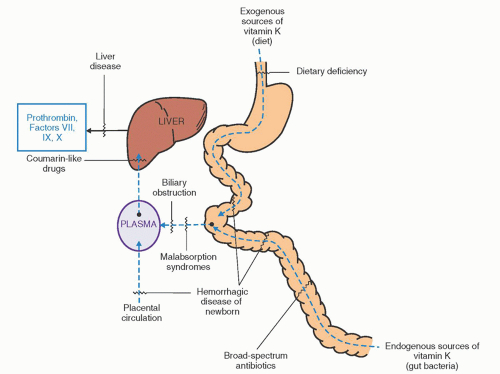
disease, drugs).4 VKDB can also be classified in terms of age of onset: Early (onset <24 hours of age), classic (onset usually between 3 and 5 days), and late (onset on or after 8 days). Early VKDB is uncommon and usually results from placental transfer of maternal drugs that antagonize vitamin K in the newborn. Late VKDB occurs almost exclusively in breast-fed infants who may also have hepatobiliary disease.4
TABLE 54.1 ACQUIRED COAGULATION DISORDERS | |||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||
prophylactic administration of vitamin K1 to infants 1 to 5 months of age, especially those who are breast-fed or who have a disorder that may impair vitamin K absorption.17 The usual dosage in such older infants is 50 to 100 µg daily or 1 mg monthly.
TABLE 54.2 LABORATORY FINDINGS IN ACQUIRED COAGULATION DISORDERS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
in such patients, and when unsuspected, they may be confused with DIC or may first be revealed by serious, unexpected postoperative hemorrhage. Antimicrobial agents presumably impair vitamin K production by inhibiting the synthesis of menadiones by gut bacteria, but they may also directly affect carboxylation reactions.21, 22 Vitamin K deficiency also may result from use of drugs other than antimicrobial agents, such as cholestyramine,23 which acts by binding bile salts, or mineral oil and other cathartics when used for protracted periods. Vitamin E may antagonize the metabolic action of vitamin K and potentiate the action of coumarins.6 When taken in large doses, this vitamin may prolong the PT.24 Antibiotic therapy, poor diet, or any of the aforementioned disorders may predispose patients to coumarin toxicity (see Chapter 55).25 Large doses of aspirin, as given to treat rheumatic disorders or in amounts associated with overdosage of the drug, may also induce vitamin K deficiency.26
TABLE 54.3 ABNORMALITIES OF HEMOSTASIS AND COAGULATION IN LIVER DISEASE | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
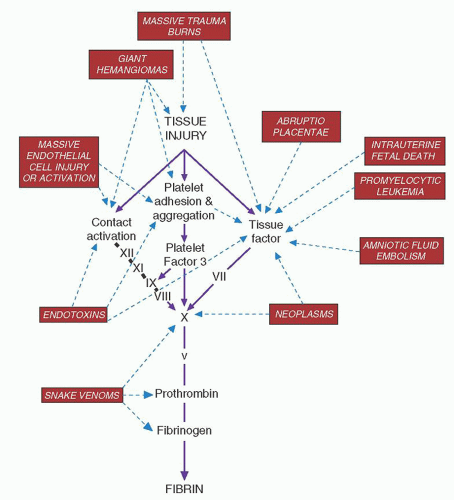
snakebite, which is probably one of the most common causes of the disorder worldwide.
TABLE 54.4 ETIOLOGIES OF DISSEMINATED INTRAVASCULAR COAGULATION | ||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||
amounts of particulate matter enter the circulation suddenly. In intrauterine fetal death, thromboplastic substances from the dead fetus are slowly but continuously absorbed, producing a picture of chronic but progressive DIC.
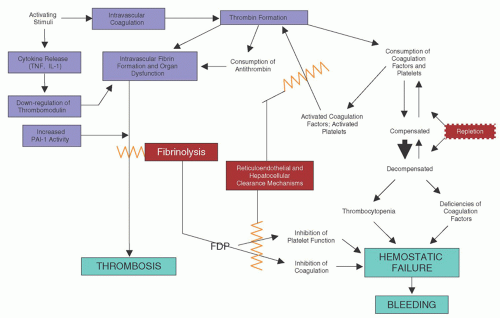
terms of anticoagulant properties (e.g., thrombomodulin activity, fibrinolysis) by stimuli relevant in the pathogenesis of DIC (see Chapter 19). This altered endothelium is referred to as activated; properties of activated endothelium include conversion of the normally anticoagulant phenotype to a procoagulant phenotype, expression of adhesion molecules, production of inflammatory mediators, and production of vasoactive agents.121 Many of these pathologic events are amplified by endothelial cell proteaseactivated receptors (PARs) (see Chapter 19). Vascular endothelium may also promote coagulation by formation of thrombogenic microparticles, which express anionic phospholipid.122 Microparticles in DIC may also originate from platelets or granulocytes.123 Thrombin generation caused by activation of factor XII in DIC appears to be less important than that initiated by tissue factor.124 Contact activation, instead, appears critical in mediating DIC-associated hypotension.125
thrombotic lesions. These processes include adhesion to denuded or damaged endothelial cell surfaces and intravascular aggregation with subsequent sequestration, which may be caused by endotoxin, antigen-antibody complexes, thrombin, particulate matter, and, possibly, fibrin-FDP complexes.
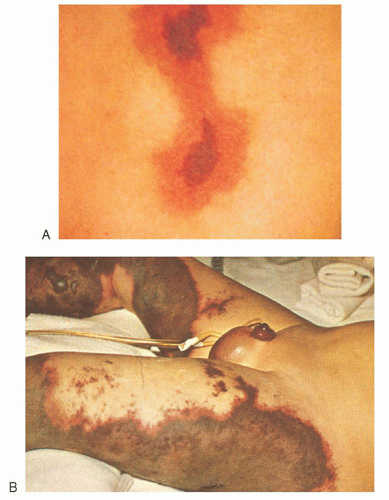
from recurrent venous thrombosis or arterial embolism98 with high levels of platelets and coagulation factors to acute DIC with severe hemorrhage.98 The clinical and experimental evidence is incontrovertible that pregnancy, the best studied form of hypercoagulability, is associated with an increased propensity for the development of DIC.160, 161 Indeed, even normal pregnancy has been suggested as a form of low-grade “physiologic” DIC,160 which, at term, becomes overt for a short time. Thus, transitory but significant elevations of FDP and corresponding diminution in fibrinogen levels are observed regularly during the first 4 hours after delivery.160 This may be triggered, in part, by the release of tissue factor into the circulation.
healthy subjects, may represent a significant decrease in a patient whose baseline level was 800 mg/dl due to acute-phase changes.
of FDP; abnormalities of the PTT, PT, and thrombin time; and deficiencies of factors V and VIIIc. The euglobulin lysis time is significantly and persistently shortened, often in association with plasminemia. However, the platelet count usually is normal, the D-dimer level should be normal or only minimally elevated, and protamine sulfate tests should be negative. Hypoprothrombinemia and deficiencies of stable coagulation factors VII, IX, X, and XI are rare. Thus, routine coagulation tests should be able to distinguish DIC from primary fibrinogenolysis.
thromboembolism, and cancer is often called Trousseau syndrome.98 Laboratory findings are variable. Evidence of chronic DIC, hypercoagulability, or acute DIC may be found. In a study of more than 1,000 patients with solid tumors, 7% were diagnosed with DIC using standard coagulation tests (platelet count, fibrinogen, D-dimer, FDPs).208 Risk factors associated with the occurrence of DIC included older age, male gender, advanced disease, breast cancer, and necrosis of the tumor specimen.208
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree