningitidis, Streptococcus pneumoniae, and Haemophilus influenzae) were isolated and described in the last two decades of the nineteenth century. Quincke introduced lumbar puncture (LP) in 1891, and the major CSF alterations associated with meningitis (pleocytosis, hypoglycorrhachia, and elevated protein concentration) were well recognized by the turn of the century.
The treatment of bacterial meningitis in the early years of this century was dominated by methods for removal of large volumes of CSF and/or direct instillation of substances (e.g., dyes, enzymes) into the SAS. After early leads from European investigators, the first truly significant therapeutic modality for this disorder on a large scale, the systemic and intrathecal administration of antimeningococcal antisera raised in horses, was documented by Simon Flexner in 1913. Though toxic, antisera therapy reduced the mortality of meningococcal meningitis (from approximately 80% to 30%) during World War I and for decades thereafter. The principles of serum therapy were applied by Dr. Hattie Alexander and others to meningitis caused by H. influenzae in the 1940s.
The approach to the patient with bacterial meningitis was profoundly altered by the advent of antimicrobial therapy. The first successful account of the therapy of meningococcal meningitis with an antimicrobial agent in this country was published by Schwentker et al. (3) in 1937; nine patients survived after receiving subcutaneous and intraspinal injections of sulfanilamide, and the sole death occurred after eradication of the organism from CSF. The introduction of penicillin and other antimicrobial agents (e.g., streptomycin and chloramphenicol) ushered in the modern antimicrobial era, likened to an industrial revolution (4). These developments led to the widespread belief that serious bacterial infections were “solved.” Despite the introduction of myriad new antimicrobial agents and the development of newer diagnostic techniques, the mortality from meningitis caused by the three major bacterial pathogens has not changed appreciably in the last four decades. However, the use of the third-generation cephalosporins during the 1980s for therapy of gram-negative aerobic bacillary meningitis has substantially reduced the mortality of this condition. Recent years have revealed an explosion of new knowledge on the pathogenesis and pathophysiology of bacterial meningitis (see later discussion), with attendant ramifications on the use of adjunctive therapy (e.g., corticosteroids, nonsteroidal antiinflammatory agents, and monoclonal antibodies) for this disease.
EPIDEMIOLOGY
During 2003 to 2007, approximately 4,100 cases of bacterial meningitis occurred annually in the United States (5), but this disease is much more common in developing countries (see later discussion). In addition, the relative frequency with which each of the various bacterial species causes meningitis is age related. Gram-negative bacilli (principally Escherichia coli K1), group B streptococci, other enteric bacilli, and much less commonly, Pseudomonas species are the major causative agents during the neonatal period. Meningitis in children and adults is primarily caused by meningococci and pneumococci, although disease caused by aerobic gram-negative bacilli is increasing in frequency, especially in the elderly. N. meningitidis is the only major cause of epidemics of bacterial meningitis.
The development of meningitis depends on a complex array of factors, including virulence properties of the organisms, the carrier state, and the host’s humoral immune response. These factors differ among the major pathogens, for which reason epidemiology, carrier state, and host immunity are considered separately for each of the three major etiologic agents in this section.
The classification of the pathogens, putative virulence factors, and the clinical settings associated with less prevalent agents are discussed later (see the section “Etiology,” later in this chapter).
Haemophilus influenzae
H. influenzae type b (Hib) was previously the leading cause of bacterial meningitis in the United States but now accounts for only approximately 7% of cases (5). Meningitis caused by Hib displays an interesting bimodal seasonal pattern in northern Europe and the northern United States, with peaks in June and September through November (6,7).
The overall annual incidence of serious Hib disease differs between geographic locales and among populations. Significant interannual variations in the incidence of meningitis caused by Hib have also been reported within a single geographic area over time (8). This is an important consideration in assessing the efficacy of Hib vaccines. Before the advent of conjugate vaccines (see later discussion), the overall rate of Hib meningitis in the United States was approximately 60 per 100,000 children younger than 5 years of age (9), greater than the figures from other countries in northern Europe. These incidence rates differ markedly among age-groups (see later discussion) and in children younger than 6 years. In a 3-year nationwide prospective study on pediatric meningitis in Israel, the incidence of Hib meningitis during the first year of life was 67.1 per 100,000, and in children younger than 5 years of age, it was 18.5 per 100,000 (10). Some studies report a higher incidence in nonwhites (7,11). For example, the incidence rate for Hib meningitis for the total population of Washington State was 2.2, 3.4, and 13.5 per 100,000 for whites, blacks, and Native Americans, respectively (11). In contrast, others have found no differences between rates for blacks and whites younger than 1 year of age (12).
Before the availability of the current Hib vaccines, 1 in every 200 children developed invasive Hib disease by 5 years of age. Meningitis caused by Hib in the first 2 months of life is rare, presumably because of placental transfer of protective concentrations of maternal bactericidal antibody. Most cases occur between 4 months and 2 years of age. The highest rate of illness occurs in children 6 to 17 months of age; children older than 2 years of age have a lower incidence (6,8,9,11–17). Approximately 80% of cases develop in unvaccinated children younger than 2 years of age in this country, but this proportion varies by geographic locale. The proportion of cases is approximately 20% lower in this age-group in northern European countries. These differences in age distribution may directly influence the efficacy of candidate Hib vaccines. Nontypeable strains of H. influenzae are commonly carried in the nasopharynx of asymptomatic individuals. Carriage of encapsulated strains (usually type b) is rare: rates are less than 5% in children and less than 1% in adults. However, the carriage rates among household contacts of an index case are much higher: 20% to 25% overall and more than 50% among children younger than 5 years of age. This varies with the clinical disease. For example, carriage rates among children 5 years old are 20% and 55% for household contacts of epiglottitis and meningitis cases, respectively. This prevalence is reflected in the increased risk for serious Hib disease among household contacts of the index case, which is age dependent: 4% for children 2 years of age, 2% for children 2 to 3 years old, and 0.1% for children 4 to 5 years of age. The risk for Hib infection among household contacts is approximately 600-fold greater than the age-adjusted risk for the population at large and is the basis for chemoprophylactic strategies. Carriage is usually asymptomatic and may occur despite the presence of circulating anticapsular antibodies or effective eradication of meningitis following antibiotic therapy. The Hib carrier state may persist for weeks to months.
The occurrence of Hib meningitis is inversely proportional to the age-related concentration of type-specific anticapsular antibodies (18). Finnish studies, measuring anti–polyribosylribitol phosphate (anti-PRP) antibodies by radioimmunoassay, confirm the age-related susceptibility to systemic Hib disease: 90% of children (3 to 12 months old) had concentrations of less than 150 ng/mL, whereas adults had higher concentrations (19). These anti-PRP antibodies, in concert with complement, are (a) opsonic and bactericidal against Hib in vitro and (b) protective in vivo. Antibodies to Hib outer membrane proteins (Omps) also appear protective, but only against the homologous subtype. The anti-PRP response to infection is age related, being poor in infants; older children and adults develop higher titers. It is also dependent on PRP concentrations and clearance rates. PRP antigenemia may persist for weeks in younger children with Hib meningitis, delaying the antibody response. Approximately 80% of children with Hib meningitis develop an antibody response within 3 months. The antibody response is blunted in children with agammaglobulinemia or immunoglobulin G2 (IgG2) subclass deficiency, as well as in all children younger than 24 months of age receiving the Hib PRP vaccine, because this polysaccharide is a poor immunogen in this age-group (9). The age-related acquisition of protective anticapsular antibodies is too rapid to be accounted for by the low incidence of carriage or disease caused by Hib alone. Cross-reacting antigens from E. coli and other bacteria within the gut are postulated to serve as the primary immunogen.
Acquisition of Hib (nasopharyngeal carriage) and the concentration of circulating anticapsular antibody are the two main factors that determine risk for disease in most patients. Some of these risk factors have been alluded to (e.g., immunoglobulin or complement deficiency, household contacts of an index case). Other conditions that also may be important in increasing susceptibility to invasive Hib infection include sickle cell anemia, postsplenectomy states, CSF fistulas, chronic pulmonary infections, alcoholism, and probably lower socioeconomic status (e.g., Eskimos and American Indians). Day care outside the home and the presence of young siblings increases the risk for invasive disease, whereas breast-feeding is protective. The risk is highest for children younger than 2 years of age in day care but is not apparent for older children, is equally high for those in a family day care setting or those in a professional day care center (mean group size, 4 and 12 children, respectively), and is significantly higher (p < .02) within the first month of attendance, especially among younger children (20). The risk ratio doubles with each additional sibling younger than 7 years of age and is higher in twins (20). New associations have also suggested that the child’s previous state of health, especially a history of otitis media and/or previous hospitalization, increases the risk for serious Hib disease. Otitis media remains significant, especially for younger children, even after controlling for confounding variables such as day care attendance (21). Pharyngitis and otitis media are associated with Hib meningitis in approximately one half and two thirds of the cases, respectively.
Because of the bimodal seasonal occurrence of Hib meningitis, at least in northern latitudes, it has been suggested that preceding viral upper respiratory tract infections predispose to the acquisition of Hib and subsequent disease, but this issue remains controversial (22). Recent studies comparing the attack rates of meningitis between two ethnic groups living together in one geographic area (Jews and Bedouins in the Negev region of Israel) suggested that community-acquired bacterial meningitis is associated more strongly with the type of morbidity most prevalent in the region at any given time (e.g., upper respiratory tract or gastrointestinal infections) rather than any specific type of infection (23).
There has been a profound reduction (from 76% to 90%) in the incidence of invasive infections caused by Hib in the United States, specifically in young children (24–27), attributed, in part, to the widespread use of conjugate vaccines against Hib that were licensed for routine use in all children beginning at 2 months of age. In a study of Hib disease rates in Los Angeles County, California, Hib disease was nearly eradicated in a fully immunized population, demonstrating the importance of promotion of widespread use of these conjugate vaccines (28). Similar results have been observed outside the United States. In Finland, there has been a marked decrease in the number of cases of Hib meningitis from a peak of 43 cases per 100,000 population in the late 1970s to no cases in 1991 in the greater Helsinki area (29). Similarly, during a prospective study of bacterial meningitis in the northeast Thames region of the United Kingdom, there was an 87% decline in the number of cases of Hib meningitis in 1993 (7 cases) compared with 1991 and 1992 (50 cases) (30). Widespread usage beginning at 2 months of age has nearly eliminated serious invasive Hib disease in children in North America, Western Europe, Japan, and in many areas of Latin America. Unfortunately, for reasons of cost, Hib conjugate vaccines are used sparingly in many resource-limited settings, and Hib meningitis remains common in children in these areas. For example, Hib still accounted for 32% of cases in children younger than 5 years of age in Bulgaria in the mid-1990s (31). In addition, there was a report of emergence of cases in Nottingham, United Kingdom of invasive Hib disease in those previously vaccinated against the disease (32). Several explanations were put forth to explain this increase, most notably that the vaccination schedule in the United Kingdom was at 2, 3, and 4 months of age, and no booster dose was given (33). In areas in the developing world, declining rates of Hib meningitis have been reported since introduction of Hib conjugate vaccines, with effectiveness ranging from 88% to 94% (34–37).
The occurrence of meningitis caused by Hib in individuals older than 6 years of age should prompt efforts to exclude common accompanying conditions, such as otitis media, sinusitis, epiglottitis, CSF leaks, an immunodeficiency state, splenectomy or asplenic states, other parameningeal foci of infection, diabetes mellitus, and alcoholism (38,39). Although the incidence in children has dramatically declined, the incidence of invasive H. influenzae disease in adults is more complex. In one population-based study of the epidemiology and outcome caused by typeable and nontypeable H. influenzae among adults in Utah during 1998 to 2008, there was an increase in incidence over the study period from 0.14 per 100,000 person-years in 1998 to 1.61 per 100,000 person-years in 2008 (40); patients older than 65 years of age accounted for 51% of the cases and 67% of the deaths.
Neisseria meningitidis
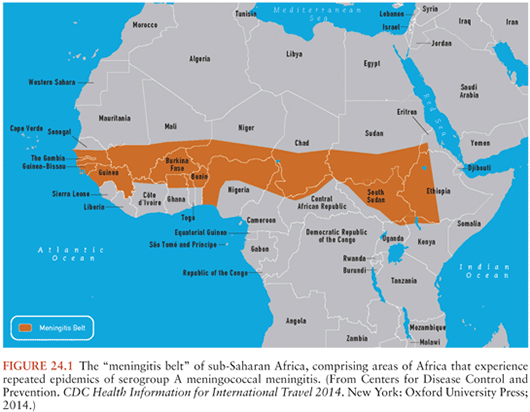
Meningococcal infections continue to pose serious problems on all continents. They are influenced by multiple factors, including geography, season, climate, meningococcal serogroup, and population demographics (2,41). Although worldwide in distribution, the incidence of epidemic meningococcal meningitis and/or meningococcemia exhibits high geographic variability. The meningitis belt of sub-Saharan Africa represents a classic endemic area (Fig. 24.1). Although meningococcal infections were not recorded in the area until the 1880s, large outbreaks still occur regularly. Although the precise effects of climatic conditions on the incidence of meningitis are unresolved, the belt lies within the 300- and 1,100-mm rainfall lines. At least 390,000 cases with 53,000 deaths occurred within the seven countries of the belt in the 10-year period 1951 through 1960. The average annual incidence since 1950 has been estimated as approximately 70 cases per 100,000 population by the World Health Organization (WHO) (41). In 1988, more than 57,000 cases of meningococcal disease were reported from the African continent; in 1989, the number of cases increased to more than 70,000, with more than 40,000 reported from Ethiopia (42). Because reporting is often delayed and may be incomplete, this figure likely underestimates the actual number of cases. More than 180,000 cases were reported by the WHO for the regions in 1996, the highest yearly total in more than 40 years. Similarly, within a 1-month period (October 1974), 4,865 patients with meningococcal meningitis were treated at the major infectious diseases hospital in Sao Paulo, Brazil. The overall mean annual incidence of meningitis caused by N. meningitidis reached 370 cases per 100,000 population in the greater Sao Paulo area in that year. The attack rate was 517 cases per 100,000 inhabitants during a group C epidemic in Upper Volta (now Burkina Faso) in 1979, and recent studies documented an attack rate of 400 to 450 per 100,000 children up to 8 years of age in the Faeroe Islands (43). In contrast, the mean annual attack rate in the United States (1975 to 1980) was approximately 1.2 per 100,000 persons but was, again, age dependent: 17.1 per 100,000 in children younger than 1 year of age, 5.2 per 100,000 in 1- to 4-year-old children, and 0.3 per 100,000 among adults. Approximately 2,500 to 3,000 cases of meningococcal infection were reported annually in the United States between 1984 and 2003. In a multistate surveillance project conducted between 1989 and 1991 in the United States, the average annual incidence of meningococcal disease was 1.1 per 100,000; 46% of cases occurred in children 2 years of age, and the highest age-specific incidence was in children younger than 4 months of age (44). A similar figure of approximately 2 per 100,000 population was reported from Finland from 1976 through 1980.
The peak incidence of meningococcal meningitis in industrialized nations occurs in winter through early spring in both epidemic and endemic periods. Similar seasonal trends may also occur in tropical areas. For example, both the group C and group A meningococcal epidemics in the Sao Paulo area from 1971 to 1974 began in May or June, the point of transition from the rainy to the dry season. African outbreaks occur during the dry season from December to June. Annual outbreaks in the sub-Saharan meningitis belt tend to peak in late April and early May, when the dry desert wind (harmattan) has ceased and temperatures are high throughout the day, and terminate abruptly with the onset of the rainy season (41). Low humidity may alter the pharyngeal mucosal barrier, thereby predisposing it to infection. Although the introduction of a new virulent strain into a susceptible population may contribute to the epidemics, many other factors—including crowding, the presence of other respiratory pathogens, poor hygiene, and poorly defined environmental features—contribute to the initiation of a meningococcal epidemic (45).
Although meningococcal meningitis may be more prevalent in men and boys, the reports are often skewed by the inclusion of military recruits and chronic alcoholics. Meningitis caused by N. meningitidis is primarily a disease of children and young adults: fewer than 10% of cases occur in patients older than 45 years of age. In the United States and Finland, children younger than 5 years of age account for approximately 55% of cases during nonepidemic conditions, whereas in Zaria, Nigeria, the peak incidence occurs in 5- to 9-year-olds (43). Major epidemics are heralded by a “shift to the right” toward older age-groups (i.e., adolescents instead of children), a predictive feature of epidemics in the meningitis belt identified by prospective surveillance. Although meningococcal meningitis is unusual in adults, 33% of sporadic meningococcal disease occurred in adults in a 5-year population-based study in Atlanta (46). Underlying conditions such as congestive heart failure, multiple myeloma, and infection with human immunodeficiency virus (HIV) were prevalent in adults older than 24 years of age with meningococcal infection but unusual in the 18- to 24-year-old group.
Large-scale epidemics caused by serogroup A meningococci have occurred at 20- to 30-year intervals throughout the world in the last and this century and continue at approximately 8- to 12-year intervals in the African meningitis belt, where approximately 1% of the population is affected. These strains infrequently cause disease in the United States, but serious outbreaks caused by serogroups A, B, or C continue in many areas (Table 24.1). A predominant serogroup circulating in the African meningitis belt since 2000 is W135, an unusual occurrence (see later discussion). Serogroups B and C now cause most focal outbreaks and endemic disease in many areas (see the section “Etiology” later in this chapter).
N. meningitidis disease is exclusive to humans. No intermediate host, reservoir, or animal-to-human transmission has been proved. The nasopharynx is the natural reservoir for meningococci; transmission is facilitated by airborne droplets or close contact. The definition of “close contact” has not been clearly elucidated but generally refers to persons who have had prolonged (8 hours or longer) contact while in close proximity (3 feet or less to the patient) or direct exposure to the patient’s oral secretions (through kissing, mouth-to-mouth resuscitation, endotracheal intubation, or endotracheal tube management) within 1 week before the onset of the patient’s symptoms until 24 hours after initiation of appropriate antimicrobial therapy (47). Meningococcal colonization may result in an asymptomatic carrier state (which is most common) or in endemic, hyperendemic (e.g., a meningitis belt between epidemics at 10 to 50 cases per 100,000 per year), or epidemic disease. Although there is no clear relationship between carriage rate and overt disease, the development of the carrier state and the host immune response are, as for Hib meningitis, important variables in the epidemiology of meningococcal infections.
Approximately 6% of the population develops nasopharyngeal colonization with N. meningitidis yearly. Nasopharyngeal carriage rates vary with age and the population under study. The carriage rate is markedly influenced by age: 0.5% to 1% in children 3 to 48 months old, approximately 5% in adolescents 14 to 17 years old, and 20% to 40% in young adults. Analogous to Hib, carriage rates are higher in close contacts of an index case. Carriage rates of meningococci of approximately 40% have been documented in close family contacts of meningococcal cases. In closed populations (e.g., military barracks during early training), carriage rates of 20% to 60% are commonplace and may reach 90% during epidemics of meningococcal disease. Nasopharyngeal carriage usually persists for weeks to months, similar to Hib carriage. Spread of meningococcal disease is usually carrier mediated (i.e., not spread by case-to-case contacts) and largely accounts for the increased risk for disease (500- to 1,000-fold above the background endemic rate) in household contacts of an index case. The organism is often introduced into the home environment by an adult family member, with subsequent transmission to others; infants are colonized last of all. Although uncharacterized host and environmental factors contribute to containment of infection to the nasopharynx (thereby preventing disseminated disease), host immunity also plays an important role. Nevertheless, those individuals most recently colonized with meningococci appear to be at the greatest risk for invasive disease. Secondary systemic meningococcal disease often develops within 5 days of recognition of the index case, with 70% to 80% of secondary cases occurring within 14 days of the primary case.
As with Hib disease, the age-specific incidence of meningococcal infection is inversely proportional to the presence of serum bactericidal antibodies against serogroups A, B, and C. More than 50% of infants possess bactericidal antibody at birth as a result of transplacental transfer. The specifics of the antibody response may be responsible for the occurrence of meningococcal meningitis during the neonatal period. The group B capsular polysaccharide is a polymer consisting of two to eight linked sialic acid residues but is immunologically identical to the oligosaccharides of several human glycoproteins, including brain gangliosides. Immunologic tolerance thus exists in this age-group; although IgM antibody can be induced, the usual switch to IgG antibody production does not occur (43). Because IgM does not cross the placenta, IgG antibody to the serogroup B polysaccharide is lacking in neonates, contributing to the occurrence of group B meningococcal disease in this patient population. In addition, group B meningococcal capsular antigen is identical to the capsular polysaccharides of E. coli K1 and certain types of group B streptococci, major causes of neonatal sepsis and meningitis. The prevalence of antimeningococcal capsular antibodies is lowest between 6 and 24 months of age, increasing to approximately 70% by early adulthood. The inverse link between occurrence of invasive disease and bactericidal antibody was first documented during an outbreak of serogroup C meningococcal meningitis among army recruits in 1968 (48). In this study, the sera of only 5.6% of the recruits who developed meningococcal disease had bactericidal activity before the onset of illness, compared with 82.2% of control sera. Notably, 5 (38.5%) of 13 recruits without bactericidal antibody developed systemic illness after colonization with the group C strain.
Although recovery from invasive meningococcal disease generally confers lifelong immunity against the homologous serogroup, this is not the major immunizing process. Nasopharyngeal colonization, particularly with serogroups B, C, or Y, may elicit the development of bactericidal activity, primarily directed against the colonizing strain but also against heterologous organisms within 5 to 12 days of acquisition. Colonization with nongroupable meningococci or Neisseria lactamica may elicit protective immunity, especially in young children. N. lactamica is virtually nonpathogenic, but nasopharyngeal carriage rates of this organism are highest (4% to 20%) in children between 3 months and 12 years of age, whereas the age-adjusted carriage rates for N. meningitidis are only 0.5% to 2%. As with Hib, the carriage rates of pathogenic meningococci are too low in children to account for antibody formation, and the importance of other cross-reacting organisms has also been proposed: Bacillus pumilus for group A polysaccharide and E. coli for group C organisms. Paradoxically, an exuberant IgA response to meningococci may actually enhance the development of systemic disease. When a large proportion of induced anticapsular antibodies are of the IgA class, complement-mediated immune bacteriolysis by IgM is blocked, thus enhancing susceptibility to invasive disease. This peculiar immunologic phenomenon is transient, lasting only a few days following asymptomatic nasopharyngeal acquisition of N. meningitidis or closely related organisms.
In addition to antibody, an intact complement system is also a component of host defense against invasive meningococcal disease. Studies of extreme phenotypes have identified genetic correlates of increased susceptibility in the complement system (49). Recurrent or chronic neisserial infections have been associated with rare isolated deficiencies of late complement components (C5, C6, C7, or C8, and perhaps C9), occasionally in concert with failure to produce antimeningococcal antibodies. Recurrent episodes of neisserial infections may occur in these patients without an increase in susceptibility to other pathogens (50), and screening for complement defects is useful in patients with these syndromes. Complement deficiency also appears to predispose to meningitis caused by nongroupable meningococci and Neisseria-related bacteria (i.e., Moraxella and Acinetobacter species) (51). In addition, complement deficiency or depletion of early components (C1, C3, or C4) because of an underlying disease such as nephrotic syndrome, hepatic failure, systemic lupus erythematosus, presence of C3 nephritic factor, or multiple myeloma may predispose to the first episode of invasive meningococcal disease. An association between homozygous C4b deficiency (present in approximately 3% of the population) and the development of childhood meningitis was demonstrated. Up to 30% of patients with invasive meningococcal syndromes display decreased complement function. Properdin deficiency, or dysfunction with normal concentrations, also predisposes to meningococcal infections; this defect is reversible by vaccination (52). The mortality associated with meningococcal meningitis in patients with complement component deficiencies is actually lower than the general population (3% versus 19%, respectively). However, since invasive meningococcal infection occurs in 39% of patients with deficiency of terminal complement components and about 6% of those with properdin deficiency, a screening test (e.g., CH50) has been suggested (53) for all individuals with serious meningococcal disease, with further consideration for determination of specific complement and/or properdin concentrations if documented. Asplenic states increase the risk for serious infections by encapsulated organisms, especially Hib or S. pneumoniae but also meningococci.
In genetic case–control studies, invasive meningococcal disease was associated with the IL1RA + 2018T → C polymorphism, with susceptibility increased in homozygous IL1RA + 2018C carriers (49). CFH, SFTPA2, CEACAM3, and CEACAM6 polymorphisms were significantly associated with an increased susceptibility to meningococcal disease, whereas other CEACAM6 and SFTPA2 polymorphisms showed a protective effect (49). A genome-wide association study identified variants in the CFH region associated with host susceptibility to meningococcal disease (54). Further studies are needed to confirm these associations before a definite conclusion can be drawn on their role because of the limited sample size and lack of correction for multiple testing.
Although all the aforementioned factors (particularly recent colonization with a pathogenic strain in a nonimmune host) undoubtedly contribute to the pathogenesis of meningococcal disease, the precise determinants contributing to overt clinical illness (as opposed to the usual outcome of asymptomatic carriage) are poorly defined. Even during epidemics, only 1 in 1,000 to 5,000 colonized patients develop disease (43). Various predisposing factors, including crowding, lower socioeconomic status, and poor general health, have been proposed to explain the increased incidence among blacks in the United States and among alcoholics in Finland or Alaska (41). However, the influence of such conditions (e.g., overcrowding) has not been supported by studies in Nigeria. An antecedent viral infection has been suggested as another predisposing factor, because approximately one third of meningococcal cases follow symptoms referable to the upper respiratory tract. An outbreak of meningococcal disease followed a large influenza epidemic in Texas in 1981, and simultaneous outbreaks of meningococcal and influenza A2 infections have been described in institutional settings. Although meningococcal pneumonia may complicate influenza (e.g., the 1918 to 1919 pandemic), the role of viral infections in the enhancement of meningococcal dissemination is unproved (22).
The time from nasopharyngeal acquisition to bloodstream invasion is short (usually approximately 10 days). The incubation period may also be short, because “secondary” cases commonly occur within 1 to 4 days of the index case. Once the organism is bloodborne, more than 90% of meningococcal disease is manifested as meningitis and/or meningococcemia.
Although a single case of meningococcemia or meningitis in a college student engenders alarm, invasive disease due to this organism is no more common in such students when compared with nonstudent age-matched 18- to 22-year-old controls but is increased threefold in freshmen living in dormitories, a target of some vaccine recommendations. Active or passive smoking and binge alcohol consumption also appear to increase the risk of meningococcal disease in this age-group. The first quadrivalent meningococcal conjugate vaccine against serogroups A, C, Y, and W135 was licensed in January 2005 in the United States for routine use, starting at age 11 to 12 years; recent recommendations are for one vaccine in this age-group, with a booster dose given at age 16 years (55,56). A two-dose primary series administered 2 months apart is recommended for those age 2 to 54 years with persistent complement component deficiency or functional or anatomic asplenia and for adolescents with HIV infection. A two-dose primary series is also recommended for children 9 to 23 months of age at increased risk for invasive meningococcal disease (57). Lack of a vaccine against serogroup B meningococcus is a significant issue, although recent investigations of a multicomponent serogroup B vaccine (4CMenB) demonstrated that this vaccine was immunogenic (58–61); 84% to 100% of infants who were administered the vaccine developed bactericidal antibodies.
Streptococcus pneumoniae
Although pneumococcal meningitis occurs in all age-groups, pneumococci remain the most common cause of bacterial meningitis in adults. The annual incidence of pneumococcal meningitis has remained relatively stable in the United States for the past three decades until recently with introduction of the heptavalent pneumococcal conjugate vaccine (see later discussion). In seven studies analyzing data from diverse geographic areas in this country from 1959 to 1978, the annual incidence was 0.3 to 2.3 per 100,000 population, with a mean of 1.3 per 100,000. An identical infection rate of 1.3 per 100,000 persons annually was recorded from the Oklahoma City area in 1984 and extrapolated from several states and Los Angeles County in a relatively recent (1995) national survey conducted by the Centers for Disease Control and Prevention (CDC) (62,63); higher rates of invasive pneumococcal disease were reported at the extremes of age (see later discussion), in men and boys, and among blacks and American Indians as compared with whites. Nearly identical incidence figures (1.2 to 1.4/100,000 population annually) were reported from the Göteborg, Sweden area from 1975 through 1980 (64) and from Örebro county in Sweden (1.0/100,000 population annually) from 1981 through 1992 (65). Pneumococcal meningitis was, again, more common in men and boys, and most cases occurred from December through May. However, higher incidence rates have been reported from other areas. For example, surveillance studies from 1980 to 1986 among the Alaskan native population in the Yukon-Kusko-Kurin delta region of southwestern Alaska documented an extremely high frequency of invasive pneumococcal disease; the annual rate for pneumococcal meningitis was 13.2 per 100,000 persons overall (66). Perhaps more importantly, the annual incidence rate was 216 per 100,000 children younger than 2 years—18 times higher than that reported from Sweden (64) and 36- to 37-fold greater than United States rates derived from both passive and active surveillance (7,62,66). These rates of bacteriologically confirmed invasive pneumococcal disease were the highest reported for any population worldwide. Although most cases of invasive pneumococcal disease occurred during the Arctic summer, pneumococcal meningitis cases clustered in the winter (66), as described in other reports (7).
The risk for pneumococcal meningitis is age dependent, with increased incidence rates occurring at the extremes of age. For example, the number of cases per 100,000 persons per year in the Göteborg, Sweden area for 1970 through 1980 were as follows: 12.0 for infants younger than 12 months old, 0.4 to 0.9 for children and adults 2 to 39 years old, 1.2 to 1.6 for persons 40 to 70 years old, and 2.2 for those older than 70 years of age (64). The dramatic incidence among Alaskan native children younger than 2 years of age (216/100,000 annually) are noted earlier in this chapter. Similarly, the annual incidence rates for all invasive pneumococcal infections (including bacteremic pneumonia) in the Oklahoma City area in 1984 were 97 per 100,000 for infants younger than 1 year of age and 87 per 100,000 for elderly adults older than 80 years of age.
As with disease caused by the other major meningeal pathogens, pneumococcal meningitis follows recent nasopharyngeal colonization by a virulent strain. The rate of asymptomatic carriage varies with age, environment, geographic locale, and the presence of an upper respiratory tract infection. Pneumococci have been isolated from the upper respiratory tract of 5% to 70% of normal adults; approximately 25% acquire a new strain annually. Carriage rates decline with age (30% to 35% for children ages 6 to 11 years and 18% to 19% in adults) and are higher in closed populations (e.g., 27% to 58% in schools and orphanages, 50% to 60% in closed military populations). The duration of pneumococcal carriage varies from weeks to months and is longer in children than in adults. Most carrier strains in the normal population are of higher numbered capsular types and only infrequently are associated with invasive disease (see later discussion). Carriage is prolonged in individuals with low serum antibody concentrations against the homologous capsular type before colonization. Spread of this organism within the family unit is influenced by crowding, the season (greater in fall and winter), and the presence of pneumococcal disease (particularly pneumonia and otitis media). The precise relationship between pneumococcal carriage and the development of protective immunity is poorly defined. More than 50% of children develop a rise in type-specific antibody concentrations following colonization; this is rarely observed in adults, perhaps because of the relatively high antibody concentrations already present in adults. Nevertheless, otitis media often occurs in colonized infants despite the presence of type-specific antibodies. Antibody concentrations generally decline with time despite persistent carriage of a given strain (67). In addition, antibody responses to different capsular types vary considerably and are generally poor in infants younger than 2 years of age. Specific antibody responses tend to be higher after intermittent periods of nasopharyngeal carriage than after continuous ones. The antibody response after pneumococcal colonization, its influence on subsequent disease, and the impact of other environmental antigens require further study.
Studies of extreme phenotypes among patients with invasive pneumococcal disease have identified genetic correlates of increased susceptibility in the complement system and the signalling cascade after toll-like receptor pathways (49). Case–control studies showed that invasive pneumococcal disease was associated with certain MBL and C3-variant genotypes (49,68).
Several factors predispose to pneumococcal meningitis (69,70):
■ Pneumonia (15% to 25% of patients)
■ Otitis media
■ Sinusitis
■ CSF fistulas, leak
■ Head injury
■ Cochlear implants with positioners
■ Alcoholism, cirrhosis
■ Sickle cell disease, thalassemia major
■ Other splenic disease
■ Wiskott-Aldrich syndrome
■ Multiple myeloma
Pneumonia coexists much more commonly with pneumococcal meningitis than with the other two major pathogens. Acute otitis media is seen in approximately 30% of patients with pneumococcal meningitis; acute sinusitis may also be an important antecedent event. Pneumococci are the most common cause of recurrent meningitis in the setting of CSF leaks. Recent or remote head trauma is found in about 10% of patients with pneumococcal meningitis. Alcoholism, cirrhosis, and spontaneous bacterial peritonitis are underlying disorders in approximately 20% to 35% of patients with this disease. Pneumococci are the most common cause of meningitis in children with sickle cell anemia and commonly cause meningitis in other asplenic states or in the setting of primary or acquired immunodeficiencies (71). S. pneumoniae causes 87% of pyogenic meningitis cases in sickle cell disease; cases in the 2- to 3-year-old group occur at a rate of 12 per 1,000 patient-years. The risk for pneumococcal meningitis in this age-group of children with sickle cell anemia is increased 36-fold as compared with that observed in control groups of black children, and it is increased 314-fold over that observed in whites. Pneumococcal meningitis also occurs with increased frequency in persons with the Wiskott-Aldrich syndrome, thalassemia major, childhood nephrotic syndrome, multiple myeloma, and chronic lymphocytic leukemia. Defects in immunoglobulin concentration or function, as well as poor alternative complement pathway–mediated opsonization of pneumococci, are common in many of these disorders. Pneumococcal meningitis is approximately 450-fold more common in patients with acquired immunodeficiency syndrome (AIDS) when compared with the general population (71), often despite prophylaxis with trimethoprim-sulfamethoxazole (TMP-SMX) to prevent other opportunistic infections. In one study of 352 episodes of community-acquired pneumococcal meningitis in adults, 245 (70%) were associated with an underlying disorder and the overall in-hospital mortality rate was 30% (72); death in patients younger than 60 years of age was more often caused by neurologic complications; in patients 60 years or older, death was more likely secondary to systemic complications. In children with cochlear implants with positioners who are beyond 24 months after implantation, the incidence of bacterial meningitis was 450 cases per 100,000 person-years compared with 0 cases in children without positioners (73); the updated overall incidence was 189 cases per 100,000 person-years, with most cases caused by S. pneumoniae (74). Outbreaks of pneumococcal meningitis have also been described during African outbreaks of meningococcal meningitis (75). In children who develop second episodes of pneumococcal meningitis, screening for congenital immunoglobulin deficiencies should be performed (53).
The rates of pneumococcal meningitis have decreased in children and adults since introduction of the heptavalent pneumococcal conjugate vaccine (from 1.13 to 0.79 cases per 100,000 between 1998 to 1999 and 2004 to 2005), although the increase in disease caused by serotypes not in the vaccine (19A, 22F, and 35B) is concerning (76). The Advisory Committee on Immunization Practices now recommends use of the 13-valent pneumococcal conjugate vaccine (77), which not only offers protection against the serotypes in the heptavalent vaccine (4, 6B, 9V, 14, 18C, 19F, 23F) but also protection against additional serotypes (serotypes 1, 3, 5, 6A, 7F, 19A).
ETIOLOGY
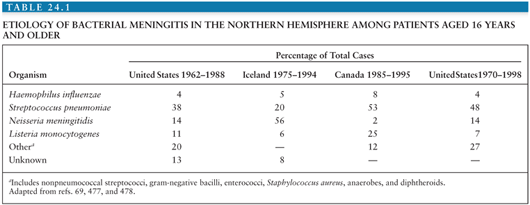
In a review of 296 episodes of community-acquired meningitis in adults reported from the Massachusetts General Hospital from 1962 through 1988, the most common pathogens were S. pneumoniae (37%), N. meningitidis (13%), and L. monocytogenes (10%) (78).
The epidemiology of bacterial meningitis changed dramatically in areas that have embraced Hib conjugate vaccines starting in the late 1980s. The influence of these vaccines from the four largest and most recent surveys conducted in the United States (7,62,79) is shown in Table 24.2. Hib was responsible for 45% to 48% of cases from 1978 through 1986 but only 7% of cases in a surveillance study (62) conducted in 1995 in laboratories serving all acute-care hospitals in 22 counties from four states (>10 million population). The incidence of Hib meningitis declined from 2.9 to 0.2 per 100,000 population from 1986 to 1995, respectively. The median age of patients with bacterial meningitis rose from 15 months to 25 years during this interval, perhaps the most dramatic change in the epidemiology of any bacterial infection in recent decades. As a result, approximately 70% of bacterial meningitis in the United States is due to pneumococci or meningococci. In the early pivotal trial in California, invasive (e.g., bacteremia and meningitis) pneumococcal disease was virtually eliminated in infants vaccinated with the heptavalent conjugate vaccine at 2, 4, and 6 months of age. Although targeted to children, recent data support a “herd” immunity phenomenon, similar to that experienced with Hib conjugate vaccines, leading to a decline in pneumococcal bacteremia and/or meningitis in the population (including adults) following introduction of conjugate pneumococcal vaccine in infancy. In another surveillance study among residents in eight surveillance areas representing 17.4 million persons from 1998 to 2007, the impact of the heptavalent pneumococcal conjugate vaccine was appreciated in which the incidence of meningitis caused by vaccine serotypes decreased from 0.61 cases per 100,000 population in 1998 to 1999 to 0.05 cases per 100,000 population in 2006 to 2007, although the number of cases of bacterial meningitis caused by nonvaccine serotypes increased by 61% (5); the mean age of all patients with meningitis increased from 30.3 years in 1998 to 1999 to 41.9 years in 2006 to 2007. However, despite the declining incidence of bacterial meningitis in the United States, the overall case-fatality rates did not change significantly (15.7% in 1998 to 1999 compared with 14.3% in 2006 to 2007; p = .50).
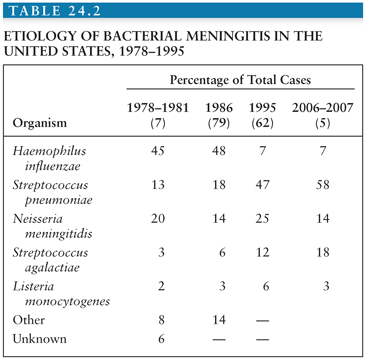
As shown in Table 24.1, the responsible organisms for community-acquired bacterial meningitis in adults older than 16 years of age are somewhat different, but S. pneumoniae, N. meningitidis, and L. monocytogenes predominate. Similar recent (e.g., after 2000) surveys for resource-limited settings are lacking and sorely needed to guide vaccine strategies in this age-group.
In the following sections, each of the major meningeal pathogens is considered, including relevant classification schemes. In addition, the potential virulence characteristics of each organism are briefly discussed.
Haemophilus influenzae
Haemophilus species are small, gram-negative, pleomorphic coccobacilli. They are facultative anaerobes that grow best anaerobically with 5% to 10% CO2 on blood-enriched media. H. influenzae requires both X factor (hematin) and V factor (nicotinamide adenine dinucleotide, nicotinamide adenine dinucleotide phosphate, or nicotinamide nucleoside) for growth under aerobic conditions and is nonhemolytic. Chocolate agar is most commonly employed for the initial isolation of H. influenzae.
H. influenzae strains are either encapsulated (typeable) or unencapsulated (nontypeable). Encapsulated strains are classified into six types, designated a through f, according to specific reactions with antisera directed against epitopes on capsular antigens. Methods for grouping include counterimmunoelectrophoresis, latex particle agglutination of culture supernatants, immunofluorescence, and the production of halos surrounding colonies on media containing antisera. Nearly all invasive H. influenzae infections are caused by serotype b (Hib). The capsule is a repeating polymer of PRP, an important virulence determinant of this organism (see later discussion). Hib also contains a lipooligosaccharide (here, designated lipopolysaccharide [LPS] for convenience) in the outer membrane, an additional virulence determinant. Hib strains have been further classified into subtypes based on electrophoretic mobility differences among Omps. Although the pathogenic role of these Omps is uncertain, subtype analysis is useful for epidemiologic studies. For example, in a survey of 256 invasive isolates from 22 states representing a variety of clinical settings, about 70% of cases were caused by strains of three subtypes (1H, 2L, and 3L) among the 21 Omp subtypes identified. In contrast, 84% of 80 invasive isolates studied in the Netherlands had the same Omp subtype pattern (type 1, identical to subtype 3L in the Granoff classification system), and no strains of subtype 1H, 1L, or 2H were found.
It has been recognized, largely on the basis of multilocus enzyme electrophoretic analysis by Musser et al. (80), that the natural populations of Hib from widely divergent geographic areas are clonal as a consequence of infrequent recombination of chromosomal genes. For example, 32 distinct multilocus enzyme genotypes, referred to as electrophoretic types (ETs), were apparent among 177 U.S. isolates by analysis of 16 metabolic enzymes, but 73% of invasive disease episodes were caused by strains belonging to only three ETs. In the largest and most comprehensive analysis (80), 2,209 encapsulated H. influenzae strains (including 1975 Hib) from 30 countries on six continents collected over a 40-year period were studied by multilocus electrophoresis of 17 chromosomally encoded metabolic enzymes, Omp subtyping, and the pattern of restriction fragment length polymorphism in the cap region (the chromosome region responsible for capsular expression). On the basis of allele profiles at the enzyme loci, 280 distinct ETs in two phylogenetic divisions were identified: the population structure is definitely clonal. Currently, nearly all invasive disease worldwide is caused by nine clones of Hib. One genetically distinct clone complex occurs with considerable frequency worldwide, but marked geographic variation occurs for other clones or clone families. Based on an extensive analysis, it appears that this distribution of clones on an intercontinental scale is largely accounted for by the patterns of racial/ethnic composition and historical demographic movements of the human host populations (80).
Neisseria meningitidis
Neisseria species are non–spore-forming, nonmotile, oxidase-positive, gram-negative cocci (measuring approximately 0.8 × 0.6 µm) that usually appear as biscuit- or kidney-shaped diplococci on smears of infected fluids. Because other organisms (e.g., Moraxella species) are similar morphologically, identification rests on biochemical or immunologic techniques. All Neisseria species are oxidase positive, and sugar fermentation reactions are usually sufficient for speciation within the genus. Gonococci ferment glucose (but not maltose or lactose) to acid, whereas meningococci ferment both glucose and maltose to acid. N. lactamica, a related organism occasionally present in throat cultures, ferments glucose, maltose, and lactose. Maltose-negative variants of meningococci have been isolated; these strains may be differentiated from gonococci by fluorescent antibody tests, coagglutination tests, or electrophoretic analysis of hexokinase isoenzymes. These strains usually acquire the ability to ferment maltose on subculture; true maltose-negative variants are rare, but this property is genetically linked to sulfadiazine resistance. Meningococci grow rapidly on blood, chocolate, gonococcal, or enriched Mueller-Hinton agar in a moist, 3% to 10% CO2 environment at 35°C to 37°C. A modified Thayer-Martin medium is employed for meningococcal isolation from contaminated sites, as in detection of the carrier state. The transoral approach for obtaining specimens is more practical and is at least as sensitive as older transnasal approaches for carrier detection. Because this organism is susceptible to drying and chilling, all specimens should be inoculated promptly.
As in other gram-negative bacteria, the ultrastructural characteristics of meningococci are complex. The surface components include capsular polysaccharide, fimbriae or pili, LPS, and Omp; several of these structures are important virulence determinants. Meningococci are classified by serogroups based on structural differences among capsular polysaccharides and agglutination reactions with specific antisera, and they are further defined by serotypes based on analysis of the Omp. Capsular polysaccharide detected by positive agglutination with reference antisera is uniformly present among invasive isolates, but 20% to 50% or more of carrier strains are unencapsulated (nongroupable). The serogroups have important epidemiologic and prevention-related implications. Thirteen serogroups are recognized (43), designated as follows: A, B, C, D, H, I, K, L, X, Y, Z, 29E, and W135. Most meningococcal disease is caused by organisms in serogroups A, B, C, and Y, although the proportion of cases caused by serogroup W135 is increasing. Although serious outbreaks of serogroup A, B, or C disease have occurred worldwide, most focal outbreaks and endemic disease in many countries are caused by serogroups B and C. For example, in the United States from 1975 to 1980, the distribution of serogroups among 12,980 cases was as follows: B, 56%; C, 19%; Y, 11%; W135, 10%; and A, 3%. Similar figures were reported for the period 1978 to 1981 (7). Serogroup W135 disease, especially among adults, has been increasing in the United States and apparently elsewhere (e.g., Senegal) since 1981. Group B organisms, especially B:15:P1.16, have emerged as important pathogens in northern Europe, causing serious local outbreaks peaking in the 10- to 20-year-old group. The continued high prevalence of serogroup B meningococcal disease has important implications because of the lack of an effective, widely available vaccine against this serogroup. An outbreak of serogroup C disease occurred in California in 1987 (81). Serious outbreaks caused by serogroup A continue in Nepal, Saudi Arabia, Chad, Sudan, Burkina Faso, and elsewhere. Approximately 40,000 cases of serogroup A disease occurred in Ethiopia in the spring of 1989 (Fig. 24.1).
There have been increases in the incidence of serogroup C disease in North America to equal or surpass the incidence of disease caused by serogroup B (42). From 1985 through 1992, a clonal serogroup C meningococcal strain (designated ET-15 and defined by multilocus enzyme electrophoresis) was associated with an increase in both the incidence and the mortality of meningococcal disease in Canada (82). This and four other closely related clonal strains were also implicated in a marked increase in outbreaks of serogroup C meningococcal disease in the United States (83). These studies suggested the emergence of a clonal group of virulent serogroup C meningococcal strains in North America, leading to an increase in the rate of meningococcal disease in some regions, an increased number of outbreaks, and a higher case-fatality rate (84).
In an analysis from the United States (85), part of an active and ongoing laboratory-based population-based surveillance for meningococcal infection from 1992 to 1996, the serogroup distribution for the three most common isolates was as follows: C, 35%; B, 32%; and Y, 26%. Similar results were noted in New York City through 2000 (86). In contrast, serogroup B accounted for 75% of meningococcal isolates in Italy (87). More than 98% of cases of invasive meningococcal disease are sporadic (88). From 1998 to 2007, a total of 2,262 cases of meningococcal disease were reported to the Active Bacterial Core surveillance sites, with an annual incidence of 0.53 cases per 100,000 population (89). The incidence decreased from 0.92 cases per 100,000 population in 1998 to 0.33 cases per 100,000 population in 2007; the incidence decreased to this historic low before the introduction of the quadrivalent meningococcal conjugate vaccine. Infants younger than 1 year of age had the highest incidence (5.38 cases per 100,000 population), although the distribution of serogroups is also different in this population (serogroup B, 3.08 per 100,000 population; serogroup C, 0.53 per 100,000 population; serogroup Y, 1.50 per 100,000 population). A recent outbreak of serogroup C disease was reported in New York City among men who have sex with men (90). During the outbreak of meningococcal disease coinciding with the Hajj pilgrimage in March 2000, the attack rate of W135 disease was 25 cases per 100,000 pilgrims (91); all outbreak-associated isolates of serogroup W135 were members of a single clone of the hypervirulent ET-37 complex, which occurred as the result of expansion of a clone that had been in circulation since 1970 (92). A high incidence of serogroup X cases was reported in Niger (93), representing 51% of 1,139 confirmed cases of meningococcal meningitis in 2006; serogroup X disease also emerged in Togo and Burkina Faso during 2006 to 2010 (94).
Serotypes within a serogroup of N. meningitidis are classified largely on analysis of Omp profiles in the cell envelope. At least 20 serotypes are recognized (95), resulting in a classification scheme that is useful in epidemiologic studies. Physicochemical characterization of the Omps, which might be candidate antigens for vaccines, has led to the designation of five major classes. Class 2 and 3 proteins are the major porins responsible for aqueous channels in the outer membrane. Class 5 proteins are surface exposed and may have a role in virulence, but the function of class 1 and 4 proteins is poorly defined. The serotype designation has important epidemiologic uses and may identify virulence characteristics among meningococci. For example, serotype 2 (2a, 2b) strains are responsible for approximately 50% of serogroup B disease (in which 15 serotypes have been described), followed by serotypes 15 and 9; in contrast, serotypes 4 and 6 have been isolated only from carriers. Serotype 2 is also responsible for about 80% of invasive serogroup C disease and is an important marker for serogroups Y and W135 pathogenic strains as well (95). All serogroup A meningococci, in contrast, are homogeneous with respect to Omp and show no homology to serotypes within other serogroups. Serotype analysis has also been linked to certain clinical characteristics (96). However, this technique is available only in research or reference laboratories. In addition, at least eight immunotypes of meningococci, classified by differences among LPS subtypes, are known to exist and may play a role in pathogenesis and disease expression. LPS immunotype analysis may also be useful in the further characterization of the epidemiology of meningococcal disease (41,43). The recent availability of monoclonal antibodies to detect variations in Omp and/or LPS will improve the resolution of these typing systems for epidemiologic analysis.
In addition to classification by serogroup, serotype, and LPS subtype, many reports have focused on multilocus enzyme electrophoresis for the characterization of the chromosomal genome of isolates and for estimation of the genetic relatedness among strains (97). These studies, similar to the analysis of Hib (80), have identified the clonal nature of N. meningitidis and have been useful for epidemiologic purposes. For example, electrophoretic variation (defined as <17% genetic distance between isolates) in seven metabolic enzymes and two Omp in 423 isolates of serogroup A strains recovered from 23 epidemics or outbreaks occurring in 38 countries on six continents over a 70-year period since 1915 identified 21 “clones” (designated A I-1 through A IV-4) containing 34 ETs (97). This technique has been useful for delineating similarities and differences among isolates from cases and carriers before, during, and after epidemics (98). It has also been useful for tracing movements of epidemic strains geographically over time (81). For example, following an epidemic in Nepal (1983 to 1984), a single clonal complex of serogroup A meningococci (III-1, representing 11 closely related ETs) was introduced into Saudi Arabia in 1987 by Muslim pilgrims traveling to Mecca for the annual hajj (98). The same strain was then introduced into sub-Saharan Africa following the hajj, causing an explosive outbreak in Chad in 1988. An analysis of 109 isolates of serogroup B meningococci in Norway has also revealed differences among carriers and cases (99). Although 78 ETs were identified, 91% of the cases of systemic disease in 1984 were caused by strains from the ET-5–ET-37 complex, whereas these isolates were recovered from only 7% and 9%, respectively, of healthy carriers. The most common clonal complex found among carriers was never isolated from patients with invasive disease, suggesting a low virulence potential for these clones. Two clones, ET-15 and ET-508, have been associated with outbreaks of meningococcal infection in North America (82,83,100). Clonal analysis will undoubtedly continue to contribute important information on the epidemiology of meningococcal infection, and it may prove useful in an analysis of virulence properties. As noted earlier, serogroup W135 has been circulating, particularly among pilgrims to Mecca during the hajj since 2000 in Africa, the Middle East, and worldwide (attack rate ≥25/100,000); all isolates were members of a single hypervirulent ET-37 complex (91,92). This occurred through the expansion of a clone that had been circulating since 1970. Vaccination with quadrivalent vaccine in hajj pilgrims from several areas (e.g., North America, Western Europe) appears to have reduced transmission of W135 meningococci to close contacts upon return (101,102).
The putative meningococcal virulence characteristics are as follows:
■ Capsular polysaccharide
■ Pili
■ IgA protease
■ LPS (endotoxin)
■ Omps
■ Outer membrane vesicles, or blebs
■ Metabolic pathways (e.g., iron)
As noted earlier, all isolates from invasive infections are encapsulated (serogroup positive), but 20% to 90% of isolates from carriers are unencapsulated (nontypeable). The capsular polysaccharide appears to be essential for meningococcal virulence, probably because of its antiphagocytic properties that allow the organism to escape host phagocytic clearance mechanisms within the bloodstream and/or CSF. Pili are protein surface appendages composed of identical pilin-repeating subunits. Pili from meningococci and gonococci are morphologically and chemically similar. Cross-reacting antibodies bind to a short peptide sequence (residues 69 to 94) of gonococcal pili that is essential for receptor-binding function. Fresh meningococcal isolates from carriers and cases contain 7 to 40 pili per diplococcus. Pili are important mediators of meningococcal adhesion to human nonciliated columnar nasopharyngeal epithelial cells, an important early step in the development of the carrier state. Extracellular proteases that cleave the IgA1 heavy chain in the hinge region are elaborated by pathogenic Neisseria species (i.e., meningococci and gonococci). Although the role of IgA proteases in the pathogenesis of disease is controversial, these enzymes are produced by only a few organisms (e.g., N. meningitidis, Neisseria gonorrhoeae, H. influenzae, S. pneumoniae, and Streptococcus sanguinis), many of which are important meningeal pathogens and may promote invasion at the pharyngeal portal of entry. Meningococcal LPS resembles H. influenzae LPS by a lack of the O-antigenic polysaccharide side chains found in enteric bacilli despite a smooth phenotype and proven virulence. LPS is clearly important in the genesis of an array of the clinical manifestations of meningococcemia and/or meningitis (see later discussion). Although the specific role for Omp in meningococcal virulence is unclear, these organisms release substantial amounts of cell surface material (in the form of outer membrane vesicles containing Omp and LPS) during growth in vitro and in vivo in the absence of cell lysis, a process exacerbated by antimicrobial agents. These outer membrane vesicles, or blebs, represent relevant vehicles for central nervous system (CNS) tissue damage during meningococcal infection (see later discussion). Tissue invasion may also be facilitated by the ability of meningococci to obtain iron from transferrin.
Streptococcus pneumoniae
Pneumococci are non–spore-forming, nonmotile, small (approximately 0.8 µm), gram-positive streptococci that typically appear as lancet-shaped diplococci with the tapered ends in juxtaposition in clinical specimens. They tend to associate in pairs rather than in short chains, although the latter morphology is facilitated by broth culture. Pneumococci are facultative anaerobes that flourish in a variety of supplemented artificial media. Optimal growth occurs in various media supplemented with serum or blood and glucose, at a pH level of 7.8 and a temperature of 37°C in an enriched CO2 environment. The organisms are catalase negative and relatively fastidious. Colonies on blood agar are initially dome shaped, but they become umbilicated with time as a result of the activity of autolytic enzymes (L-alaninemuramylamidase). Pneumococci are α-hemolytic (i.e., a greenish discoloration surrounds colonies on blood agar), although β-hemolysis occurs under anaerobic conditions. S. pneumoniae must be separated from other α-hemolytic streptococci in the laboratory; this is usually accomplished with a disk susceptibility test using the unique susceptibility of pneumococci to Optochin (ethylhydrocupreine hydrochloride). Pneumococci, but not other streptococci, are also sensitive to bile or bile salts (e.g., 10% deoxycholate), but the bile solubility test is now rarely performed in hospital laboratories.
Pneumococci are classified within serotypes on the basis of antigenic differences among capsular polysaccharides. These capsular substances are complex polysaccharides that form hydrophilic gels on the surface of the organism. At present, at least 90 serotypes have been identified, classified by two systems of nomenclature (American and Danish). Capsular polysaccharides are identified by agglutination; the Neufeld quellung reaction, characterized by capsular swelling and increased refraction in the presence of antisera, also useful for identifying pneumococci in clinical specimens (e.g., CSF); precipitation; and counterimmunoelectrophoresis. It is important to emphasize that cross reactions exist between individual pneumococcal serotypes, as well as with other bacteria (e.g., E. coli, Klebsiella pneumoniae, Hib, and S. sanguinis). For example, serotype 14 pneumococcal capsular polysaccharide cross-reacts with type III group B streptococci and with certain human ABO blood group isoantigens.
Capsular polysaccharide is essential for pneumococcal virulence, and a few serotypes are associated with most invasive infections. Encapsulated organisms (smooth colonies on agar) are virulent for humans and experimental animals, whereas unencapsulated (rough colonies on agar) strains are avirulent. Capsular polysaccharide enhances virulence through its antiphagocytic properties, as in Hib and meningococci.
Of the more than 90 known pneumococcal serotypes, only a few (usually the lower number types rather than the higher number types commonly found in the carrier state) account for most invasive pneumococcal infections. The predominant capsular types were types 1, 2, and 3 in the preantibiotic era. A different pattern has emerged in the past 40 years, and it differs between adults and children. A few capsular types cause the majority of bacteremic cases among adults: types 1, 3, 4, 7, 8, 9, 12, and 14, and less commonly types 6, 18, and 19 (these are not listed in rank order). Approximately 65% of cases are caused by eight serotypes, although no single serotype predominates among bacteremic adults. Capsular types 14, 6, 18, 19, 23, 1, 4, and 9 cause approximately 85% of serious infections in children, a pattern different from that observed in adults. Perhaps more important with respect to this discussion, there is a very strong correlation between serotypes causing pneumonia (or bacteremia) and those responsible for pneumococcal meningitis (69). For example, 76% to 86% of blood and CSF isolates were included among the 14 serotypes represented in the original pneumococcal vaccine. However, marked geographic variations exist among pneumococcal serotypes causing invasive disease, and the serotype distribution may change over time in a given locale. Furthermore, some serotypes may be more virulent than others and associated with less favorable outcomes, including death. Older studies of pneumococcal meningitis in the 1950s (summarized by Scheld [69]) noted an increased mortality with disease caused by serotypes 2, 8, and 12. Although type 12 caused only 2.5% of cases, it was responsible for 30.7% of the deaths. Similarly, serotype 6 was associated with four of eight deaths in a more recent survey.
The role of other cell surface components (such as C-polysaccharide antigens, cell wall antigens, M- or R-protein antigens) and putative toxins (such as hemolysin, neuraminidase, purpura-producing principle) in the pathogenesis and pathophysiology of pneumococcal meningitis remains poorly defined. Nevertheless, CSF inflammation is induced by pneumococcal cell wall and its constituents (particularly lipoteichoic acid) but not by purified pneumococcal capsular polysaccharide (see later discussion).
Gram-Negative Bacilli
Approximately 84% of cases of neonatal meningitis and sepsis attributable to E. coli are caused by strains bearing the K1 capsular polysaccharide antigen; this capsular type serves as a marker of neurovirulence. In addition to the K1 capsule, multiple other potential virulence factors for blood–brain barrier (BBB) traversal and meningitis have been documented in E. coli from phylogenetic groups A, D, and B2 (e.g., O45:K1 and O18:K1 strains) (103).
Aerobic gram-negative bacilli have become increasingly important in patients with bacterial meningitis (104). Beyond the neonatal period, aerobic gram-negative bacillary meningitis occurs in three major clinical settings: head trauma (approximately 30% of cases, especially in conjunction with CSF rhinorrhea or otorrhea); after neurosurgical procedures (approximately 50% of cases); and in association with other conditions (approximately 20% of cases), including strongyloidiasis, gram-negative bacteremia, ruptured brain abscess, or impairment of host defense mechanisms (e.g., steroids or AIDS). Many of these infections are nosocomial in origin, although community-acquired gram-negative bacillary meningitis is increasing in frequency, particularly in the elderly older than 71 years and in debilitated, alcoholic, or diabetic adults (105–107). The most common causes of gram-negative aerobic bacillary meningitis beyond the first month of life include Klebsiella species (about 40% of cases), E. coli (roughly 15% to 30%), and Pseudomonas aeruginosa (about 10% to 20%). In a recent study from Korea of 91 adult patients with nosocomial meningitis, Acinetobacter species accounted for 32.5% of cases (108).
Streptococcus agalactiae
Group B streptococci are the most common cause of invasive neonatal disease in the United States, accounting for approximately 11,000 cases of meningitis and/or bacteremia yearly. The incidence of group B streptococcal neonatal infections has remained relatively stable in this country until recently (109). The use of antimicrobial agents (e.g., ampicillin) in pregnant women with vaginal colonization with group B streptococci at the time of delivery has led to a decline in the incidence of neonatal invasive group B streptococcal disease, perhaps, as suggested by one study, with an increase in ampicillin-resistant E. coli early-onset sepsis in low-birth-weight infants (110). However, it appears to be increasing in frequency in the developing world.
Group B streptococci are classified into six main serotypes (designated Ia, Ib/c, Ia/c, II, III, and IV) based on the expression of type-specific capsular polysaccharide antigens and various surface proteins as additional antigenic markers (111); additional candidate serotypes are under evaluation. Although all serotypes have been isolated from neonates with invasive disease, type III is responsible for the vast majority of meningeal infections, suggesting a high virulence potential and/or CNS tropism for this serotype. The chromosomal genetic diversity of S. agalactiae was studied and the clonal nature of the native bacterial populations was again demonstrated (112). A collection of 128 isolates representing all six serotypes, including 44 type III isolates from invasive episodes (18 recovered from CSF), were subjected to multilocus enzyme electrophoresis, an analysis based on electrophoretically demonstrable allelic profiles at 11 metabolic enzyme loci, all encoded at the chromosome level. Nineteen distinct ETs were identified in two primary phylogenetic divisions, each representing a multilocus clonal genotype. A single ET (ET-1), comprising 40 isolates of serotype III group B streptococci, formed the first phylogenetic division. These strains produced greater amounts of neuraminidase and were more virulent than the other type III isolates found in several of the 18 ETs in the second division. This newly evolved clone (ET-1) is responsible for most invasive disease episodes caused by group B streptococci type III in the United States (112). Overall, approximately 52% of group B streptococcal meningitis cases in the United States occur during the first month of life. In one recent review of 444 cases of neonatal bacterial meningitis over a 7-year period, group B streptococcus was the most common etiology in early-onset (occurring between day 0 to 4 of birth) and late-onset (occurring between day 5 and 28 of birth) disease, responsible for 77% and 50% of cases, respectively (113). In the United States, the overall mortality rate ranges from 7% to 27%. Survivors of group B streptococcal meningitis also have substantial long-term morbidity (114), indicating the need for ongoing developmental follow-up and the development of preventive strategies (see later discussion).
In addition to the common conditions of neonatal meningitis and postpartum fever and/or bacteremia in parturient women caused by group B streptococci, these organisms also cause serious infections in adults, including meningitis (115,116). Risk factors in adults include age older than 60 years, diabetes mellitus, parturition, cardiac disease, collagen-vascular diseases, malignancy, alcoholism, hepatic failure, renal failure, corticosteroid therapy, decubitus ulcers, neurogenic bladder, previous stroke, and AIDS. No underlying illnesses were found in 43% of patients in one review (116).
Listeria monocytogenes
L. monocytogenes remains an important cause of neonatal meningitis; the source is the genital tract or subclinical infection of the mother. Although L. monocytogenes may cause meningitis in normal adults, most patients are diabetic, alcoholic, elderly, or immunosuppressed. L. monocytogenes had been the major cause of bacterial meningitis among renal transplant recipients, but this is decreasing in frequency as a result of the use of TMP-SMX prophylaxis.
L. monocytogenes is widespread. Although clusters of nosocomial cases and focal outbreaks are reported, most cases of human listeriosis are sporadic. The incidence of L. monocytogenes infections is difficult to quantitate. Many countries in northern Europe have reported annual incidence figures of approximately 2 to 3 per million. After a large (142 cases) food-borne outbreak in Los Angeles County, California, in 1985, mandatory reporting of L. monocytogenes isolates by clinical laboratories was instituted. During the first year of active surveillance, 94 cases of listeriosis were reported (117), for an annual crude incidence of 11.7 per million persons, similar to figures (11.3 per million annually) reported from France in 1984. Listeriosis is undoubtedly underreported.
Approximately one third of cases in the United States are in neonates and/or their mothers (39% in the Los Angeles survey) (117). The proportion of perinatal infections in Europe is higher. Among the nonperinatal cases, various risk factors for listeriosis were identified, including immunosuppression as a documented history of steroid ingestion or chemotherapy (35% of cases, the single most important risk factor); age older than 75 years; renal disease; cancer; alcoholism and/or cirrhosis; and AIDS. Nevertheless, serious Listeria infections, including meningitis, remain uncommon in patients with AIDS (118,119), but diagnosis of Listeria meningitis in anyone younger than 50 years of age should prompt testing for HIV, if not done previously. Of the nonperinatal cases, only 2 of 57 had no definable underlying risk factor; 21 of 57 had meningitis (117). As stated earlier, L. monocytogenes remains a distinctly unusual cause of meningitis in developing countries. Listerial infection is most common in infants younger than 1 month of age (up to 10% of cases), adults older than 60 years of age, alcoholics, cancer patients, those receiving corticosteroid therapy, and immunosuppressed adults (e.g., renal transplant recipients) (120–122). In one recent study, patients with chronic lymphocytic leukemia had a greater than 1,000-fold risk of acquiring listeriosis (123). Other predisposing conditions include diabetes mellitus, liver disease, chronic renal disease, collagen-vascular diseases, pregnancy, and conditions associated with iron overload. Listeria meningitis has also been reported with use of anti–tumor necrosis factor-α (TNF-α) agents, such as infliximab in patients with Crohn disease (124) and ulcerative colitis (125), and etanercept in a patient with adult Still disease (126). Meningitis can also occur in immunocompetent children and adults (127,128). In nonperinatal cases, the route of transmission is often unknown. At least 1% of normal individuals excrete the organism in their stools, but contacts of symptomatic patients have much higher excretion rates (approximately 25%). Nevertheless, the true carriage rate, its duration, and its relationship to invasive disease are poorly defined. Although often considered a zoonosis, most patients do not report animal exposure. Reports have emphasized food-borne transmission of L. monocytogenes, a route of transmission that accounts for the overwhelming majority of sporadic cases. Many foods have been implicated, including coleslaw, Mexican-style cheese, raw vegetables, seafood, pasteurized milk, Swiss cheese, raw hot dogs, undercooked chicken, alfalfa sprouts, cantaloupe, diced celery, hog head cheese (a meat jelly made from hog heads and feet), and processed meats, thus pointing to the intestinal tract as the usual portal of entry (120,122,129–134). In one outbreak, 57 cases of listeriosis occurred in western Switzerland in association with the consumption of soft cheese (135); 40% of these cases were meningitis and 39% were meningoencephalitis. Some studies report a higher frequency of listeriosis in summer, opposite to the seasonal pattern seen with most other forms of bacterial meningitis. However, the incidence of invasive listeriosis has been decreasing, likely a result of a decrease in the prevalence of L. monocytogenes contamination of ready-to-eat food (136); this has been associated with a decrease in nonperinatal listeriosis-associated deaths (137).
L. monocytogenes is a gram-positive, non–spore-forming, catalase-positive, aerobic rod that may be difficult to culture on initial isolation but that, once grown, passes readily on a variety of laboratory media. The organisms may appear coccoid on Gram stains of clinical specimens, particularly CSF, and are often mistaken for pneumococci. More importantly, L. monocytogenes resembles diphtheroids and may thus be dismissed as a “contaminant,” a grave error. The presence of α-hemolysis and a characteristic tumbling motility at room temperature are used to separate L. monocytogenes from similar diphtheroid-like organisms. A hemolytic and cytolytic toxin (listeriolysin 0) of 52 kd appears essential for virulence; the toxin is expressed under conditions of low pH and low iron concentration and may facilitate phagolysosomal disruption and growth within mononuclear phagocytes. At least 11 serotypes are recognized, but more than 90% of invasive infections are caused by three serotypes: Ia, Ib, and IVb. The rate of unfavorable outcome among adults with Listeria meningitis was recently found to increase over a 14-year period from 27% to 61%, with the emerging L. monocytogenes serotype ST6 identified as the main factor leading to a poorer prognosis (138).
Staphylococcus epidermidis
Coagulase-negative staphylococci are very rare causes of bacterial meningitis in children and adults, except in the setting of an indwelling CSF shunt, where these organisms are the most prevalent pathogen. Therefore, this group is discussed elsewhere in this volume.
Staphylococcus aureus
Meningitis caused by Staphylococcus aureus is relatively unusual; this organism was responsible for 0.8% to 8.8% of cases in various surveys (139,140). S. aureus is the second most common cause of CSF shunt infections, accounting for 12% to 36% of cases. This organism is also frequently isolated from patients with nosocomial meningitis and is responsible for approximately 20% of such cases. Although secondary meningitis in the setting of infective endocarditis is an uncommon event, most of these infections are caused by S. aureus. Other important associated conditions have been recognized, including head trauma, neurosurgical procedures, various abscesses (cerebral, epidural, oral, abdominal), sinusitis, osteomyelitis, decubitus ulcers, pneumonia, cellulitis, injection drug use, malignancy, and infected intravascular grafts or shunts. Several other predisposing factors have been proposed. In a retrospective review of 28 cases of S. aureus meningitis seen from 1972 to 1982 at three North Carolina teaching hospitals, 22 occurred beyond the neonatal period (140). Among the adult patients (n = 20; mean age 52 years), 45% had an underlying condition (diabetes mellitus, malignancy, renal failure, immunosuppression), 35% had had head trauma or undergone neurosurgery (ventriculoperitoneal shunt, craniotomy), and about 20% developed meningitis in association with endocarditis or a paraspinal infection. Mortality was high (50% in adults), especially when S. aureus meningitis complicated a distant extracranial focus of infection (five of the six patients with purulent meningitis during active endocarditis died) (140). The prognosis for S. aureus infections of CSF shunts is more favorable. In a review of clinical and bacteriologic data from 61 postoperative and 43 hematogenous cases of S. aureus meningitis from Denmark, postoperative cases had a lower mortality rate (18%) than cases resulting from hematogenous spread (56%); hematogenous S. aureus meningitis had a higher mortality rate related to age, presence of shock, and infections with strains of phage type 95 (141). Hospital-acquired cases are often caused by methicillin-resistant strains (142). In one series from 1999 to 2008 (143), S. aureus accounted for approximately 5% of cases of culture-proven bacterial meningitis in adults; since 2005, more than 75% of all cases were caused by methicillin-resistant Staphylococcus aureus (MRSA) and 52% (11 of 21 cases) of hematogenous cases were seen in injection drug users. In a multicenter review of 86 cases of MRSA meningitis in adults (144), the infection was nosocomial in 93% of cases; in those patients with postoperative meningitis, the most common predisposing conditions were the presence of CSF devices, neurosurgery, CSF leaks, and head trauma.
Anaerobic Bacteria
Meningitis caused by anaerobic bacteria is rare, accounting for fewer than 1% of pyogenic cases, except following the intraventricular rupture of a brain abscess. Anaerobic meningitis may be underrecognized because CSF is not routinely cultured anaerobically. Enriched media and proper transport of CSF to the laboratory, which are essential for isolation of anaerobes, are not uniformly performed. Only five cases caused by strict anaerobes were reported among 18,642 patients analyzed by the CDC in one study (7).
Anaerobic meningitis is associated with a variety of clinical conditions, including rupture of brain abscess or extension to the surface of the brain; chronic otitis, mastoiditis, or sinusitis; head trauma; neurosurgical procedures (e.g., craniotomy, laminectomy); congenital dural defects; abdominal trauma or surgery; gastrointestinal disease; head and neck cancer; suppurative pharyngitis; CSF shunts; and immunosuppression (particularly corticosteroid administration) (145,146). Most cases arise from spread of infection secondary to a contiguous focus of disease; anaerobic meningitis rarely complicates bacteremia from a distant extracranial focus.
A variety of bacterial species are responsible for anaerobic meningitis. Only nine cases of Bacteroides fragilis meningitis unaccompanied by a brain abscess had been reported in the modern era through 1987 (147). Seven occurred in premature infants or neonates (median age, 20 days), thereby complicating congenital defects or gastrointestinal disease such as necrotizing enterocolitis (148). Meningitis caused by Fusobacterium species, usually Fusobacterium necrophorum, generally occurs in older children (median age, about 5 years) or adults as a complication of acute or chronic otitis media (148). A variety of anaerobic gram-positive cocci have been isolated in a few cases, particularly peptostreptococci. Meningitis caused by Clostridium species almost always develops following head trauma or a neurosurgical procedure. For example, in a summary of 17 cases caused by Clostridium perfringens (149), only 3 were not associated with CNS trauma or surgery. Although the disease course is highly variable, some cases of clostridial meningitis are characterized by intracranial gas formation, visible on plain skull radiographs or computed tomography (CT); CSF white blood cell (WBC) concentrations exceeding 20,000/mm3; and death within hours of presentation. A few cases of meningitis caused by Actinomyces species (in the absence of brain abscess formation) and Propionibacterium acnes (usually subacute with a predominantly monocytic CSF pleocytosis) have also been reported. In approximately one eighth of patients, the infection is mixed, with anaerobic plus aerobic or microaerophilic organisms recovered from CSF.
Unusual Etiologic Agents
CNS infections caused by higher bacteria (e.g., Mycobacterium species, Nocardia species, Actinomyces species), spirochetes (e.g., Treponema pallidum, Borrelia burgdorferi, Leptospira species), Brucella species, and so on, are discussed elsewhere in this volume. A plethora of bacteria have been documented as the cause of meningitis in isolated case reports or in small numbers of patients, including group A streptococci usually in association with pharyngitis, otitis media, and/or sinusitis (150); nonpneumococcal α-hemolytic streptococci such as Streptococcus mitis (151); enterococci (152,153); Streptococcus gallolyticus; diphtheroids (although P. acnes is an important etiologic agent in patients with CSF shunt and drain infections); N. gonorrhoeae (approximately 30 cases reported in the past 20 years); Neisseria subflava; Gardnerella vaginalis (one case report); many members of the Enterobacteriaceae in addition to E. coli and Salmonella species; Flavobacterium meningosepticum; Haemophilus species other than H. influenzae; and many others. Fewer than 0.5% of adult cases of bacterial meningitis are caused by group C streptococci but may occur in humans after contact with domestic animals (especially horses) or their unpasteurized products (154,155); however, mortality is high, perhaps because of the unpredictable susceptibility of this organism to β-lactam agents.
Despite the frequency with which the viridans streptococci cause bacteremia, they are unusual causes of meningitis (0.3% to 5% of culture-proven cases) (156). Streptococcus salivarius meningitis has been reported following spinal anesthesia (157,158) and myelogram procedures (159), supporting the importance of appropriate infection control practices (i.e., masks, proper aseptic technique, and safe injection practice) in those who perform spinal procedures. Streptococcus suis is the most frequent cause of bacterial meningitis in southern Vietnam and is associated with significant morbidity attributable to hearing loss (160); the pig is the natural reservoir of this microorganism and the main source of human infection. Risk factors for S. suis meningitis include eating “high-risk” dishes (such as undercooked pig blood or pig intestine) popular in parts of Asia, occupational exposure to pigs, and exposures to pig or pork in the presence of skin injuries (161).
Polymicrobial bacterial meningitis, with simultaneous recovery of two or more bacterial species from CSF, is unusual. Mixed infections account for about 1% of bacterial meningitis cases. In a review of 34 series encompassing 11,281 cases of bacterial meningitis, 116 cases (1%) were mixed (162). This condition appears to be evolving in the antibiotic era. Before 1950, nearly all cases occurred in children and were caused by combinations of bacteria commonly associated with meningitis (the three major meningeal pathogens). Since 1950, most cases have occurred in adults, with a wider spectrum of etiologic agents, particularly gram-negative aerobic bacilli. Approximately one third of cases were nosocomially acquired. Common predisposing conditions in the older population affected since 1950 include contiguous foci of infection, tumors in close proximity to the neuraxis (e.g., head and neck), rectal carcinoma, or fistulous communications with the CNS. The mortality rate is 63% for cases occurring after 1950. Several cases of meningitis caused by mixed bacterial and mycobacterial or fungal agents have also been reported.
Simultaneous isolation of viruses and bacteria from the CSF is rare; only seven well-documented cases were reported prior to 1988 (163). However, in a 1-year retrospective review from the Ohio State University published in 1986, 5 (2.8%) of 176 children with CSF enteroviral isolates also had bacterial meningitis (163). Conversely, CSF samples from 5 (4.8%) of 105 children with bacterial meningitis also grew an enterovirus. All the patients presented in late summer at the peak of the enterovirus season, and each case was caused by a different bacterial pathogen. Because the CSF formula was indistinguishable from that of patients with typical bacterial meningitis, and because the clinical course and response to therapy were similar to those of patients with typical bacterial meningitis, this condition may be underrecognized, as CSF viral cultures are rarely performed when bacterial meningitis is the likely diagnosis.
PATHOGENESIS AND PATHOPHYSIOLOGY
Despite the availability of effective antimicrobial therapy, bacterial meningitis continues to be a potentially fatal illness. Several investigators have examined the pathogenic and pathophysiologic mechanisms operating in meningitis, with the aim of improving the outcome of patients with this disorder. These pathogenetic and pathophysiologic mechanisms are discussed in detail in Chapter 23 and are detailed in a number of excellent reviews (164–174).
PATHOLOGY
Adams et al. (175) described the pathology of bacterial meningitis in 1948 based on examination of autopsy material from patients with H. influenzae meningitis. Experimental models of bacterial meningitis, knowledge of host defense mechanisms, and the pathophysiology of associated complications have subsequently allowed for a more complete understanding of the pathologic processes operating in this disorder.
Bacteria reach the meninges through one of the following pathways: (a) hematogenous dissemination from a distant site (e.g., nasopharynx, lung, skin, and genitourinary tract); (b) spread from an adjacent suppurative focus of infection (e.g., otitis media, sinusitis, and mastoiditis); and (c) a congenital or an acquired structural defect (176).
Once bacteria gain access to the SAS, an inflammatory process ensues (Fig. 24.2). Neutrophils migrate into the SAS, producing a purulent exudate. On gross examination, the exudate has a gray-yellow or yellow-green appearance (Fig. 24.3). It is most abundant in the cisterns at the base of the brain and over the convexities of the hemispheres in the rolandic and sylvian sulci (175) (Fig. 24.4). The tendency for exudate to accumulate in the cisterns at the base of the brain is explained by the anatomy of the SAS, which is deepest at the base of the brain. The various cisterns are expansions of the SAS, with the largest of these areas lying between the cerebellum and medulla and extending downward below the foramen magnum, the so-called cisterna magna or cerebellomedullary cistern (177). Purulent exudate accumulates in this cistern and extends into the other basal cisterns and onto the posterior surface of the spinal cord (Figs. 24.5 and 24.6). The exudate also extends into the arachnoidal sheaths of the cranial nerves and into the perivascular spaces of the cortex. A small amount of exudate may be found in the ventricular fluid and attached to the ventricular walls and choroid plexus; thus, the appearance of the ventricular fluid is usually cloudy by the end of the first week of the infection (175).
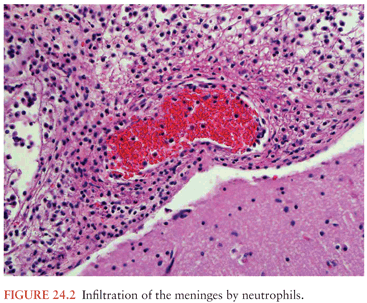
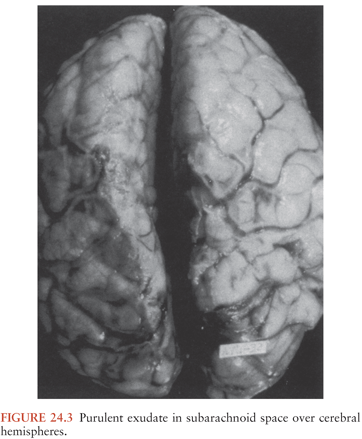
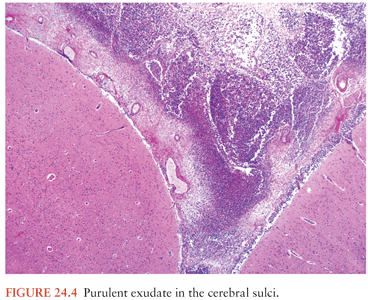
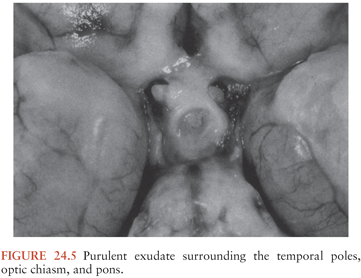
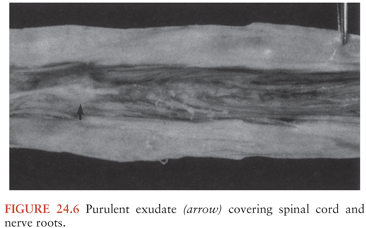
Microscopic examination of the subarachnoid exudate in the early stages of infection demonstrates large numbers of neutrophils and bacteria (lying either free in the exudate or within neutrophils) (Fig. 24.7) (176). The role of the neutrophil in eradicating infection at this stage is unknown. The presence of free-living bacteria in the exudate suggests that phagocytosis by neutrophils is incomplete as a result of deficient opsonic activity in CSF; however, low CSF leukocyte concentrations in the presence of high CSF bacterial concentrations have been associated with a poor prognosis in both experimental and human meningitis (178). These observations suggest that the neutrophils have a beneficial role in partial control of the early stages of the infection. The presence of large numbers of neutrophils in the SAS and vessel walls may, however, also be detrimental to the host, as is discussed in the previous section.
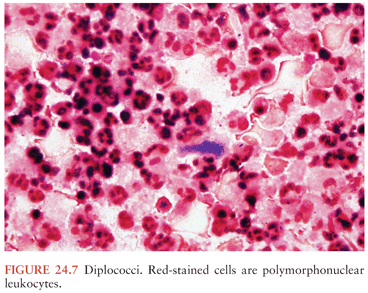
Within the first 48 to 72 hours of infection, there is evidence of inflammation in the walls of the small and medium-sized subarachnoid arteries (Fig. 24.8). The endothelial cells swell and multiply, narrowing the lumen. The adventitia is infiltrated by neutrophils, and neutrophils and lymphocytes form a layer beneath the intima (Fig. 24.9). Subintimal arterial infiltration by neutrophils and lymphocytes is relatively unique to infection of the meninges, and it may be related to the anatomy of the meningeal arteries. It is only rarely observed in inflammatory processes in other organs (175). The adventitia of the subarachnoid vessels, as they enter the brain parenchyma, is formed by the arachnoid membrane. As arteries and veins enter the brain parenchyma, they carry with them a sleeve of arachnoid immediately surrounding the vessels and a sleeve of pia mater external to this. Between these two layers lies an extension of the SAS, known as the perivascular space or Virchow-Robin space, which is filled with CSF (177) (Fig. 24.10). Because the vessel wall is enveloped by the arachnoid membrane, it is affected early by any inflammatory process in the meninges. However, as shown in animal models of bacterial meningitis, the arachnoid membrane generally remains intact.
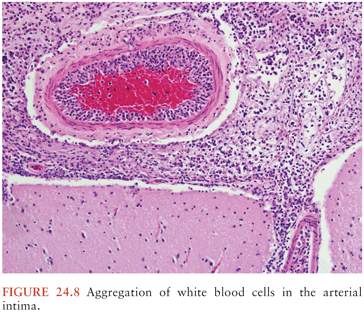
The meningeal veins become distended and develop mural inflammation during bacterial meningitis. There may be focal necrosis of the vessel wall, along with mural thrombus formation in the lumen of the vein or in the dural sinus (175) (Fig. 24.11). Hemorrhagic cortical infarction is the result of cortical venous and dural sinus thrombosis.
Toward the end of the first week of meningeal infection, there is a change in the cellular composition of the subarachnoid exudate. Neutrophils begin to degenerate and are removed by macrophages, which are derived from meningeal histiocytes. Lymphocytes and fibroblasts proliferate in the exudate. Microscopic changes in the brain parenchyma may also be present. The nuclei of neurons and glial cells become shrunken, pyknotic, and darkly staining (Fig. 24.12). Rod-shaped microglial cells and astrocytes increase in number in the cerebral and cerebellar cortex, brainstem, and spinal cord (Fig. 24.13). Astrocytic processes become swollen (Fig. 24.14). There is a loss of myelinated fibers in the subcortical white matter, cerebellum, and brainstem (175). Similar morphologic changes are seen in ischemic and hypoxic cortical injury, suggesting that ischemia and/or hypoxia may contribute to the pathologic changes from bacterial meningitis at this stage.
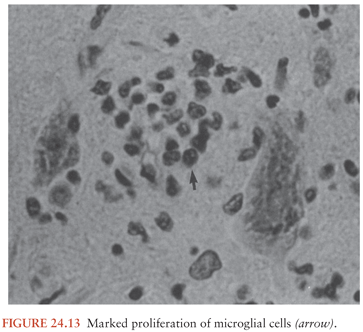
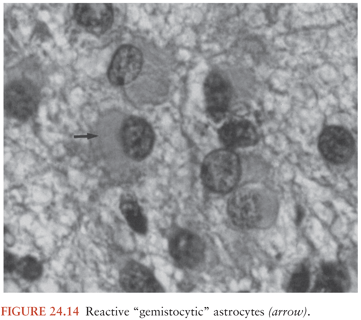
Also at the end of the first week of the infection, there is infiltration of the subependymal tissues and perivascular spaces by neutrophils and lymphocytes. The ependymal and subependymal tissues become edematous, and cells begin to die; desquamation of the ependymal lining also occurs. Rod-shaped microglial cells and swollen astrocytes proliferate and overgrow the remnants of the ependymal lining. An inflammatory infiltrate in the walls of the subependymal arteries may occlude the vessel, leading to tissue necrosis (175).
As the infection progresses, the subarachnoid exudate continues to accumulate. In some areas, it may become several millimeters thick (Fig. 24.15). Toward the end of the second week, the exudate separates into two layers. The outer layer, just beneath the arachnoid membrane, is composed of neutrophils and fibrin. The inner layer, which is contiguous with the pia, is composed of lymphocytes, plasma cells, and macrophages (175). As the subarachnoid exudate continues to accumulate, the flow of CSF may become obstructed.
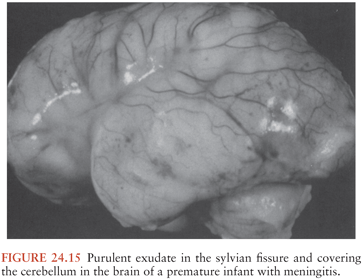
The dynamics involved in obstruction of CSF flow are as follows: the bulk of CSF is formed by the choroid plexus in the lateral and third ventricles, and it flows through the cerebral aqueduct into the fourth ventricle. CSF leaves the fourth ventricle through the midline foramen of Magendie and the lateral foramina of Luschka to reach the SAS (177). When the foramina of Magendie and Luschka are blocked by exudate, the spinal fluid cannot circulate to the convexities of the brain, where it is normally absorbed. The flow of CSF is blocked at the level of the fourth ventricle, resulting in noncommunicating or obstructive hydrocephalus (Fig. 24.16).
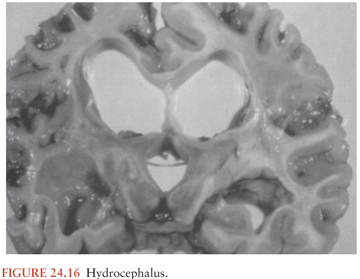
From the fourth ventricle, CSF normally flows to the SAS at the base of the brain. From here, CSF flows up over the convexity of the hemispheres to be absorbed by the arachnoid villi in the intracranial venous sinuses (177). The presence of a fibrinopurulent exudate in the SAS interferes with the absorption of CSF by the arachnoid villi. This obstruction to CSF resorption resulting from inflammatory changes in the arachnoid granulations results in communicating hydrocephalus. When the subarachnoid exudate has been present for several weeks, there are (a) marked fibrosis of the arachnoid villi and (b) pockets of exudate walled off by adhesions between the arachnoid membrane and dura (175). These fibrotic changes produce further mechanical obstruction to the resorption of CSF by the arachnoid villi. The end results are (a) transependymal movement of CSF from the ventricular system into the brain parenchyma and (b) the development of interstitial cerebral edema.
The development of diffuse cerebral edema and increased ICP further complicates the pathologic changes already described. Cerebral edema is defined as an increase in the volume of the brain resulting from an increased water content (179) (Fig. 24.17). The cerebral edema in meningitis is a combination of vasogenic, cytotoxic, and interstitial edema (170). Vasogenic edema is a result of increased permeability of brain capillaries with the subsequent accumulation of water and protein molecules in the extracellular space, mainly in the subcortical white matter. Cytotoxic edema is caused by an accumulation of intracellular water and sodium with subsequent swelling of cells. Membrane polyunsaturated fatty acids and other toxic factors released from leukocytes contribute to the development of cytotoxic edema (69). Interstitial edema is a result of obstruction to CSF resorption, as discussed earlier in this chapter.
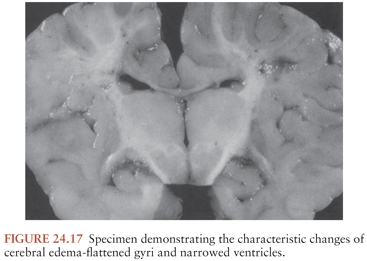
Cerebral edema leads to an increase in ICP, and increased ICP adversely affects cerebral perfusion pressure (CPP), defined as the difference between the systemic mean arterial pressure (MAP) and the ICP: CPP = MAP − ICP (180). Cerebral blood flow may be maintained at normal or near-normal levels in the presence of increased ICP, provided that the CPP is maintained at a range of at least 50 to 60 mm Hg. As ICP continues to rise, or if systemic arterial pressure decreases, cerebral ischemia and infarction may result.
Experimental evidence exists for a loss of autoregulation of cerebral blood flow in bacterial meningitis (181). This is another potential contributing factor to the development of cerebral ischemia in this infection. Cerebral blood flow is normally constant within a range of mean systemic arterial pressure from 50 to 150 mm Hg. When autoregulation is disturbed, systemic hypotension results in decreased cerebral blood flow and cerebral ischemia (182).
Cerebral edema may lead to herniation of brain tissue. Herniation may compress intracerebral arteries, leading to ischemia and infarction; it may also compress the surface of the brain against the dura, leading to necrosis of brain tissue. Tonsillar herniation, the downward displacement of the cerebellar tonsils through the foramen magnum, can result in apnea, hemodynamic instability, coma, and death (177,183).
The pathologic lesions described are typical of meningitis caused by bacteria, but some distinctions among lesions caused by H. influenzae, N. meningitidis, and S. pneumoniae infection have been observed. The subarachnoid exudate in H. influenzae meningitis is very thick and purulent, with loculated pockets of pus in the basilar cisterns and cerebral sulci. In contrast, the exudate in pneumococcal meningitis tends to be more extensive over the convexities of the hemispheres than in the basilar cisterns (Fig. 24.18). In meningococcal meningitis, the pathologic changes depend on the severity and duration of the infection. In acute fulminating meningococcemia, death may occur before pus can accumulate in the SAS. At autopsy, severe hyperemia and swelling of cerebral tissue are evident, with petechial hemorrhages in the gray and white matter (Fig. 24.19) and in the subependymal regions of the lateral ventricles. A hemorrhagic ependymitis is typical of severe lethal meningococcal infection (Fig. 24.20). The presence of pus in the SAS may be evident only by microscopic examination (183). The pathologic changes in meningococcal meningitis of longer duration are similar to those described for meningitis caused by pyogenic organisms in general.
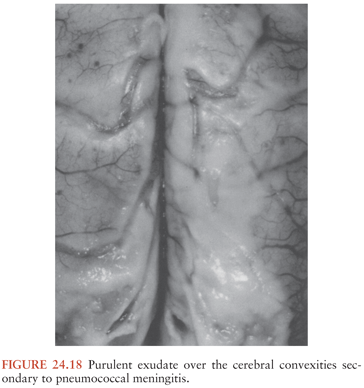
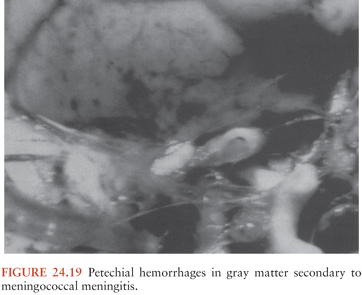
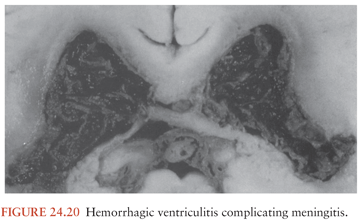
Cranial and spinal nerve deficits, focal neurologic deficits, seizure disorders, and subdural effusions are well-recognized complications of meningitis. The cranial and spinal nerve deficits are usually transient and caused by exudate in the SAS surrounding the nerves. Focal neurologic deficits and seizure activity arise from cortical and subcortical ischemia and infarction (bland and hemorrhagic), which are the result of inflammation and thrombosis in arteries and veins. Subdural effusions are relatively common in the course of bacterial meningitis in children; they are the result of an increase in the permeability of the thin-walled capillaries and veins in the inner layer of the dura, with leakage of fluid into the subdural space.
Bacteremia also contributes to the pathologic features of this disease. Bacteremia is present in 30% to 90% of cases of bacterial meningitis. It can be either the primary event leading to development of meningitis or a secondary event arising from the clearance of bacteria from the SAS through the arachnoid villi to the bloodstream. Pneumococcal cell walls activate the alternative complement pathway, with the generation of chemotactic peptides in the systemic circulation and in CSF. The principal component of this activity is C5a, a highly chemotactic peptide that is a stimulus for an intense CSF accumulation of neutrophils. By this process, neutrophils also become sequestered in the pulmonary vascular bed, leading to cardiopulmonary dysfunction, neutrophil-mediated vascular damage, and the development of the acute respiratory distress syndrome and thereby further contributing to the morbidity and mortality of meningitis (69).
CLINICAL MANIFESTATIONS
Neonates
Clinical clues to the presence of meningitis in neonates are temperature instability (hypothermia or hyperthermia), listlessness, high-pitched crying, fretfulness, lethargy, refusal to feed, weak suck, irritability, jaundice, vomiting, diarrhea, or respiratory distress (184,185). Nuchal rigidity is not typically found in the neonate. A change in the child’s affect or state of alertness is one of the most important signs of meningitis. A bulging fontanelle (seen in one third of cases) usually occurs late during the course of illness; seizures are observed in 40% of neonates with bacterial meningitis.
Infants and Children
The symptoms and signs of acute bacterial meningitis in infants and children depend on the age of the child, duration of illness, and host response to infection (186); the clinical features can be subtle, variable, nonspecific, and even absent. The initial symptoms of bacterial meningitis in infants and children may be any of the following: fever, stiff neck, headache, lethargy, irritability, nausea, vomiting, and photophobia (Table 24.3). In children 1 to 4 years of age, fever (94%), vomiting (82%), and nuchal rigidity (77%) are the most common initial symptoms.
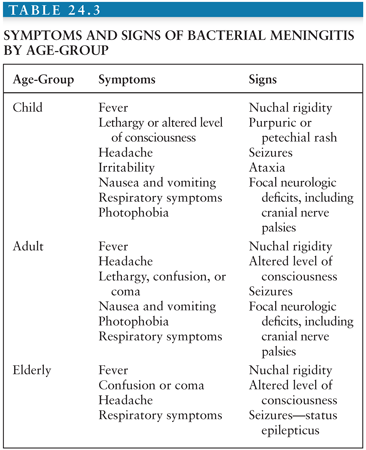
Although the symptoms are nonspecific, the combination of one or more of these symptoms with signs of meningeal irritation should suggest the diagnosis of meningitis. The classic signs of meningeal irritation are nuchal rigidity and Brudzinski and Kernig signs. Brudzinski actually described several signs of meningeal irritation, including the nape-of-the-neck sign, the identical contralateral reflex sign, and a reciprocal contralateral reflex sign, as well as others (187,188). The nape-of-the-neck sign is Brudzinski’s best-known sign and is universally recognized today as “Brudzinski sign.” The nape-of-the-neck sign is positive when passive flexion of the neck results in flexion of the hips and knees. The identical contralateral reflex sign is elicited with the patient in the supine position by passively flexing the hip and knee on one side. This sign is positive when the contralateral leg flexes with this maneuver. The reciprocal contralateral reflex sign is positive when the leg that has flexed in response to passive flexion of the other leg begins to extend spontaneously, resembling a “little kick.” The identical and reciprocal contralateral reflex signs are not elicited as often as the nape-of-the-neck sign (188). The manner in which Kernig sign is elicited and the interpretation of the results of the maneuver as it is done today are different from those originally described by Kernig (189). The maneuver as described by Kernig was performed with the patient in a seated position while the physician attempted to extend the knee passively. In the presence of meningitis, knee extension was resisted so that a “contracture of the extremities” was maintained (188,189). Today the sign is elicited with the patient in a supine position. The thigh is flexed on the abdomen, with the knee flexed. The leg is then passively extended. When meningeal inflammation is present, the patient resists leg extension (190). Nuchal rigidity and Brudzinski and Kernig signs are observed in fewer than 50% of children with bacterial meningitis.
The possibility of bacterial meningitis should be considered in every child with fever, vomiting, nuchal rigidity, and lethargy or an altered mental status. In a review of 110 cases of culture-proven bacterial meningitis in children, fever (≥38.5°C) was the most common symptom, being present in 94% of patients. The absence of fever, particularly hypothermia, was associated with a worse prognosis, perhaps related to the slower rate of bacterial replication in CSF when temperatures are elevated. Apart from fever, the most common symptoms were (a) vomiting (82%) and nuchal rigidity (77%) in 1- to 4-year-old children and (b) headache (92%) in children ages 5 to 12 years. Vomiting and nuchal rigidity were present in 80% of the children who were 12 months or older. Nuchal rigidity is a classic sign of meningitis but can be absent early in the course of this illness; therefore, the absence of nuchal rigidity should not exclude the diagnosis of bacterial meningitis (191).
In a review of 709 LPs done on children in an outpatient setting in which there was a concern for meningitis, the CSF was abnormal in 16% (192). There were 30 cases of bacterial meningitis, 70 of viral meningitis, and 12 of unknown etiology. Lethargy was more common in children with bacterial meningitis than in children with viral meningitis: 50% of the children with bacterial meningitis were lethargic, and 32% of the children with viral meningitis were lethargic (p >.14). Although vomiting is a symptom of meningeal irritation, it is a nonspecific symptom in children. Vomiting occurred in 336 children, 84 of whom had bacterial or viral meningitis. Fever was present in every child with meningitis. The temperature elevations were higher in bacterial meningitis than in viral meningitis; 80% of the children with bacterial meningitis had temperatures of 38.8°C or higher. In children with viral meningitis, 40% had temperatures of 38.8°C or greater (193). The possibility of meningitis in a child who does not or cannot complain of headache or stiff neck and who does not have meningeal signs should be suspected when fever accompanies changes in behavior, changes in mental status, or new onset of seizures.
In one recent review of children aged 2 months to 15 years who presented with suspected meningitis, the classic clinical signs had limited value in establishing the diagnosis (194). Clinical examination revealed nuchal rigidity in 65% of those with meningitis; Brudzinski and Kernig signs were elicited in 51% and 27% of those with meningitis, respectively. Therefore, physicians should have a low threshold for LP in patients at high risk for bacterial meningitis, given the serious nature of this disease.
In a review of 1,064 cases of bacterial meningitis in infants and children, there were no signs of meningeal irritation in 16 patients (1.5%). Eight patients were older than 2 years of age. LP was performed because of unexplained fever associated with an altered level of consciousness, behavioral changes, seizure activity, or petechial skin lesions. Meningitis was caused by N. meningitidis in seven patients, H. influenzae in six, S. pneumoniae in two, and Salmonella enteritidis in one. Most patients had a peripheral leukocytosis with a left shift. The peripheral WBC count was greater than 10,000 cells/mm3 in 12 patients and greater than 20,000 cells/mm3 in 7 patients (195). The results of this review suggest that although meningitis may occasionally occur without meningeal signs, there will usually be other signs or symptoms of intracranial infection and a peripheral leukocytosis.
Observational data that are useful in predicting the presence of serious illness (e.g., meningitis) in a febrile child include the following: (a) quality of cry, (b) reaction to parent stimulation, (c) level of consciousness, (d) color, (e) hydration, and (f) response to social stimulation. These six items were identified as significant and independent predictors of serious illness from a list of 14 observational items, scored by pediatricians, for 312 febrile children 24 months of age or younger (196). The quality of the cry in a child with a serious illness was weak, moaning, or high pitched. A healthy child was either not crying or had a strong cry with normal tone. Reaction to parental stimulation was judged based on the parent holding the child, talking to the child, or giving the child a bottle. The child with a serious illness did not stop crying or barely responded to stimulation by its parent. Consciousness was impaired in children with serious illnesses. They were lethargic, stuporous, or obtunded. Sick children were described as pale, cyanotic, or ashen. Signs of dehydration were present. The response to social stimulation was judged according to whether the child would smile when talked to or smiled at. Sick children did not respond to social stimulation. These six items, when used together, had a specificity of 88% and a sensitivity of 77% for the presence of serious illness. When combined with history and physical examination, the sensitivity of the six-item model increased to 92%. If all six of the observation items were normal in a child, the probability of that child having a serious illness was only 4.7% (196).
The possibility of meningitis in a febrile child may also be suggested by the tempo of the illness. The presentation of meningitis in children is that of either a subacute infection or an acute fulminant illness. Children with a subacute presentation have fever, lethargy, and nuchal rigidity that progresses over 1 to several days and is usually preceded by an upper respiratory tract infection or otitis media (197). Children with meningitis may also present with an illness that has been progressive over 24 to 72 hours or a fulminant illness that develops over several hours.
Children with a more rapidly progressive illness have signs and symptoms of meningeal irritation and increased ICP on initial presentation. CSF pressures exceeding 300 mm H2O are common in acute bacterial meningitis, and ICPs exceeding 500 to 600 mm H2O are not unusual (197,198). Increased ICP in bacterial meningitis in children is the consequence, in part, of vasogenic and cytotoxic cerebral edema, altered CSF resorption, and the inappropriate secretion of antidiuretic hormone (199) (see Chapter 23). The clinical manifestations of increased ICP include (a) an altered level of consciousness; (b) dilated, poorly reactive or nonreactive pupils; (c) abnormalities of ocular motility; (d) pathologically brisk lower extremity reflexes; and (e) bradycardia and hypertension, also known as Cushing reflex. The development of elevated ICP should be anticipated and monitored in a child with bacterial meningitis. The absence of papilledema does not exclude the presence of increased ICP. Papilledema is rarely observed early in the course of increased ICP and is usually not evident until increased ICP has been present for at least several hours (197,198). For this reason, the presence of papilledema at the time of the initial presentation should raise suspicion of a focal intracranial process such as a brain abscess or other localized mass lesion, and it is an indication for CT prior to LP.
Seizures occur in 30% to 40% of children with acute bacterial meningitis, usually during the first 3 days of illness (200). In one review of 52 cases of H. influenzae meningitis in children, seizures occurred in 44% (23 cases) (198). There has been a long-standing controversy about whether to do an LP in febrile children with new-onset seizures. The vast majority of children who present with a new-onset seizure associated with fever and who have a normal neurologic examination do not have meningitis. One series reviewed the results of LP performed on 328 children presenting with their first febrile convulsion. None of the children had meningeal signs. Meningitis was diagnosed by LP in four children (1.2%). Three of the children had viral meningitis, and one had H. influenzae meningitis. All four children were younger than 18 months of age. All the children in this series who were older than 18 months of age had unequivocal signs of meningitis (201). A similar observation was made in a review of LP performed on 304 children for evaluation of new-onset seizures associated with fever. There were 15 cases of meningitis, and in only one case were there no meningeal signs. In that case, the child had viral meningitis and recovered fully without treatment (202). These studies suggest that LP should not necessarily be routinely performed in children for evaluation of simple febrile convulsions in the absence of meningeal signs.
Convulsive seizure associated with fever is a problem unique to young children. A simple febrile seizure, as defined by the Consensus Development Meeting on Long-term Management of Febrile Seizures (1980), occurs between ages 3 months and 5 years in association with fever and is of brief duration (<15 minutes), nonfocal, nonrepetitive, and without associated neurologic deficits. If the seizure fits this definition and the child is awake and alert after the seizure, the yield of an LP is very low. If, however, there are clinical signs of meningitis, an LP is indicated. If the seizure has a focal onset or there is a focal neurologic deficit on examination, CT is indicated before LP is performed. All children with new-onset febrile convulsions in whom LP is not performed should be reexamined 1 to 4 hours after the initial examination to be sure that serious disease is not present (203).
The presence of a diffuse erythematous maculopapular rash may be an early manifestation of meningococcemia or may represent a viral illness. The presence of a purpuric or petechial rash on the trunk and lower extremities is suggestive of meningococcemia, although petechiae are sometimes seen in echovirus type 9 meningitis, acute staphylococcal endocarditis, and rarely pneumococcal or H. influenzae meningitis (198,204). Petechiae are found in the skin, mucous membranes, or conjunctivae, but never in the nailbeds, of patients with meningococcemia; they usually fade in 3 or 4 days (205). Petechiae and/or purpura occurs in 50% to 75% of children with meningococcal meningitis. Children with fulminating meningococcal septicemia may have the Waterhouse-Friderichsen syndrome, characterized by the following: (a) sudden onset of a febrile illness, (b) large petechial hemorrhages in the skin and mucous membranes, (c) cardiovascular collapse, and (d) disseminated intravascular coagulation. Of all patients with a meningococcal infection, 10% to 20% have a fulminant meningococcal septicemia (206) (Color Figs. 24.21 to 24.26).
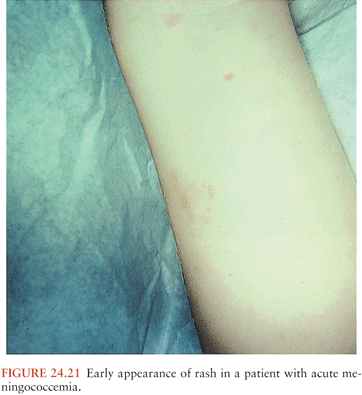
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree