3.1 Acute Respiratory Failure
Acute onset of shortness of breath (SOB) or acute respiratory decompensation is a common emergency condition. This can occur due to multiple etiologies, as following:
Exacerbation of asthma or chronic obstructive pulmonary disease (COPD).
Respiratory infection (viral/bacterial).
Heart failure and pulmonary edema.
Respiratory depressants such as opiate poisoning or barbiturates.
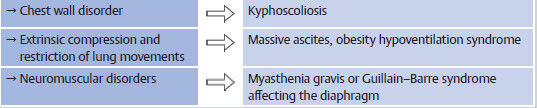
Tiring diaphgramatic muscles which can occur in all causes of severe persistent hypoxemia, e.g., in later stages of asthma exacerbation or pulmonary embolism.
Acute neuro-muscular weakness with involvement of diaphgram, that can occur in conditions like myasthenic crisis or Guil-lain–Barre syndrome.
Lung auscultation is a very powerful diagnostic tool
Increased breath sounds (inspiration and expiration equally heard = bronchial breath sounds) | Consolidation of lung segments due to lobar pneumonia Additional findings include:
| |
Bronchospasm, which can be caused by | ||
Stridor = noisy breathing (often so loud that it can be heard without a stethoscope) | Due to incomplete upper or middle-respiratory tract obstruction | |
aThese exam findings are due to increased conduction of vibration by consolidated lung. In the consolidated part of the lung | ||
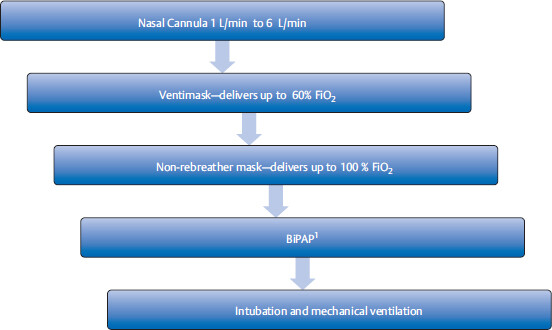
Management steps for acute respiratory failure
• Pulse oximetry (which measures oxy-hemoglobin saturation), chest X-ray (CXR), and on occasions arterial blood gas (ABG), are important initial diagnostic tools to evaluate the severity and the cause of respiratory compromise.
Step up of supplemental oxygen therapy: This is used to maintain oxygen saturation (SaO2) to > 88 to 92% or PaO2 > 60 mmHg.
3.1.1 Bilevel Positive Airway Pressure aka Noninvasive Positive Pressure Ventilation
Bilevel positive airway pressure (BiPAP) is a noninvasive method of ventilation that can deliver up to 100% oxygen with application of positive airway pressure through a tight-fitting mask. The mask is very easy to put on and take off (since it is a mask), in contrast to invasive intubation. In acute pulmonary edema, BiPAP has the added benefit of decreasing preload. BiPAP increases intrathoracic pressure and decreases venous return to right heart.
1 Note that BiPAP cannot be used in following cases:
• Obtunded patient who cannot control their airway.
• Patients with excessive secretions.
• Agitated or uncooperative patient.
3.1.2 Mechanical Ventilation
Indications
Severe hypoxemia, hypercarbia, or respiratory acidosis unresponsive to general noninvasive measures.
Patients who have hemodynamic instability (such as hypotension).
Patients who have altered mental status and cannot protect their airways.
An endotracheal tube is inserted in the patient’s airway and connected to a ventilation machine.
In most ventilator settings, the following parameters are looked at
It is the volume of air delivered through ventilation machine in each breath. Recommended tidal volume is 6–8 cc/kg of predicted body weight. | ||
Too low TV can cause atelectasis, and hypoventilation (respiratory acidosis) | ||
| ||
Generally preferred at < 60% to decrease risk of oxygen toxicity | ||
Minimal PEEP of 5 cm H2O is used in all patients on mechanical ventilation to prevent end expiratory alveolar collapse. As PEEP is increased, it helps to recruit collapsed alveoli to improve gas exchange and oxygenation. Higher levels of PEEP (15–20 cm H2O) may be required to improve oxygenation, with the goal to reduce higher FiO2 to prevent oxygen toxicity. | ||
a Auto-PEEP can occur when the respiratory rate is set too high and/or if there is increased airway resistance (due to COPD, asthma, 3 To prevent auto-PEEP in COPD/asthma, it is recommended to use lower TV and RR, even at the expense of mild hypoventilation and respiratory acidosis, as long as pH is > 7.2. This strategy is known as permissive hypercapnia. In this case, the lungs do not have enough time to expire the inhaled air, and hence, more and more air gets trapped in each consecutive respiratory cycle, incrementally increasing the intrathoracic pressure. This can result in barotrauma and hypotension, similar in pathophysiology to high PEEP. | ||
An intubated patient is found to be hypoxic and hypotensive. Exam reveals severe wheezing. It appears that ventilator settings needs to be adjusted. What is the likely cause of hypoxia and hypotension in this patient?
An intubated patient is found to be hypoxic and hypotensive. Exam reveals no breath sounds on one side of the lung. What is the likely cause of hypoxia and hypotension in this setting?
Intubated patient has hypoxia and absent breath sounds in one side of the lung.
3.1.3 Modes of Ventilation
Assisted-control mode of ventilation (ACMV): This mode is commonly used for starting patient on mechanical ventilation and when patient is ready for weaning
4 Do not choose synchronized-intermittent mandatory ventilation as a weaning mode for ventilation as this method is not used anymore.
If patient’s own breathing rate is 10, then patient will get additional 2 breaths delivered with TV of 500 cc each. In this case minute ventilation = 0.5 L × 12/min = 6 L/min.
If patient’s own breathing rate is 15, then the extra 3 breaths that patient initiates, he/she will get 500 cc of preset TV in each breath (minute ventilation = 0.5 × 15). In this case, patient is said to be breathing over the vent.
Pressure control ventilation (PCV): The ventilator delivers preset pressure support in each breath. Tidal volume is not specified.
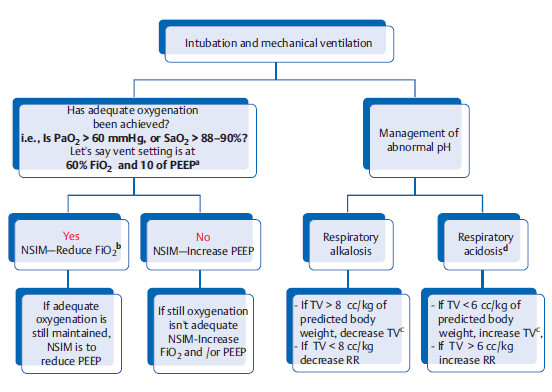
Stepwise management of ventilation
a Try to keep the ratio of 60% FiO2 to 10 of PEEP, and 40% FiO2 to 5 of PEEP. Most of the time exam CCS will involve a question with 60 FiO2 with 10 of PEEP scenario.
b Reducing FiO2 is the first step to reduce risk of oxygen toxicity. But note that if PEEP was mistakenly set at 20 and FiO2 is set at 60%, and if oxygenation is adequate then NSIM is to decrease PEEP in this case; but this is an extreme example only.
c Recommended tidal volume is 6 to 8 cc/kg of predicted body weight. FYI an average adult’s normal tidal volume is 500 mL.
d Remember permissive hypercapnia in ARDS, COPD, and asthma.
5. Patient is intubated for ARDS. His vent settings are PEEP of 10, FiO2 of 80% and RR of 12. ABG reveals PaO2 of 90. What is the NSIM?
6. A 65-year-old female with asthma exacerbation is currently intubated. RR of 20. ACMV mode is set at RR of 13 per minute and TV of 500 cc. ABG shows pH of 7.26, PaCO2 of 60 mmHg, PaO2 70 mm Hg. What is the NSIM?
Ventilator Weaning
Consider weaning when the patient
Can maintain oxygenation with FiO2 < 40 to 50%, PEEP of ≤ 5 cm H2O.
Does not have excessive secretions.
Is awake enough to control airway.
Is hemodynamically stable (no or minimal vasopressor support).
To determine whether patient is ready for extubation, a spontaneous breathing trial is used with ventilation mode changed to low-pressure support ventilation. It is very similar to BiPAP, with positive pressure given during inspiration and expiration and TV/RR is completely dependent on the patient’s own respiratory effort.
During the first few hours of low-pressure support ventilation, rapid shallow breathing index (RSBI) ratio is calculated.RSBI = RR/TV (in liters). It is a ratio that is used to assess readiness of a patient to get extubated.
Other complications of mechanical ventilation
Stress-induced peptic ulcers: all patients on mechanical ventilation are recommended to get prophylaxis with proton pump inhibitor or H2 receptor antagonist (e.g., famotidine).
Ventilator-acquired pneumonia: placing patient on semirecumbent position has been shown to decrease incidence.
Pneumomediastinum, pneumoperitoneum, and subcutaneous emphysema are milder forms of barotrauma and generally require supportive care only.
A 65-year-old male patient is on 5 PEEP and 40% FiO2 on ACMV mode. ABG done showed PaO2 of > 60 mmHg. Prior to morning intensive care unit (ICU) rounds the ventilator setting is changed to lowpressure support ventilation. During rounds he is found to have RR of 30 per minute and TV of 200 cc.
Next morning his RSBI is < 105 on low-pressure support. Extubation is attempted after rounds.
Intubated ARDS patient develops swelling of face and neck and eyelids. Exam reveals crepitus over the neck and face.
9. What is the NSIDx (next step in diagnosis)?
VQ mismatch
Ventilation(V) in a lung zone should be matched with perfusion (Q) in that zone. The following are the types of VQ mismatch:
When perfusion is decreased to a well-ventilated area of the lung, then that part of the lung is defined as a dead space, where the ventilated volume of air will not participate in gas exchange. Think of this as dead air (space), which is useless. Classical example is pulmonary embolus | Anatomic or vascular shunts: blood passes from right side of heart to the left side bypassing the lungs/alveoli. This is known as right to left shunt To counteract this mismatch, reactive hypoxic pulmonary vasoconstriction does occur, but remember that compensation is never complete etc.1 |
Dead space = high V/Q ratio as denominator (perfusion) is low | |
aHypoxemia due to anatomic/vascular shunts canot be corrected by supplemental oxygen. Physiologic shunting, on the other hand, is usually not a pure shunt. Supplemental oxygen will help in these cases. | |
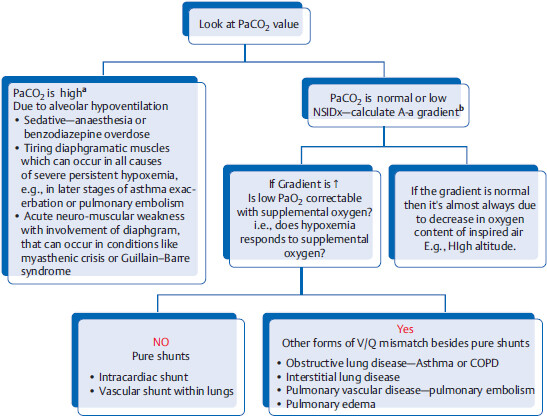
How to find out the cause of acute respiratory failure by looking at ABG?
aIn patients with acute respiratory distress and acute hypercapnic respiratory failure, NSIM is usually BIPAP or intubation. Patients at this stage are usually lethargic or comatose.
6 On the other hand, chronic hypercapnia +/- hypoxemia can occur in COPD, obesity hypoventilation syndrome and extrinsic lung disease (e.g., severe kyphoscoliosis). It is usually well compensated by chronic metabolic alkalosis.
bA-a gradient = Alveolar (PAO2) – arterial (PaO2) gradient. As alveolar (PAO2) is not easily obtainable, clinically the following formula is used to calculate A-a gradient = (150 – 1.25 × PaCO2) – PaO2 (the normal value is < 15 mmHg). Increased gradient (in between the oxygen content of the alveoli and the oxygen content of the systemic arteries) means that there is intrinsic lung pathology. A-a gradient is not increased in hypoventilation or high altitude.
3.1.4 Acute Respiratory Distress Syndrome
Background: Any severe local lung injury (e.g. drowning) or severe extrapulmonary pathology (e.g. severe sepsis) can cause oversecretion of inflammatory mediators into the blood stream that can activate neutrophils, which in turn release proteases and free radicals, damaging the endothelial and epithelial layers of alveoli. This leads to high protein vascular fluid leakage into the alveoli which eventually fills most of the intra-alveolar space, thereby causing significant areas of shunting. This condition is called ARDS. Histopathological picture is of diffuse alveolar damage.
Diagnostic criteria
Acute onset: within 1 week of initial insult.
Bilateral infiltrates present on CXR.
No clinical evidence of elevated left atrial pressure, or pulmonary capillary wedge pressure (PCWP) is ≤ 18 mmHg.
Work-up: Transthoracic echocardiogram (TTE), and serum Brain natriuretic peptide (BNP) are good initial tests, as the major differential dx for ARDS is cardiogenic pulmonary edema. TTE is generally adequate to assess for evidence of heart failure. If large body habitus makes it hard to get a good quality TTE images and if assessment of volume status is needed, a Swan-Ganz catheter can help in direct measurement of cardiac pressures.
Rx: Always treat the underlying etiology. Mechanical ventilation is usually necessary. Recommended TV is 6 mL/kg of predicted body weight and plateau pressure (end inspiratory) of ≤ 30 cm H2O, even if it results in mild hypercapnia and respiratory acidosis (permissive hypercapnia is tolerated until pH is < 7.2).
In ARDS of newborns (aka hyaline membrane disease [HMD] of the prematurity), the primary pathology is generalized collapse of alveoli due to deficient surfactant. So CXR picture in HMD of newborn will show ground-glass appearance of the lung as opposed to ARDS of adults that will show bilateral opacities.
In patients with similar presentation, but PaO2/ FiO2 ratio is > 200 (and < 300), the dx is acute lung injury (ALI).
3.2 Pulmonary Function Tests
Indications
• Unexplained pulmonary symptoms.
Monitoring known pulmonary disease.
Preoperative assessment prior to lung resection.
Differentiating between obstructive and restrictive lung disease.
9 If a patient presents with vague respiratory complaints and has hx of smoking as well as high-risk occupation (such as working in mining industry), test of choice is spirometry. It can differentiate between obstructive (COPD) and restrictive (asbestosis associated interstitial lung) disease.
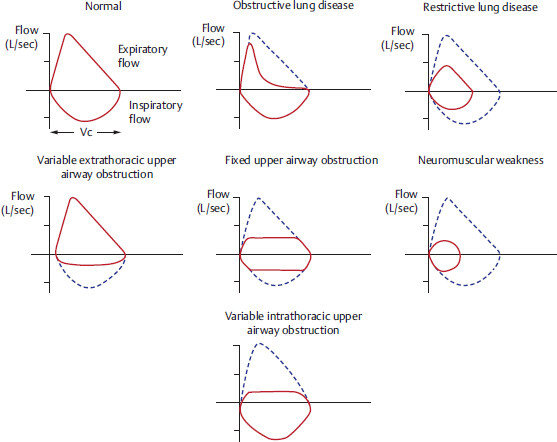
Pulmonary function test values in obstructive and restrictive lung disease
It is characterized by decrease in expiratory flow rates in PFT. Narrowing of airway disproportionately affects expiratory phase, as airway is normally narrower in expiration | One of the classic examples of restrictive lung diseases is interstitial lung fibrosis. Imagine the lung as a thick, stiff, small balloon which is very hard to inflate (to inhale) and easy to deflate (exhale), as the recoil pressure of the stiff-fibrosed lung is very high | |||
FEV1/FVCa ratio | ||||
DLCOb | Emphysema, bronchiolitis obliteransc and bronchiectasis | Primary pulmonary restrictive disorder, e.g., diffuse parenchymal lung disease (aka interstitial lung disease) | ||
Asthmad and chronic bronchitis | Extraparenchymal lung disorderse | |||
a FEV1/FVC = Forced expiratory volume of air in 1 sec/forced vital capacity (most important parameter we need to look at) | ||||
b DLCO is a measurement of alveolar diffusion capacity. This diffusion capacity is affected by decrease in surface area (e.g., emphysema) or decreased permeability (e.g., interstitial lung disease). 11 High DLCO occurs in polycythemia and left to right shunt. Decreased DLCO and normal PFTs can be seen in severe anemia, or primary pulmonary vascular disorder with no parenchymal involvement (e.g., pulmonary hypertension). | ||||
c Bronchiolitis obliterans (aka obliterative bronchiolitis) is inflammatory condition of smallest airways. It can be either sudden in onset or slowly progressive. It presents with dry cough, SOB, and wheezing. Etiologies include lung, or bone-marrow transplantation, infection, connective tissue disorders, toxic fume inhalation, etc. Treatment is supportive as disease is irreversible and patients may go on to require lung transplantation. | ||||
d NSIDx after PFTs suggest obstructive airway disease is to do bronchodilator challenge (e.g., albuterol). If FEV1 increases by ≥ 12% and/or increases by 200 mL from baseline, it indicates reversible airway obstruction suggestive of asthma. | ||||
Note: These values (FEV1, FVC, etc.) obtained from PFT are used for diagnosis, prognosis, evaluating progression of disease, and treatment response. So, PFT is “the” diagnostic test of choice for most of the chronic respiratory disorders.
Abbreviations: DLCO, diffusion capacity of lung for carbon mono oxide; RV, residual volume; TLC, total lung capacity Residual volume.
Lets practice some values
First trick is to look only at percentage of predicted value. The normal value is usually 80 to 120% (100 +/- 20). Low is <80%. The only exception is forced expiratory volume1/forced vital capacity (FEV1/FVC) ratio, where the cut off for low value is < 70%.
Second trick is to first look at FEV1/FVC to categorize it as obstructive or restrictive and then look at DLCO to find out the subtype.
3.2.1 Flow Volume Loop
Commonly used medications for obstructive airway disease
Fluticasone, beclomethasone, mometasone, budesonide, flunisolide, ciclesonide | |||
Frequent use (e.g., in patients with multiple exacerbation) may lead to development of drug-induced Cushing’s syndrome | |||
3.3 Asthma
Pathophysiology: A disorder of hyperactive airways due to inflammatory response to generally innocuous stimuli (such as allergens, cold, exercise, etc.), which lead to the following changes:
Depending upon the stimuli, asthma can be divided into the following categories:
Classic presentation: Acute sudden onset of wheezing and shortness of breath
that comes and goes. In earlier stages, patients are mostly asymptomatic in between the attacks. If asthma is not properly controlled, it can progress to fixed airway obstruction and at this stage patients can have persistent SOB or wheezing.
PFT is most helpful in confirming the dx of asthma
If FEV1 or FEV1/FVC is reduced (in moderate to severe asthma), NSIM is bronchodilator challenge (e.g., albuterol). If repeat PFT shows ≥ 12% increase in FEV1 and/or increase in 200 mL from baseline, this indicates reversibility of airway obstruction, suggestive of asthma.
If FEV1 and FEV1/FVC is normal, then NSIDx is either
3.3.1 Principles of Asthma Management
Prevent exposure to stimuli and treat other associated comorbidities that can worsen asthma (e.g., gastroesophageal reflux disease [GERD], morbid obesity, sinusitis, etc.).
Prevent asthma attacks (long-term management).
Treat acute asthma exacerbation.
Long-term management of asthma
Intensity of therapy depends on frequency and timing of attacks, as shown in the table below.
Daily occurrence of symptom (acute SOB and wheezing occurring at least once every day) | LDIS + LABAa | |||
Continuous symptoms (SOB and/or wheezing throughout the day) | High dose inhaled steroids (HDIS) + LABAb | |||
aIn asthma, LABA is not used alone and is always given in combination with inhaled steroids. Using LABA alone has been shown to increase risk of asthma-related complications. In contrast, LABA can be given alone in COPD. 13 In asthma, inhaled steroids have been shown to have the maximal benefit on the patient’s long-term quality of life. They work by reducing the airway reactivity, edema, and inflammation. | ||||
bWhen patients are on HDIS evaluate for appropriateness of omalizumab (monoclonal IgG antibody to IgE). Omalizumab is indicated in patients who have evidence of allergy in skin testing and IgE levels between 30 and 700 IU/mL. If IgE levels are too low, giving antibody to IgE is useless. If IgE levels are too high, antibodies to IgE are overwhelmed by the amount of IgE . | ||||
cSome patients with severe asthma might require chronic use of systemic steroids. In these patients an FDA approved procedure, called bronchial thermoplasty can be considered, which consists of bronchoscopy-mediated application of heat to reduce smooth muscle hypertrophy and obstruction. | ||||
Abbreviations: LABA, long-acting β2 agonist; SABA, shortong-acting β2 agonist; SOB, shortness of breath. | ||||
Few notes on long-term management of asthma
• Use of short-acting β2 agonist (SABA) > two times/week for acute symptoms indicates poor asthma control and signals the need for step-up of therapy.
If asthma is well controlled, always attempt to reduct dosage or discontinue inhaled steroids. This strategy will reduce incidence of side-effects (step-down management)
Consider subcutaneous allergen desensitization therapy in patients with persistent atopic asthma.
In patients with persistent asthma, alternative therapies such as mast cell stabilizers (cromolyn or nedocromil), theophylline, and leukotriene receptor antagonist (zafirlukast and montelukast) can also be considered.
13. Patient has hx of asthma and is on low-dose inhaled steroids (LDIS). He complains of wheezing and SOB every day for the last one week. What is the severity of asthma? What is the NSIM?
14. Patient has hx of asthma and is on LDIS+ long-acting β2 agonist (LABA). Currently patient reports that for the last few months, he has had to use his rescue inhaler (albuterol) only two times per week. What is the NSIM?
15. Patient has hx of asthma and is on LDIS. Patient has FEV1 of 50 in office. She reports that this is her baseline and feels SOB throughout the day. What is the severity of asthma? What is the NSIM?
16. Patient has hx of moderate persistent asthma and is on LDIS +LABA. Patient reports waking up at least two times per week with SOB. What is the NSIM?
3.3.2 Acute Asthma Attack
Example CCS: Patient with hx of asthma comes to emergency department (ED) with acute onset of shortness of breath(sob). Exam reveals diffuse wheezing. Peak expiratory flow rate (PEFR) at beside is 220 L/minute. Patient’s predicted PEFR value is 350. Patient’s PEFR is 62% of predicted (as 220/350 = 0.62).
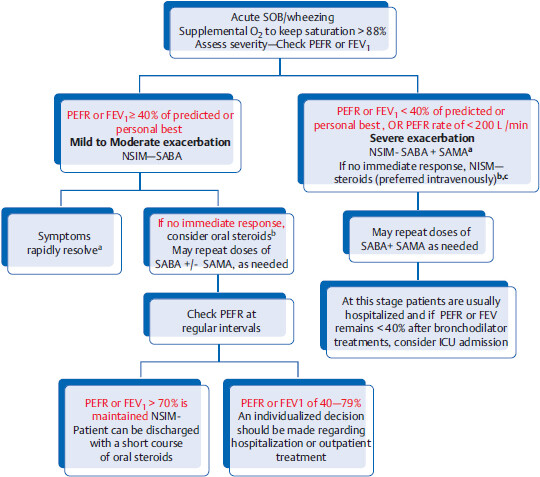
Algorithm for guidelines on acute asthma exacerbation management
Legend: NSIM, next step in management; SABA, short-acting B2 agonist (albuterol); SAMA, short-acting muscarinic antagonist (e.g., ipratropium); PEFR, peak expiratory flow rate.
aIf patient improves after the first bronchodilator treatment with PEFR of > 70% of predicted or personal best, patient can be discharged without systemic steroids.
bAfter first round of SABA +/- SAMA, if patient still has persistent wheezing and no improvement, NSIM is to give steroids (oral or intravenous). Time to order CXR.
cIf any of the following signs/symptoms are present, even after adequate bronchodilator therapy, NSIM is to consider intubation and IV magnesium sulfate.
15 There is so much lung hyperinflation that it is increasing the intrathoracic pressure, leading to greater than expected drop in systolic pulse pressure (> 20 mmHg) on inspiration. (The pathophysiology is similar to auto-PEEP.)
• Inaudible or decreased breath sounds on lung exam: in this scenario, the airway obstruction is so severe that there is no air movement (clinically called silent chest).
• PaCO2 is N or ↑ : PaCO2 is low in acute asthmatic attack, due to tachypnea and hyperventilation, but if PaCO2 is N or ↑ then it is either due to severe obstruction or patient’s respiratory muscles are tiring.
16 Decision to intubate should not be made based on ABG alone. If ABG shows PaO2 of > 60 mmHg and high PaCO2, but patient is speaking in full sentences and clinically appears better, then intubation is likely not necessary. In this case, patient is likely improving from severe asthma attack and is responding to treatment.
Acute asthma management—additional points
Prior to discharge remember the indications for vaccination which include flu and pneumococcal vaccination (same schedule as in COPD). Also, assess underlying severity of asthma (to prescribe LDIS or MDIS or LABA, if needed).
Antibiotics are only indicated if there is hx suggestive of bacterial bronchitis or pneumonia.
Do not give mast cell stabilizers (nedocromil and cromolyn sodium) for acute episode.
3.3.3 Other Forms of Asthma
Conditions presenting with asthma-like symptoms (low yield)
Patients with the following conditions can present with difficult to control asthma or worsening asthma symptoms in a previously well-controlled patient:
3.4 Chronic Obstructive Pulmonary Disease
Pathophysiology: Long-term exposure to irritants, such as tobacco smoking, air pollution, exposure to poorly ventilated cooking and heating fires (particularly in developing world), and genetic factors can lead to an inflammatory response in the lungs, causing COPD.
17 Of all chronic smokers, 10 to 15% develop COPD. Ninety percent of COPD patients have smoking history.
COPD comprises of emphysema and chronic bronchitis.
18 Patients with COPD may not have a clearcut mphysema or chronic bronchitis. These frequently coexist and the management is similar.
Destruction of alveolar walls and respiratory bronchioles with subsequent loss in diffusion surface area | Chronic airway inflammation with mucosal hypersecretion (chronic productive cough) and hypertrophy (obstruction) | |
Patients have normal oxygenation (pink) but as the lungs are hard to deflate (due to poor lung recoil and diaphragmatic flattening), patients typically must puff out the air (puffers). Hence known as “Pink puffers” | Early stage: chronic sputum production (look for this to make a clinical dx of chronic bronchitis) | |
Main differentiating feature in PFTa | ||
a The diagnosis of COPD is made with PFT: FEV1/FVC ratio remains < 70% after bronchodilator challenge (signifying irreversible obstruction). | ||
Abbreviations: DLCO, diffusion lung capacity for carbon monoxide; HTN, hypertension; PFT, pulmonary function test. | ||
3.4.1 General Principles of COPD Management
Smoking cessation (most important intervention that increases long-term survival rate).
Symptomatic control to improve quality of life.
Pulmonary rehabilitation program is recommended in patients with FEV1 < 50% of predicted. This has been shown to improve SOB and quality of life.
Pharmacological measures (see section below).
Treatment of acute exacerbation of COPD.
Long-term home oxygen therapy (increases long-term survival rate) in select patients.
Vaccination: flu vaccine is given every year. For pneumococcal vaccine give PPSV23 alone at diagnosis. After 5 years or at the age of 65 (whichever comes late) give PCV13 followed by PPSV23 (booster dose).
19 All chronic lung diseases have this same schedule (e.g., asthma, bronchiectasis, interstitial lung disease, etc.)
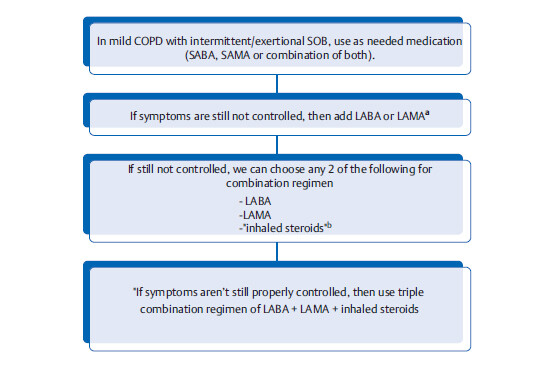
Pharmacological measures to improve symptoms in COPD
aLAMA is generally preferred because it is associated with reduced rate of exacerbation and it is also a once daily medication. Unlike asthma, in COPD inhaled steroids isn’t the 1st line therapy. In COPD, inhaled steroids have not been shown to alter long-term decline in FEV1; in-fact in old patients with advanced disease, inhaled steroids may actually increase the risk of pneumonia.
20 In COPD, inhaled steroids should not be used alone: use only in conjunction with either LABA or LAMA. In asthma, LABA should not be used alone: use only in conjunction with inhaled steroids.
bIn patients with evidence of reversibility of airway obstruction (asthmatic component), a combination regimen with LAMA or LABA + inhaled steroids might be preferred.
Abbreviations: NSIM, next step in management; SA BA/LABA, short-acting/long-acting B2 agonist; SAMA/LAMA, short-acting/long-acting muscarinic antagonist.
Other treatment modalities for refractory disease
Theophylline: It can be considered in patients with poor exercise tolerance despite triple therapy.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree