IMAGING OF INTRACRANIAL INFECTIONS
CARRIE P. MARDER AND KATHLEEN R. FINK
Intracranial infections are usually diagnosed by clinical assessment and laboratory investigations, particularly cerebrospinal fluid (CSF) analysis, combined with radiologic findings. Imaging plays an important role by providing or narrowing the differential diagnosis and occasionally identifying a particular entity that has a characteristic appearance, such as herpes simplex virus encephalitis, pyogenic abscess, or empyema. Imaging is also crucial for identifying complications of disease and assessing response to treatment. Finally, imaging contributes to the evaluation of opportunistic infections in immunocompromised patients and other patients at high risk for infection.
Here, we emphasize the overall strategy for imaging intracranial infections and highlight specific entities in which imaging findings contribute to diagnosis or management. We stress the major complications of intracranial infections and address special considerations for immunocompromised patients.
IMAGING STRATEGY
Patients suspected of harboring intracranial infection who present with altered mental status, seizures, or focal neurologic deficits should emergently undergo noncontrast computed tomography (NCCT) to exclude life-threatening conditions. In the acute setting, NCCT is the test of choice to assess for hydrocephalus, cerebral edema, mass lesions, or hemorrhage and is often performed prior to lumbar puncture (LP) to exclude impending brain herniation. NCCT is widely available, and the images are rapidly acquired, making the examination well tolerated even by critically ill patients.
Clinically stable patients in whom immediately life-threatening conditions have been excluded by NCCT often require further evaluation with contrast-enhanced magnetic resonance imaging (MRI), which has a greater sensitivity for leptomeningitis, ventriculitis, cerebral abscess, and empyema as well as downstream complications of infection such as infarctions. MRI lacks ionizing radiation, so it is relatively safe to perform, but specific contraindications include pacemakers and other implanted metallic devices or metallic foreign bodies. The risks and benefits of MRI should be weighed carefully in pregnant patients. Studies have not proven any negative effects of MRI to the fetus, but the American College of Radiology recommends deferring MRI until after pregnancy if possible (1). Because MRI acquisition time is much longer than CT, MRI may not be feasible in critically ill patients who require intensive monitoring. Additionally, if a patient is unable to lie still, motion artifact may significantly degrade the images obtained.
Gadolinium contrast agents improve the sensitivity of MRI but are generally avoided in patients with severe renal dysfunction and a glomerular filtration rate of less than 30 mL/min/1.73 m2 due to the risk for nephrogenic systemic fibrosis (2). Administration of gadolinium-based contrast should be avoided in pregnancy due to the unknown effects of exposure to free gadolinium ions on the developing fetus (1).
According to the American College of Radiology Appropriateness Criteria for headache (3), both NCCT and contrast-enhanced MRI of the head are usually appropriate for patients presenting with new headache and suspected meningitis or encephalitis, with the choice of test depending on local preference and availability. When MRI is unavailable or contraindicated, contrast-enhanced CT may be a suitable alternative. Cerebrovascular complications of infection are relatively frequent, and therefore magnetic resonance angiography (MRA) and magnetic resonance venography (MRV), either with or without contrast, may also be appropriate tests. Computed tomography angiography (CTA) may be performed when there is strong suspicion for vascular disease or to further evaluate abnormalities detected by MRA. Other advanced imaging techniques such as computed tomography perfusion (CTP) or magnetic resonance perfusion (MRP), magnetic resonance spectroscopy (MRS), and nuclear medicine studies such as single-photon emission computed tomography (SPECT) and 18F-fluorodeoxyglucose positron emission tomography (FDG-PET) or PET/CT are usually only performed for problem solving after the standard imaging evaluation has been performed.
For evaluation of a new headache in an HIV-positive or immunocompromised individual, MRI with or without contrast is usually appropriate as the initial imaging test (ACR Appropriateness Criteria “Headache” [3]). For an indeterminate cerebral mass in an immunocompromised patient, additional problem-solving techniques such as FDG-PET/CT, SPECT, MRS, and MRP may be helpful to narrow the differential diagnosis.
In neonates in whom the anterior fontanelle is patent, cranial ultrasound may be used to evaluate for hydrocephalus, subdural and epidural collections, and parenchymal masses (4).
Uncomplicated rhinosinusitis is usually managed clinically without the need for imaging, but for suspected intracranial or orbital complications of sinonasal disease, both NCCT and contrast-enhanced MRI of the head, orbits, and paranasal sinuses are usually appropriate, with CT and MRI serving as complementary examinations (ACR Appropriateness Criteria “Sinonasal Disease” [5]). Brain imaging is critical if there is a concern for intracranial extension and is best accomplished with contrast-enhanced MRI. If the patient is unable to tolerate gadolinium contrast, noncontrast MRI augmented with contrast-enhanced CT of the head and sinuses is recommended. Immunocompromised patients with acute or subacute rhinosinusitis are at high risk for developing intracranial or orbital complications, and therefore the threshold for obtaining imaging should be lower than for immunocompetent patients.
PYOGENIC INFECTIONS
Meningitis
Meningitis refers to inflammation of the pia and arachnoid membranes. LP with CSF analysis is the test of choice for diagnosis (6), and imaging plays an ancillary role.
Meningitis may be classified based on the pattern of involvement. Pyogenic and viral meningitis typically involve the cerebral cortices. Granulomatous or chronic meningitis typically involves the basal surfaces of the brain (basilar meningitis) and may be due to infectious or noninfectious causes. Pyogenic infections may also produce a basilar pattern of meningitis.
The primary goals of imaging are to evaluate for contraindications to LP and to exclude unexpected clinical mimics or complications. NCCT can satisfy these goals, but not all patients with suspected meningitis require CT. Reported risk factors for an abnormal CT in patients with suspected bacterial meningitis include age 60 years or older, immunocompromise, recent seizure, focal neurologic deficits, and impaired consciousness (7). In the absence of these clinical indicators, CT may not be necessary.
Five percent of patients with acute bacterial meningitis suffer brain herniation, and herniation accounts for 32% of deaths (8). A causal relationship between LP and brain herniation has not been proven, but generally accepted imaging contraindications to LP include midline shift, effacement of the basilar cisterns, and posterior fossa mass effect (8, 9). Clinical signs of increased intracranial pressure are also a contraindication to LP.
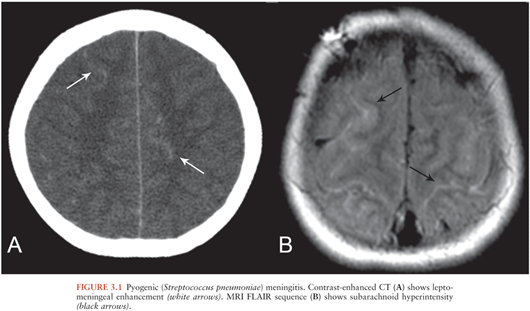
Imaging is usually normal in cases of bacterial meningitis (10). Imaging findings supporting the diagnosis include cerebral edema, inflammatory material in the subarachnoid spaces, and leptomeningeal enhancement. Cerebral edema manifests as narrowed or compressed sulci, ventricles, and basilar cisterns. Inflammatory material in the subarachnoid spaces manifests as hyperdense or enhancing material on CT (Fig. 3.1A) and abnormal fluid-attenuated inversion recovery (FLAIR) signal hyperintensity (Fig. 3.1B), enhancement, or restricted diffusion (11) on MRI. Leptomeningeal enhancement manifests as thin, linear enhancement extending along the sulci and basilar cisterns in a gyriform pattern (12). Leptomeningeal enhancement should not be confused with pachymeningeal enhancement, which refers to enhancement of the dura mater.
Imaging is also helpful to assess for complications of meningitis, including extensive cerebral edema resulting in brain herniation, infarcts, hydrocephalus, extraaxial pus collections, ventriculitis, cerebritis, and cerebral abscess.
Sterile subdural effusions occur with meningitis but, unlike infected extraaxial collections, generally resolve spontaneously. Sterile subdural effusions may develop internal membranes or septations and occasionally become infected, resulting in subdural empyema (SDE) (13). Subdural effusions occur more often in children with bacterial meningitis and typically develop over the frontal and temporal lobes (13). Sterile effusions appear similar to CSF in density or signal intensity or may be mildly proteinaceous, resulting in slight signal hyperintensity on FLAIR compared to CSF. They may be mistaken for prominent subarachnoid spaces, a normal finding in infants. One distinguishing feature is the finding of bridging vessels crossing the collections, which are present in prominent subarachnoid spaces but not subdural effusions (14).
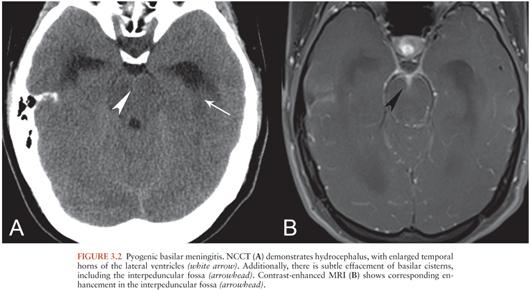
Hydrocephalus is a potentially life-threatening complication of meningitis resulting from impaired resorption of CSF by the arachnoid granulations or by diminished CSF outflow due to viscous material in the ventricles or basilar cisterns. Hydrocephalus may be the only imaging finding in patients with meningitis, particularly those with basilar meningitis (Fig. 3.2). In some cases, proteinaceous or enhancing material may also be evident in the basilar cisterns on CT (Fig. 3.2A) or MRI (Fig. 3.2B).
Cerebritis
Cerebritis refers to focal brain inflammation due to any cause, including pyogenic infection (15). Unlike meningitis, which is localized to the pia and arachnoid, cerebritis involves the brain parenchyma and may occur adjacent to infected subdural or epidural collections.
Cerebritis has a nonspecific imaging appearance. CT findings include focal low attenuation (16) (Fig. 3.3A) without enhancement or with nodular or peripheral enhancement, which may resemble infarct or mass lesion. MRI findings include hyperintensity on T2 and FLAIR (Fig. 3.3B) with variable enhancement (Fig. 3.3C). There may be hemorrhage or restricted diffusion. MRI appearance may be similar to that seen in status epilepticus, ischemia, or neoplasm.
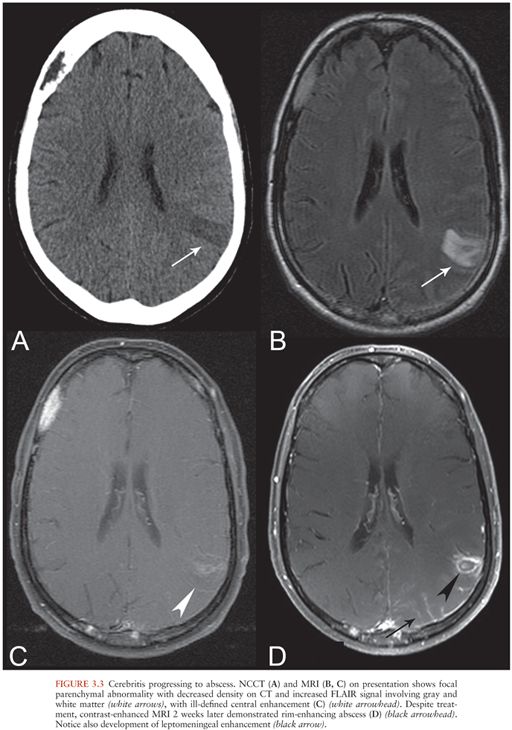
If inadequately treated, cerebritis may develop into a cerebral abscess, following a well-described progression through the stages of early cerebritis, late cerebritis, early abscess formation, and late abscess formation (16,17) (Fig. 3.3C and D). Features suggesting the formation of an abscess within an area of cerebritis include development of a ring-enhancing mass with restricted diffusion of the central cystic or necrotic core. This is in contrast to the restricted diffusion that may be seen with cerebritis, which affects the brain parenchyma itself.
Abscess
Cerebral abscess refers to a focal pus collection within brain parenchyma with a surrounding capsule (15). Abscesses may result from direct extension of local infection or from hematogenous spread. Local infections associated with cerebral abscesses include otomastoiditis, sinusitis, and odontogenic infections (18). Bloodborne infections may be associated with intravenous drug use, bacterial endocarditis, pulmonary infections, pulmonary arteriovenous malformations, congenital heart disease, and other causes (18–21). Cerebral abscesses may also occur after trauma or neurosurgical intervention.
Abscesses from bloodborne infections tend to be multiple and located at the gray–white junction, most commonly in the frontal lobes (18–21). Abscesses arising from local spread are often spatially related to the primary infection. For example, a frontal abscess may develop adjacent to frontal sinusitis. In these cases, the primary infection is usually visible on imaging.
Regardless of etiology, abscesses share common imaging features. On NCCT, abscesses appear as hypodense masses with surrounding vasogenic edema, sometimes with a hyperdense rim corresponding to the abscess capsule (16) (Fig. 3.4A). MRI also shows a localized mass with a T2-hyperintense necrotic core and a markedly T2-hypointense rim surrounding the collection that corresponds to the capsule (22) (Fig. 3.4B). The central core demonstrates markedly restricted diffusion (hyperintense on diffusion-weighted image [DWI] sequence [Fig. 3.4C] and dark on apparent diffusion coefficient [ADC] maps). Quantitative ADC values of the necrotic core are significantly lower for abscesses than necrotic neoplasms (23).
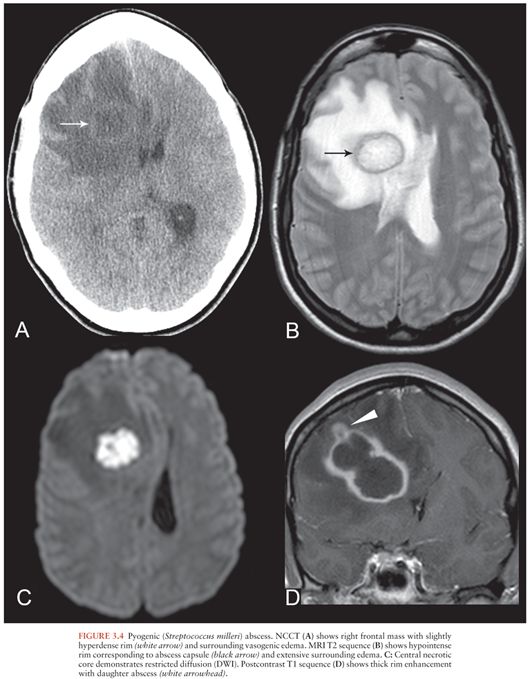
On both CT and MRI, cerebral abscesses demonstrate thick smooth rim enhancement, sometimes with thinning of the medial wall. Focal wall rupture results in formation of a daughter abscess (22) (Fig. 3.4D). In immunocompromised patients, ring enhancement may be absent and vasogenic edema may be mild (15), requiring a high index of suspicion for diagnosis.
Complications of cerebral abscess include mass effect and brain herniation. It is important to evaluate for intraventricular rupture of the abscess with resulting ventriculitis because this is a marker of poor prognosis that requires aggressive treatment (21). Imaging findings indicating ventricular rupture include layering debris in the lateral ventricles and enhancement of the ependymal lining.
The differential diagnosis of a ring-enhancing mass includes high-grade glial neoplasm, metastasis, and less commonly, tumefactive demyelination or subacute infarction. Usually, the clinical scenario helps distinguish these entities, but in difficult cases, MRP and MRS may be helpful.
MRP allows comparison of the cerebral blood volume of the lesion with that of contralateral normal white matter, resulting in a measure of relative cerebral blood volume (rCBV). The enhancing component of high-grade tumors demonstrates elevated rCBV (increased perfusion) compared to normal white matter, whereas pyogenic abscesses demonstrate significantly reduced rCBV, which is less than that of contralateral normal white matter (23,24). It is important to evaluate the enhancing rim of these lesions rather than the central necrotic regions, which do not statistically differ.
MRS evaluates the presence and relative ratios of metabolites present in a region of interest. Metabolites commonly evaluated include choline, a marker of cell membrane turnover; N-acetylaspartate (NAA), a marker of neuronal integrity; lactate, a marker of anaerobic metabolism; and lipid, a byproduct of necrosis. Creatine, a marker for energy metabolism, is often used as an internal control against which other metabolite peaks are compared. In the central necrotic core of both tumors and abscesses, the metabolite peaks of NAA, choline, and creatine are all depressed or absent (25). In brain abscesses, additional metabolite peaks may be present, including amino acids, alanine, acetate, succinate, and lactate/lipid (26). Although lactate and lipid may be present in necrotic tumors, the other metabolites are more specific to abscesses (23,25,27). Treatment alters the metabolite profile of the central abscess cavity and must be considered in the evaluation.
Ventriculitis
Ventriculitis may result from intraventricular rupture of an abscess, severe pyogenic meningitis (13), or as a complication after ventricular drainage procedures. Imaging findings include layering debris within the ventricles, which appears hyperdense on CT, hyperintense on FLAIR, and hypointense on T2 compared to CSF (28). Infected ventricular debris may also show markedly restricted diffusion, similar to the central core of an abscess. Hydrocephalus is usually present, and there may be enhancement of the ependymal lining. Ventricular septations may develop as a late sequela of ventriculitis (28).
Subdural Empyema
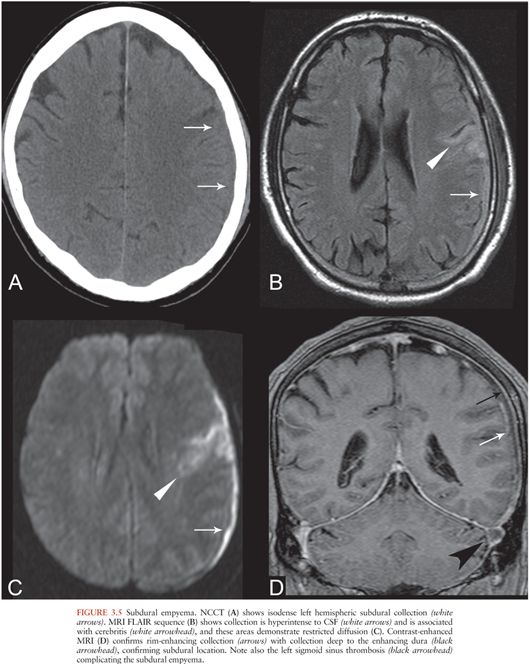
SDE refers to an infected subdural collection, occurring between the dura and arachnoid membranes. SDE is often associated with direct spread of infection from sinusitis or otitis media but also occurs as a complication of meningitis, trauma, or neurosurgical procedures. Seeding of subdural effusions in infants with meningitis, or seeding of subdural hematomas, also leads to SDE (29,30). Early recognition of SDE is vital because urgent surgical decompression is usually required (31).
On CT, SDE appears as a hypo- or isodense crescentic subdural collection (Fig. 3.5A) with rim enhancement (31), which may be subtle. On MRI, SDE appears as a proteinaceous subdural collection, hyperintense on T1 and FLAIR relative to CSF (Fig. 3.5B). This is in contrast to a subdural effusion, which follows CSF signal intensity on all sequences. SDEs are usually hyperintense on DWI (11) (Fig. 3.5C) and demonstrate rim enhancement (Fig. 3.5D), similar to other pus collections. Sterile subdural effusions may demonstrate mild rim enhancement but do not typically demonstrate restricted diffusion (32). Complications of SDE include dural venous sinus thrombosis (Fig. 3.5D), cerebral edema, cerebritis, and cerebral abscess (Fig. 3.5B and C).
Epidural Abscess
Epidural abscesses are usually the result of direct extension of adjacent infections, particularly sinusitis or otomastoiditis (33), but may also occur after trauma (30) or neurosurgical procedures. Infected material collects between the dura and calvarium. Epidural abscesses may extend to involve the subdural space and may be associated with cerebritis or cerebral abscesses.
Cranial epidural abscesses appear on NCCT as hypo- or isodense extraaxial collections in the epidural space (33) that may contain gas (Fig. 3.6A). On MRI, epidural abscesses are T2 hyperintense and T1 iso- or hypointense relative to brain, depending on the viscosity of the infectious material. There is usually intense enhancement of the dura (Fig. 3.6B).
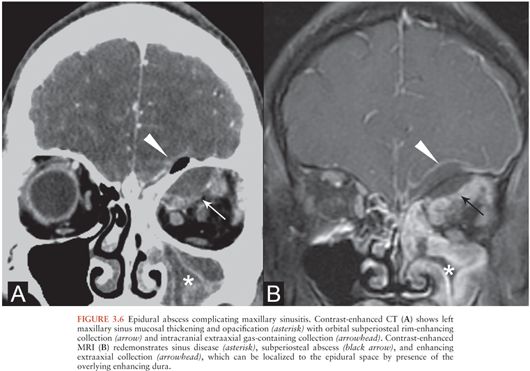
Coexistence of subdural and epidural collections is common, and differentiating them is sometimes difficult, particularly by CT (31,34). MRI may help by allowing delineation of the thickened or enhancing dura with respect to the collection (34). Additionally, like epidural hematomas, epidural abscesses tend to be lentiform and can freely cross dural reflections such as the falx but are bounded by the cranial sutures.
SPECIFIC ENTITIES
Tuberculosis
Tuberculous meningitis typically involves the basilar cisterns, which become filled with a thick inflammatory exudate (35). NCCT findings may be subtle and hydrocephalus may be the only finding, although isodense material in the basilar cisterns may be evident. Contrast-enhanced CT may demonstrate enhancing material in the basilar subarachnoid spaces (36), which may involve the pachymeninges. However, the absence of basilar meningeal enhancement should not preclude the diagnosis (37). Additional findings that support the diagnosis include infarcts and tuberculomas (36,37). HIV patients with tuberculous meningitis are more likely to have tuberculomas and infarcts rather than basilar enhancement or hydrocephalus (35).
MRI is useful in the assessment for tuberculous meningitis and allows better visualization of basilar meningeal enhancement (Fig. 3.7A), infarcts, pachymeningeal involvement, and tuberculomas than CT (38). MRI may also show enhancement of cranial nerves.
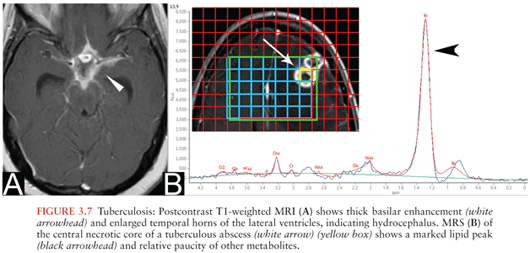
Tuberculomas are punctate or large granulomatous lesions that have a variable appearance depending on the extent of central caseation (39). On CT, tuberculomas may appear target-like with central calcification surrounded by an enhancing rim. On MRI, tuberculomas may be uniformly T2 hypointense or may appear target-like, with a T2-hyperintense core surrounded by a low T2 rim (39). Enhancement may be solid, nodular, or ringlike.
Tuberculous abscesses are encapsulated masses containing pus and viable mycobacteria that occur more commonly in immunocompromised patients (40). Unlike tuberculomas, tuberculous abscesses are not primarily granulomatous (35). By imaging, differentiating a tuberculoma with central caseation from a tuberculous abscess is difficult. Tuberculous abscesses also resemble pyogenic abscesses on conventional imaging, with rim enhancement and a central necrotic core demonstrating restricted diffusion. MRS may help differentiate a tuberculous abscess from pyogenic abscess or necrotic tumor. Metabolites specifically associated with tuberculous abscesses include high lipid and lactate peaks (Fig. 3.7B). Unlike pyogenic abscesses, amino acid, succinate, acetate, and alanine peaks are absent (25,26).
Lyme Disease
Lyme neuroborreliosis refers to central nervous system (CNS) involvement of Lyme disease. Although no specific imaging findings exist, neuroborreliosis is often included in the differential diagnosis for nonspecific white matter lesions and may share overlapping features with multiple sclerosis. Most patients with neuroborreliosis appear normal on MRI. When imaging abnormalities exist, leptomeningeal enhancement and cranial nerve root enhancement may be equally as common as white matter lesions (41). The seventh cranial nerve is most commonly involved, followed by the third and fifth cranial nerves (42).
Syphilis
Neurosyphilis presents clinically in several discrete phases, with variable imaging findings in each stage. A high index of clinical suspicion is needed for diagnosis. Imaging may be normal or may show cerebral atrophy, nonspecific white matter lesions, parenchymal masses, or vascular complications including infarcts (43,44). T2/FLAIR-hyperintense lesions measuring less than 1 cm occur in the deep periventricular and subcortical white matter (43,44).
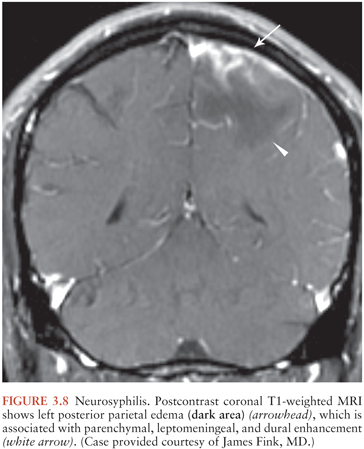
Syphilitic gummas are granulomatous lesions of the meninges that subsequently involve brain parenchyma or dura or both and that may contain Treponema pallidum. Gummas are hypodense on CT and T1 hypointense and T2 hyperintense on MRI, with mass-effect, enhancement, and surrounding vasogenic edema (44,45) (Fig. 3.8). Dural thickening indicates dural involvement. Gummas typically occur in the cerebral hemispheres but may also appear in unexpected locations, such as the pituitary gland (45).
Meningeal syphilis may manifest as meningeal enhancement on CT or MRI. Cranial nerve enhancement may also occur, often involving cranial nerves VII and VIII (46). Syphilitic vasculitis (meningovascular syphilis) may affect medium and large vessels (Heubner arteritis) or small vessels (Nissl-Alzheimer type) (46). Infarctions can complicate either type. Angiographic findings of infectious vasculitis are discussed further later in this chapter.
Neurocysticercosis
Neurocysticercosis results from CNS invasion by the parasitic organism Taenia solium and may involve brain parenchyma, ventricles, or subarachnoid spaces. The imaging appearance varies with location and stage of infection.
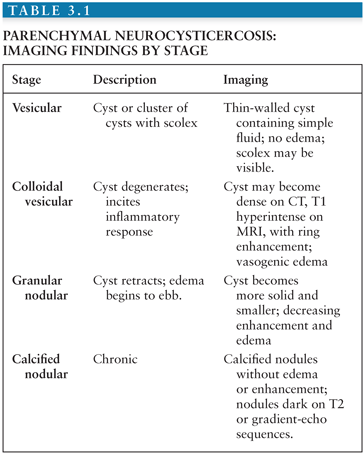
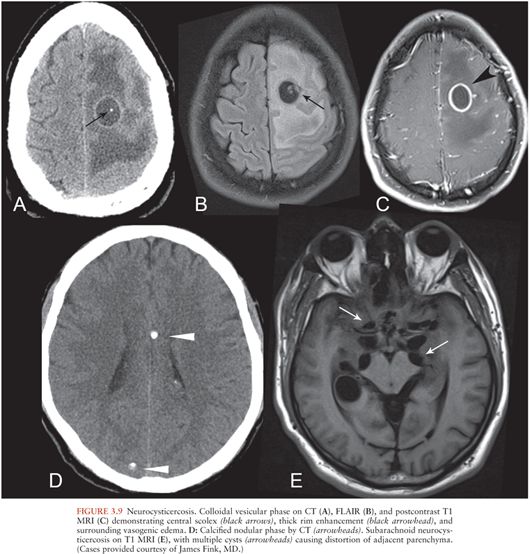
Parenchymal neurocysticercosis can be classified into four stages from acute to chronic (47), as summarized in Table 3.1. In the vesicular stage, a thin-walled cyst forms containing the invaginated scolex, which may be visible on FLAIR and contrast-enhanced sequences (48). During the colloidal vesicular stage, the cyst degenerates, becomes proteinaceous, and the scolex may disappear (Fig. 3.9A–C). The granular nodular stage occurs as the cyst retracts. Vasogenic edema lessens, although nodular or ring enhancement persists. Differentiating the colloidal vesicular stage from the granular nodular stage may be difficult (47). Finally, during the nodular calcified stage, edema and enhancement subside, leaving a small calcified nodule (Fig. 3.9D). MRI is best for identifying lesions in the vesicular, colloidal vesicular, and granular nodular stages. CT is excellent at detecting lesions in the nodular calcified stage (47), as is MR gradient-echo sequence.
Intraventricular neurocysticercosis may occur alone or in conjunction with parenchymal neurocysticercosis. Imaging findings include cystic lesions within the ventricles, commonly in the fourth ventricle (47). There may be associated noncommunicating hydrocephalus. Cysts usually are thin walled and contain CSF-like fluid, making them difficult to see on standard MRI sequences. High-resolution heavily T2-weighted MRI sequences (MR cisternography) may help to delineate the cyst walls.
Neurocysticercosis may also involve the subarachnoid spaces, particularly the basilar cisterns, and appears as multilobular cystic lesions (“racemose” neurocysticercosis) (Fig. 3.9E). The scolex is often not visible.
Creutzfeldt-Jakob Disease
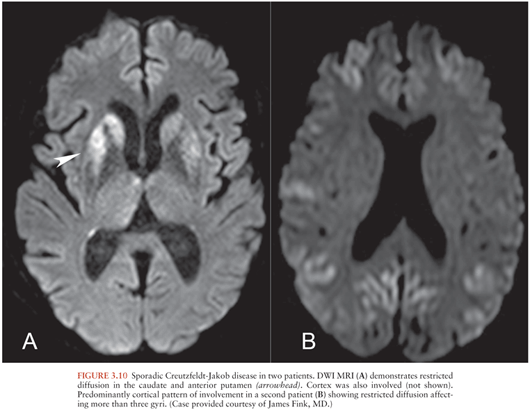
Creutzfeldt-Jakob disease (CJD) is a prion disease that may be sporadic, hereditary, or acquired from exposure to infected CNS tissue. Variant CJD (vCJD) most commonly occurs after consumption of meat from cows infected with bovine spongiform encephalopathy. Kuru occurs with cannibalism. Iatrogenic CJD occurs with surgical exposures such as corneal transplantation. Most cases of CJD are sporadic (sCJD) (49). The imaging appearances of sCJD and vCJD have been best described.
MRI is the preferred imaging modality (49) to support the diagnosis and exclude other etiologies. Criteria to support the diagnosis of sCJD include DWI or FLAIR signal hyperintensity in the caudate nucleus and putamen (Fig. 3.10A), with involvement of at least one cortical gyrus or involvement of more than three cortical gyri (50) (Fig. 3.10B). The precentral gyrus is usually spared (51). Globus pallidus, thalamus, and periaqueductal gray matter may be involved (49). Cortical atrophy occurs with disease progression.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree