COMPLICATIONS OF INFECTIVE ENDOCARDITIS
STEFANO GIULIERI, RETO ANTOINE MEULI, AND MATTHIAS CAVASSINI
Central nervous system (CNS) complications of infective endocarditis (IE) occur in about 30% of patients, with the highest incidence among patients referred to tertiary care centers and intensive care units, as well as patients with mitral valve endocarditis and IE due to virulent microorganisms (e.g., Staphylococcus aureus, gram-negative bacilli, fungi). Ischemic stroke accounts for up to two thirds of CNS complications, followed by intracranial hemorrhage, meningitis, brain abscess, intracranial infectious aneurysm (ICIA), and encephalopathy. Embolization of infected vegetations is the main pathogenetic mechanism. Imaging (both computed tomography [CT] and magnetic resonance imaging [MRI], coupled with noninvasive angiography) plays a pivotal role in the diagnosis of CNS complications. Optimal management includes early institution of antimicrobial treatment, which has the highest impact on the risk of further emboli. Anticoagulation should be cautiously continued in patients with established cardiac indications (e.g., prosthetic valve). In the presence of an established indication, patients with IE and ischemic stroke should undergo cardiac surgery without delay, whereas it should be postponed for at least 4 weeks in case of intracerebral hematoma. Unruptured ICIA may be managed medically with radiologic follow-up, whereas enlarging or ruptured ICIA should be treated surgically or by an endovascular approach. Despite improvement in the management, patients with CNS complications generally carry a worse prognosis compared to patients without neurologic complications.
HISTORY
Infective endocarditis (IE) was probably recognized as early as 1646 by Rivière, but the full clinical spectrum of “malignant endocarditis” was first comprehensively described by Sir William Osler (1) in his Gulstonian lectures at the Royal College of Physicians in London in 1885. He not only described “the different modes of onset, and the extraordinary diversity of symptoms which may arise,” but carefully analyzed the pathophysiology of distant complications, particularly of central nervous system (CNS) manifestations such as meningitis: “The meningeal complications of endocarditis have not received much attention, considering the frequency with which it has occurred . . . somewhat over 12 per cent” (1). He also related IE and mycotic aneurysms to a common infectious etiology and later emphasized the central importance of blood cultures in diagnosis (2).
EPIDEMIOLOGY
In developed countries, the reported incidence of IE is 3 to 7 per 100,000 per year (3–6). This rate has remained fairly constant over time (7). Incidence of IE increases sharply with age, with a peak of 15 episodes per 100,000 per year in patients older than 70 years (4,8). The male:female ratio is 2:1. In-hospital and 6-month mortality in recent series are 15% to 20% and 25%, respectively (9). These apparently stable overall rates of incidence and mortality mask the continually evolving spectrum of IE and the contrasts that exist between various types of IE. For instance, subacute IE due to viridans streptococci has an early mortality rate of only 5% to 10%, whereas acute prosthetic valve endocarditis (PVE) in an elderly patient with congestive heart failure can be fatal in up to 70% of patients. The overall figures reflect shifting frequency of predisposing factors. In developed countries, the decreasing prevalence of chronic rheumatic heart disease is counterbalanced by an increased number of patients with prosthetic valves and intravascular devices, injection drug use, and elderly patients with degenerative heart disease (10). The mean age of patients presenting with IE has increased, from 34 to 43 years in the early antibiotic period to 52 to 55 years more recently, and has even passed 60 years in some reports (3,11,12).
PREDISPOSING FACTORS
Degenerative valve disease has replaced rheumatic heart disease as the main underlying condition in patients with IE (10). Degenerative valvular disease with calcified atheromatous deposits is associated with IE in a proportion of cases that increases with age and may be a predisposing factor in up to 50% of the IE cases in elderly patients (13). Mitral valve prolapse (MVP) associated with valve dysfunction is another recognized risk factor for IE, found in 10% to 30% of patients (14). In a case–control study, the risk of IE was eight times higher among patients with MVP (15), but, given the high incidence of MVP (5%) in the general population, the absolute risk per patient is low, estimated to be 0.0175% per year (14).
Although it remains a common predisposing condition in developing countries, rheumatic heart disease is associated with IE in less than 5% of cases in the Western countries (16). Congenital lesions are found in 6% to 20% of cases (17), mostly ventricular septal defect, bicuspid aortic valve, patent ductus arteriosus, and tetralogy of Fallot. A bicuspid aortic valve is an important factor in elderly persons in whom it may be present in up to 20% of IE cases; this is probably because of associated valvular sclerosis and abnormalities of blood flow (18).
Prosthetic valves account for 7% to 25% of the cases of IE and the annual incidence of IE in patients with prosthetic valves is approximately 1% (19). In-hospital mortality rate ranges from 20% to 30% (20).
IE related to hospitalization and ambulatory invasive treatments (health care associated) now accounts for one third of cases (21). The proportion of IE associated with other intracardiac devices such as pacemakers or implantable cardioverter-defibrillators was 6.4% in the ICE-PCS (22). Nosocomial bacteremias (mainly Staphylococcus aureus) represents as many as 25% of the cases in certain series (23,24). IE is reported in 2% to 6% of patients undergoing long-term hemodialysis (25). Intravenous drug users (IVDUs) are at high risk for IE. Moreover, 20% to 40% of IVDUs suffering from IE have preexisting cardiac lesions, often caused by previous infection (26).
PATHOGENESIS
Certain lesions of the endothelium of the valve or heart cavities can easily be infected should a bacteremia occur. This has been shown in animal models: An intravascular polyethylene catheter placed across the aortic or the tricuspid valve will induce the deposition of platelets and fibrin and lead to the formation of nonbacterial thrombotic endocarditis (NBTE) (27). Many factors can induce endothelial lesions, which promote the formation of NBTE. The most important are valvular organic lesions with associated perturbations of blood flow and prosthetic valves. Even microscopic lesions are prone to infection by circulating bacteria (28), so not surprisingly, up to 50% of patients with IE have no known predisposing heart condition at the time of diagnosis (29). Transient asymptomatic bacteremias occur frequently, often following minor mucosal trauma induced by daily domestic activities such as chewing or tooth brushing. Bacteremia may also be triggered by various iatrogenic procedures. Among the many bacterial species that can be recovered during transient bacteremias, streptococci account for most IE cases, probably because of the frequency with which they enter the bloodstream and the adherence properties of their surfaces.
Circulating bacteria readily attach to NBTE. The attachment process is probably mediated on the host side by receptor-like structures such as fibronectin, fibrinogen, laminin, or collagen, which interact with the surface of bacteria (8). Platelets also play a role in these initial events (30,31). On the microbial side, only bacteria with the ability to adhere to valvular lesions can produce IE. Gram-positive cocci (e.g., staphylococci, streptococci) adhere more strongly than enterobacteria to heart valves (32).
Once microorganisms have attached to NBTE, they start to multiply and stimulate further deposition of fibrin and platelets through the activation of tissue factors or procoagulant activity. This thrombotic process buries some bacterial colonies deep within the vegetation where they are protected from circulating neutrophils. This has led to the concept of the vegetation as an area of “localized agranulocytosis.” Bacteria deeply seated in the vegetation are metabolically inactive and thus resistant to the action of some antibiotics. Furthermore, some antibiotics do not fully penetrate into the vegetation. This combination of factors favors the bacteria and helps explain why cure of endocarditis requires the prolonged administration of bactericidal antibiotics.
Because the infected vegetation is located within the bloodstream, persistent bacteremia is a hallmark of bacterial endocarditis, permitting the diagnosis through blood cultures in most patients. Moreover, further deposition of platelets, fibrin, and circulating bacteria will compensate for the fragmentation, embolism, and/or resorption of the vegetation by the inflammatory response, in a delicate and complex balance. IE also induces immunologic responses that contribute to some clinical manifestations of IE such as glomerulonephritis or vasculitis (30).
ETIOLOGIC AGENTS
The list of microorganisms that can cause IE includes most human pathogenic bacteria, as well as rickettsiae, Bartonella, chlamydiae, and fungi (19). However, gram-positive cocci predominate. The pattern of distribution of these etiologic agents differs profoundly according to underlying risk factors, the valve type (native vs. prosthetic), and geography (33).
Native Valve Endocarditis
S. aureus is now as common as streptococci as a causative pathogen of native valve endocarditis (NVE) in developed countries (6). In the International Collaboration on Endocarditis-Prospective Cohort Study (ICE-PCS), S. aureus and streptococci both accounted for 32% of 1,881 cases of definite NVE (16). The increase in frequency of S. aureus IE is explained by several invasive medical procedures associated with S. aureus bacteremia (e.g., hemodialysis, long-term intravenous catheters) (24). The clinical course of S. aureus IE is usually acute and complicated. In-hospital mortality rate was 22% in a recent prospective study (24). Coagulase-negative staphylococci rarely cause NVE but appear to be increasing in frequency and can pursue an aggressive clinical course (34). Special attention has been drawn to Staphylococcus lugdunensis, being commonly associated with valve destruction (35).
In a recent French series of 497 patients with definite IE (79% with NVE), streptococci, although less frequent compared to a previous study (4), were still the most common cause of IE and were responsible for 48% of cases (6). The viridans group (e.g., Streptococcus sanguis, Streptococcus oralis, Streptococcus mutans, and Streptococcus mitis) accounts for at least two thirds of these cases. The clinical course is generally subacute and an underlying cardiac condition is often present. Streptococcus milleri, an occasional cause of IE, has a peculiar propensity to cause distant abscesses and local perivalvular invasion (36). Streptococcus bovis group (including S. gallolyticus subsp. gallolyticus, S. gallolyticus subsp. pasterianus, and S. infantarius subsp. coli) accounts for up to 20% of streptococcal IE (37) and is often associated with neoplasms of the gut, of which it may be the first manifestation (38,39). Streptococcus pneumoniae and streptococci of the Lancefield groups A, B, C, G may also occasionally cause endocarditis (40,41). Enterococci account for 10% of all IE (42); these strains are particularly resistant to the bactericidal activity of antibiotics. Older people are usually affected and outcome can be fatal in approximately 16% of patients (42).
Numerous other bacteria are well known but uncommon causes of NVE, for example, Coxiella burnetii, Brucella, and bacteria of the “HACEK” group (Haemophilus species, Actinobacillus actinomycetemcomitans, Cardiobacterium hominis, Eikenella corrodens, Kingella species) (43). Actinobacillus actinomycetemcomitans has now been reclassified as Aggregatibacter actinomycetemcomitans (44). Bartonella is an unusual cause of endocarditis in homeless people (45).
Prosthetic Valve Endocarditis
PVE cases with onset within 2 months after surgery are called early PVE (46). Early PVE cases are presumably related to intraoperative contamination. S. aureus is responsible for 35% of early PVE cases; coagulase-negative staphylococci are found in 17% of cases. The frequency of gram-negative bacilli (10% to 20%), diphtheroids (0% to 3%), and fungi (5% to 10%) is higher in PVE than in NVE (47), and they are usually isolated in early PVE.
Cases occurring 2 months or more after surgery are called late PVE and are largely community acquired. As compared to early PVE, streptococci are more common and are responsible for approximately 20% of late PVE cases (46).
Intravenous Drug Users
S. aureus accounts for more than 50% of IVDU cases probably because of its high frequency on the skin in local infections at the injection site (cellulitis, abscesses, suppurative thrombophlebitis) and on drug paraphernalia (48). In some regions, methicillin-resistant S. aureus (MRSA) have been responsible for IE among IVDUs. Several other bacterial species such as Pseudomonas aeruginosa, enterococci, and Serratia species or fungi are all more common in addicts than in the general population (49). In 40% to 70% of the cases, the tricuspid valve is affected (50). Right-sided IE among IVDUs has a mortality rate of less than 10% (48).
Culture-Negative Infective Endocarditis
Patients with a clinical presentation suggestive of IE but with negative blood cultures account for 5% to 10% of the cases (8). The most common cause for culture-negative IE is prior administration of antibiotics. In the absence of prior antibiotic administration and with appropriate blood culture media, most bacteria will eventually grow but may require repeated blood culture and prolonged incubation periods (51). Some microorganisms, such as Aspergillus species, C. burnetii, Legionella species (usually Legionella pneumophila), or Tropheryma whippelii, cannot be recovered from the blood, so diagnosis relies on other methods such as tissue culture, in situ hybridization, polymerase chain reaction (52), or special serologic tests (53). By applying a standardized diagnostic algorithm, Fournier et al. (33) were able to identify an etiologic agent among 476 out of 759 (63%) patients referred for blood culture–negative IE. C. burnetii (229 cases) and Bartonella sp. (86 cases) were the most common etiologic agents. A noninfectious origin of culture-negative IE (e.g., marantic endocarditis, systemic lupus erythematosus) was diagnosed in 19 patients.
CLINICAL MANIFESTATIONS
The signs and symptoms of IE are extremely variable because of the diversity of the etiologic agents and the various organs involved. Clinical presentation may range from a chronic disease with unspecific systemic symptoms to acute life-threatening sepsis. Fever with a heart murmur is the most common clinical presentation; it may be the only clue to the diagnosis, especially early during the course of the disease and with microorganisms of low virulence. In a prospective study of 109 episodes of IE, fever was present in 98% of cases (54). A new murmur and skin lesions were found in 59% and 32%, respectively. Splenomegaly and musculoskeletal manifestations were present in 16% (54). Congestive heart failure complicates one third of episodes in recent studies (4,16,54). Distant complications such as major emboli, lumbar pain, or rupture of a infectious aneurysm may be the first presenting manifestations of IE. All the various clinical manifestations of IE arise from one or more of four main mechanisms: bacteremia, embolization (55), immunologic manifestations (56), and local complications (57).
DIAGNOSIS
The diagnosis of IE requires the integration of clinical, microbiologic, and echocardiographic data. Hematologic abnormalities are frequently observed: Anemia, leukocytosis, and thrombocytopenia are present in 66%, 50%, and 18% of cases, respectively. Urinalysis shows abnormalities in 30% or more of patients, mainly evidence of proteinuria or microscopic hematuria, one third of patients has elevated creatinine (54). Circulating immune complexes may be detected, but they are nondiagnostic (56). Repeated electrocardiography can reveal new atrioventricular block, particularly in the setting of aortic valve endocarditis, suggesting perivalvular invasion (58).
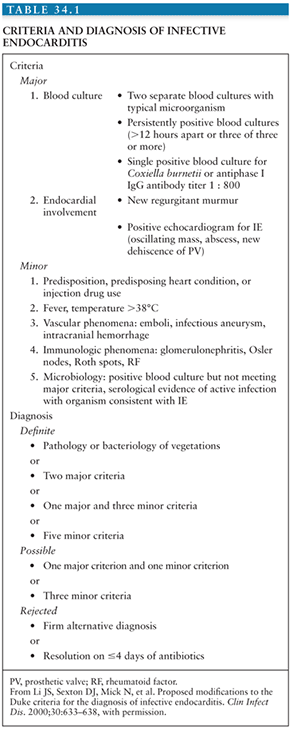
The Duke criteria combine predisposing factors to IE, the blood culture isolate or histologic examination, and echocardiographic findings (59). Based on the number of major and minor criteria, any case of suspected IE is classified as “definite,” “possible,” or “rejected” IE. These criteria have been primarily developed for research purpose, but they can help integrate diagnostic findings (Table 34.1)
Echocardiography has proven to be a valuable diagnostic tool in patients with suspected IE. Transesophageal echocardiography (TEE) has substantially better ability to detect vegetations, perivalvular extension, and myocardial abscesses than transthoracic echocardiography (TTE). TTE has a sensitivity of 40% to 63% for detecting vegetations in NVE (60). TEE increases the sensitivity for detecting vegetations in NVE to more than 90% (61). TEE is particularly useful in PVE, with which vegetations are detected in more than 80% of the cases compared with less than 30% with TTE. A negative TEE has a negative predictive value for IE of 86% to 97%.
TREATMENT AND PREVENTION
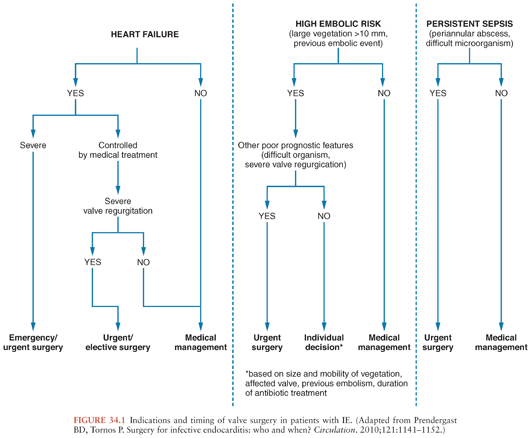
Once the diagnosis of IE has been made, antibiotic treatment should be started promptly to eradicate the infecting microorganisms as soon as possible. It is well established that a prolonged course of antibiotics is needed for cure even if the microorganisms are highly sensitive to the drug used. The antibiotic agent must be bactericidal and should be selected according to in vitro susceptibility test results and the experience gathered from experimental and clinical studies (10,62). Surgery has an important role in the management of IE, as an optimal therapeutic approach requires operative intervention during the course of medical treatment in almost 50% of patients with IE (63). In the ICE-PCS, the rate of surgical intervention (48%) was comparable among patients with NVE and PVE (16). Nearly all patients with fungal endocarditis require combined surgical and medical treatment (64). Hemodynamic deterioration, local complications, and uncontrolled infection are the major indications for surgery (10,65). Although the need for surgery in patients presenting with hemodynamic deterioration and uncontrolled infection is widely recognized (66), it remains controversial whether surgery is indicated for prevention of systemic embolism. In a randomized study from Korea, patients at high risk for systemic embolism (i.e., with severe valvular regurgitation and large vegetations) had significantly less systemic embolic events if operated early during the course of disease (67). Figure 34.1 summarizes current indications for valve surgery in patients with IE. Other interventions that should be considered in patients with IE are the removal of intravascular devices (e.g., pacemakers, intracardiac cardioverter defibrillators, central venous catheters) (68), and the drainage of extracardiac infections (e.g., septic arthritis).
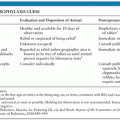
Attempted prevention of IE is aimed at patients with known underlying predisposing factors who undergo a procedure that may result in bacteremia. Possible portals of entry, such as oral and dental lesions or urinary or gastrointestinal tract pathology, should be sought and treated if necessary. Recommendations for use of prophylactic antibiotics have been updated, and indications for prophylaxis have been considerably restricted to four high-risk conditions: prosthetic cardiac valve, history of previous IE, some forms of congenital heart disease (CHD) (e.g., unrepaired CHD), and heart transplantation recipients with valvulopathy (69).
CENTRAL NERVOUS SYSTEM COMPLICATIONS OF INFECTIVE ENDOCARDITIS
Among the various manifestations of IE, neurologic complications are particularly important for three main reasons: They occur often, they may be the first or the predominant manifestation of the disease, and they are a leading cause of death and complications due to IE (70).
Incidence
The reported overall incidence of CNS complications of IE varies greatly. In most series, the incidence of CNS involvement during the course of IE ranges between 20% and 40%, with an average of 30%. The incidence has not changed much over time, despite wide differences in the diagnostic criteria employed in various studies and many evolutionary changes that have occurred in the natural history of IE over the past 60 years. Among 743 patients pooled from seven series published before the advent of antibiotics, 176 (24%) were noted to have neurologic manifestations (1,71–76). Among 1,622 patients from eight studies reported between 1947 and 1978, 457 (28%) had such complications (77–85). Among 1,329 episodes of IE from seven series published between 1981 and 1993, 437 (33%) were accompanied by neurologic manifestations (11,86–93). And among 2,162 episodes of IE from eight series published between 1996 and 2007, 512 (24%) were complicated by neurologic events (70,94–101). Recent studies may indicate a lower rate of neurologic complications: The incidence of stroke in a French cohort of 390 patients and in the ICE-PCS was 18% and 17%, respectively (4,16). However, data on other neurologic complications were not reported. Finally, among 513 patients presenting with complicated native-valve IE, focal neurologic findings, and altered mental status were described in 18% and 16%, respectively (9).
Incidence of asymptomatic CNS events is much higher. By systematically using resonance imaging of the brain, Snygg-Martin et al. (102) identified cerebrovascular complications in 65% of patients; about half of them were asymptomatic. In another study of systematic brain imaging in IE, radiologic evidence of brain embolism was detected in 80% of patient, whereas 25% had clinical stroke (103).
Factors Influencing the Incidence of Central Nervous System Complications
The reported incidence is understandably higher in studies devoted primarily to the neurologic aspects of IE and in studies based on autopsies, because brain damage often causes or contributes to death in IE (11,78,79). In one autopsy series of 69 cases of IE, cerebral emboli were found in up to two thirds of the young adults and in 46% of the total cases (104). Frequency of CNS complications also tends to be higher in series gathered from referral centers (105) or specialized units. For example, among 198 patients with IE hospitalized in 33 intensive care units (ICUs) in France, 108 (55%) presented with neurologic complications (106). In contrast, the rate of CNS complications was only 22% in a series of IE observed in a large community hospital (11). This referral bias would alter not only the number but also the type and severity of the complications because referral is often precipitated by CNS involvement. On the other hand, in a series gathered from six hospitals of different types (university, private, and Veterans), the frequency of CNS complications was very similar (92).
Age and Sex
In many studies of adults with IE, the age of the patients did not appear to greatly influence the overall frequency of CNS complications. In a recent series, however, major neurologic events were recorded in 22%, 28%, and 34% of patients younger than 40 years, 40 to 60 years, and older than 60 years, respectively. In elderly patients, neurologic manifestations tend to be more common as a presenting clinical sign of IE (107); as many as one fourth to one third of them may present with neurologic signs (86), compared with 5% to 17% in the general population. If disorientation is included as a neurologic finding, the figure can be as high as 45%. Neurologic complications in children reported in five series ranged from 15% to 32% (108). Because major neurologic events are uncommon in children or young adults, everyone should recall the dictum, “In hemiplegia in young adults or children, always think of infective endocarditis.”
Generally, the sex distribution of the patients with or without neurologic complications appears to be similar in both sexes (83).
Location of Vegetations in the Heart
Neurologic complications are a hallmark of left-sided valvular abnormalities. This association was noted as early as 1852, before IE was recognized as a clinical entity (109). The incidence of neurologic complications is markedly lower in isolated right-sided IE. In a series of 40 IVDUs with tricuspid IE due to S. aureus, none presented with cerebral manifestations, whereas 2 of 5 of those with left-sided involvement had cerebral septic emboli (110). Of 97 episodes of IE in drug addicts, none of the nine major CNS events that were present on initial evaluation occurred in the 32 cases that involved the right side of the heart (111), although the infection was caused by highly virulent organisms such as S. aureus. CNS complications were observed in 54 (41%) of 133 patients with aortic and/or mitral valve involvement and in only 4 (12%) of 33 of those with tricuspid IE (92). When neurologic manifestations occur in right-sided IE, they present as meningitis, cerebral abscesses, or encephalopathy, rather than as strokes, and are most often related to virulent pathogens such as S. aureus or S. pneumoniae (112). Systemic embolization is rare in right-sided IE but does occasionally occur either from septic thrombi arising in the pulmonary veins or via paradoxical embolization through a patent foramen ovale, which is present in 18% of the normal population.
Mitral valve endocarditis is associated with a higher rate of neurologic complications (83,106,113,114). For example, in a recent French study of 198 patients with IE, mitral valve involvement was independently associated with neurologic complications (OR 1.54, CI 1.07 to 2.21) (106). A study from Duke University including 707 patients with IE found a twofold risk of stroke among patients with mitral valve endocarditis as compared to aortic valve endocarditis (114). This association was not reported by older studies (115), probably because of small size (88).
Neurologic complications in the context of PVE are of special concern because of the threat of superimposed intracerebral hemorrhage, which might be caused or promoted by anticoagulation. Most published data are derived from PVE complicating mechanical prosthetic valves. The overall incidence of focal neurologic events associated with PVE varies from 11% to 44% (46,116–122), which is considerably higher than the overall thromboembolic rate of 1% to 4% per year observed for anticoagulated patients with uninfected prosthetic valves (123).
Studies comparing the incidence of CNS complications between NVE and PVE have given conflicting results. In one study, 11 (13%) of 82 patients with NVE, compared to 6 (33%) of 18 with PVE, presented with such complications (117); however, another study reported 40 (35%) of 113 for patients with NVE and 24 (39%) of 62 for those with PVE (88). Other series did not report any statistically significant difference regarding the number of NVE and PVE CNS complications (46,98,124).
It is unclear whether the incidence of embolic events is affected by the time of onset of PVE in relation to the placement of the prosthetic valve. In one study, CNS embolic episodes were reported in 0% to 11% of patients with early onset PVE and in 23% to 28% of those with late-onset PVE (120). The higher rate in late-onset PVE was confirmed in another study in which peripheral manifestations occurred in 10% of early onset PVE cases, compared to 34% in late-onset PVE cases (125). However, in a recent prospective study of 78 cases of PVE, the rate of CNS complications did not differ between early and late PVE (126).
The incidence of CNS complications in PVE is influenced by anticoagulant therapy. In patients insufficiently anticoagulated, CNS complication rates of 38% to 71% were noted, compared to only 8% to 10% in patients on appropriate anticoagulant therapy (116,119). In one series of 61 patients, there was no difference in the rate of embolism or risk of bleeding between patients receiving no anticoagulation or subtherapeutic doses and those who were adequately anticoagulated (121). When anticoagulated patients develop neurologic complications, they are at high risk for major hemorrhage (83,120,127).
The type of prosthetic valve may influence the rate of CNS manifestations. Of 33 patients with bioprosthetic valve infection gathered from three studies, 4 (12%) had neurologic events (117,120,121), a figure lower than that with mechanical valves. However, in another study of 62 patients with PVE, there was no statistical difference when the type of prosthetic valve involved was compared among patients with and without neurologic complications (88).
In summary, patients with PVE do not appear to be at a much higher risk for CNS complications than patients with NVE, provided that anticoagulation is maintained and carefully controlled for complications associated with mechanical valves. However, those presenting a CNS complication are particularly threatened by the possibility of a massive cerebral hemorrhage (83,116,120).
Microbiology
An important factor in determining the rate, type, and severity of the neurologic complications is the nature of the microorganism causing the IE. This may account for some of the differences in the incidence noted between the various studies (Fig. 34.2). In series that have correlated the incidence of neurologic complications with the infectious agent causing IE, the frequency of CNS involvement ranged from 53% to 71% for S. aureus and was significantly higher than that observed with some other bacteria, particularly group D and non–group D streptococci, which ranged from 25% to 47% (83,88,92,97,113).
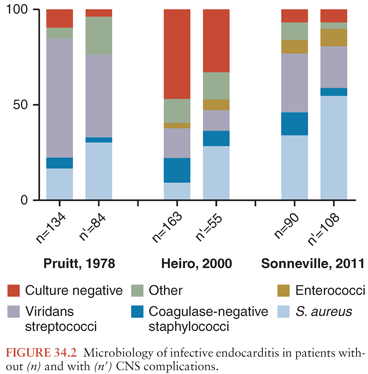
Some species of bacteria other than S. aureus, such as Enterobacteriaceae or anaerobic bacteria, have been associated with a high rate of neurologic complications (83,128). Moreover, certain microorganisms are prone to a high rate of certain types of neurologic involvement. For example, although S. pneumoniae has become rare in IE, causing only 1% to 3% of cases (129), in these few cases, associated pneumococcal meningitis is common, being found in 40% to 60% (130,131). Purulent complications are also frequently encountered with S. aureus IE, either as meningitis or as brain abscess. Certain bacteria such as the Haemophilus species, members of the genus Abiotrophia (formerly nutritionally variant streptococci), or fastidious organisms have been associated with large emboli; these emboli may occlude major vessels including cerebral arteries. In patients with culture-negative IE, major embolic phenomena, including those to the brain, were noted twice as often as in patients with culture-positive IE. Cases due to Candida spp. are also associated with large vegetations and a high rate of embolic phenomena (132,133). Of 27 patients with IE caused by Aspergillus species, a total of 14 neurologic manifestations secondary to emboli were recorded in 11 (41%) patients (134). In contrast, the rate of CNS complications in IE caused by some other bacteria, such as viridans streptococci or coagulase-negative staphylococci, appears to be lower. Of 128 patients with IE due to the latter bacteria, only 15 (12%) developed stroke (34).
Intravenous Drug Abuse
In IVDUs, as in all patients with IE, the frequency of cerebral manifestations is dependent on the side of the heart involved. Therefore, the reported incidence of neurologic complications will depend on the proportion of patients with exclusively right-sided involvement. Isolated right-sided involvement in IE has been reported in 9% to 72% in series of endocarditis in addicts (50,135). In a study comparing the clinical manifestations of S. aureus IE in addicts and nonaddicts, neurologic involvement was found in 51% of the nonaddicts, compared to only 9% of the addicts (110). Although neurologic complications were not categorized according to the side of the heart involved, 76% of the addicts had only tricuspid valve involvement, contrasted to 9% in the nonaddict population, indicating that the side involved plays an important role in the occurrence of neurologic complications (110). The frequency of neurologic complications in IVDUs with left-sided endocarditis appears to be higher than that in the nonaddict population, ranging from 45% to 58% (136). The higher complication rate may partly be due to the failure of some of the addicts to undergo a full course of antibiotics, or it may be due in part to a different pattern of etiologic agents.
Pathogenesis of Central Nervous System Complications
Neurologic complications of IE can arise through several mechanisms, as follows: occlusion of cerebral arteries by emboli, infected or not, derived from endocardial vegetations; infection of the meninges of the brain or of the walls of cerebral arteries by septic emboli or bacteremia; and microbial toxic effects or immune-mediated injuries. These events may result in various secondary lesions, including bland or hemorrhagic infarcts; intracerebral, subarachnoid, or subdural hemorrhages; focal expanding lesions such as abscesses or infectious aneurysms; and brain dysfunction due to one or multiple factors.
When embolization is the ultimate cause of the neurologic involvement, the lesions either may affect a single vessel and give focal signs or may affect multiple vessels and produce multifocal signs. Depending on whether ischemia is reversed before permanent changes occur, the clinical picture may be that of a transient ischemic attack (TIA) (137) or of a longer lasting obstruction resulting in brain damage. Because emboli of untreated or partially treated IE contain bacteria, the lesions produced may be ischemic, inflammatory, suppurative, or mixed. This may result in septic or aseptic meningitis, brain abscesses, microabscesses, or meningoencephalitis. If the wall of an artery or its vasa vasorum is involved, a infectious aneurysm may develop. Arterial rupture can occur in the absence of a detectable intracranial infectious aneurysm (ICIA) (88,138–140). Critical factors that may determine whether a septic embolus results in a bland infarct, a hemorrhagic infarct, a infectious aneurysm, or an abscess include the site where the embolus lodges, the virulence and number of the microorganisms, and importantly, the delay between the event and the initiation of antibiotic therapy.
Multiple other factors can cause or contribute to the neurologic manifestations of IE such as hypoxia, metabolic disturbances, drug toxicity, and toxic phenomena secondary to the systemic infection. Immune injury to small arteries is also likely to be involved (141), and proliferative endarteritis in the absence of local infection or embolization has been described.
In patients with tricuspid valve endocarditis, the sustained bacteremia rarely results in intracranial hemorrhage (ICH), even when due to virulent organisms, supporting the hypothesis that embolic fragments are a required factor in the pathogenesis of bleeding (138).
Clinical Presentation
Neurologic manifestations of IE constitute the presenting symptoms of IE in 16% to 23% of the cases (83,97,113). When a neurologic event is the presenting symptom, approximately two thirds are due to major cerebral emboli. The remaining third are divided among other manifestations including seizures, meningismus, subarachnoid, intracerebral, or subdural hemorrhage, personality change, visual disturbances, or weakness of the extremities (83,97).
Interestingly, the mean duration of prodromal symptoms prior to diagnosis was found to be similar in patients with and in those without neurologic complications (88).
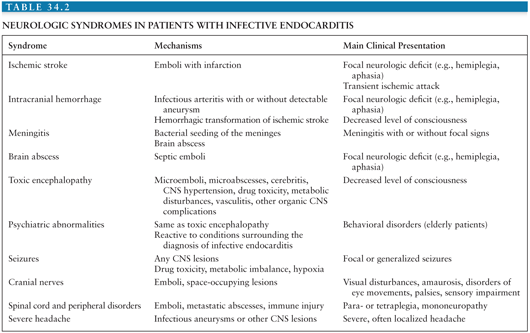
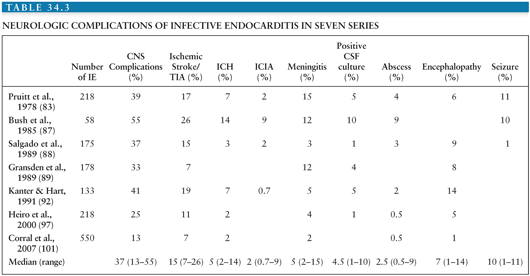
Neurologic complications occur after the initiation of antibiotic treatment in 30% of patients (88). In most of these, the neurologic events tend to occur soon after treatment has begun, usually within the first 2 weeks (88). However, cerebral emboli or rupture of ICIA rarely occurs from several months to up to 2 years after the completion of successful treatment. More than one complication is often observed in a given patient; thus, a total of 160 neurologic manifestations were recorded among 84 patients studied by Pruitt et al. (83). The neurologic complications of IE are numerous (Tables 34.2 and 34.3) and can mimic many neurologic diseases of other etiologies.
Stroke is the most common presentation and accounts for one half to two thirds of the neurologic manifestations (83,88,97,106,138). Most of these cases are due to cerebral emboli with infarction, but some are also due to intracerebral hemorrhage or even abscesses.
Meningitis, either septic or aseptic, is found in fewer than 10% of patients, with neurologic complications reported in most general reviews, although a rate as high as 37% has been reported (89). Meningeal symptoms or signs were encountered in 35 (42%) of 84 patients with neurologic complications reported by Pruitt et al. (83).
Decreased level of consciousness can be seen in association with embolism or hemorrhage. This can also occur without any specific identifiable cause, in which case nonspecific terms such as toxic encephalopathy or acute brain syndrome are often used (142). In one study of 63 patients with CNS complications of IE, 13% had a diffuse encephalopathy (i.e., alteration of the level of consciousness without focal brain lesions or meningitis) (101). These cases may be caused by various and often combined neurologic and nonneurologic mechanisms, such as microabscesses, microemboli, hypoxia, metabolic disturbances, bacterial toxins, or drug toxicity. The patients may present with symptoms of varying severity including impaired concentration, irritability, drowsiness, vertigo, or lethargy.
Seizures occur in 1.5% to 15% of the patients with neurologic manifestations (83,88). Among 141 children with IE, seizures were the most common neurologic manifestation, occurring in 10%. When seizures occur, they are often part of the presenting complex of symptoms (83). Generalized seizures occurred as the only neurologic symptom in 4 of 110 patients with CNS complications of IE (143). Three of these four patients also had focal components to the seizure activity. Focal seizures are usually the consequence of cerebral infarction. Generalized seizures can be the result of any of the organic lesions complicating IE and may be associated with other predisposing factors, such as hypoxia, metabolic disorders, or drug toxicity, especially when renal failure is present (83).
Mild, intermittent, diffuse headache is a common complaint in IE, occurring in 20% to 43% of the patients (87,144). However, severe or localized headache is found in only about 3% of patients with IE (92,144); this may be the initial symptom leading to the diagnosis of IE (145) or may indicate a disastrous complication. Indeed, 3 of 14 patients with IE and severe headache in one series (143), as well as 4 of 7 patients in another study (144), had ICIAs. Conversely, six of eight patients with ICIA in the latter study had severe localized headache, which should, therefore, prompt further investigation. This is also suggested by another study, in which 8 of 58 patients with IE complained of headache; a neurologic complication occurred in 7 of these 8 (87).
A wide variety of psychiatric abnormalities from minor personality changes to major psychiatric syndromes have been described in association with IE. This might be more common in elderly patients in whom confusion may be a presenting feature in up to 32% (96) or in patients with other underlying diseases, such as drug abuse or alcoholism (107). These psychiatric abnormalities may be caused by the same mechanisms that caused the toxic encephalopathy or they may only be reactive to the conditions surrounding the diagnosis of IE. In these cases, the neurologic examination and the cerebrospinal fluid (CSF) might be entirely normal, and fever and a cardiac murmur may be the only clue to the diagnosis of IE.
Various dyskinesias have been described, the most common being tremor, parkinsonism, ataxia, and myoclonus (146). Cases of chorea in the absence of evidence of rheumatic activity have been described (146).
Visual disturbance is a common manifestation of IE and may be due to retinal emboli or involvement of the peripheral or central pathways of cranial nerves II, III, IV, and VI. This may result in impairment in eye movements and varying degrees of visual loss (146,147). Iridocyclitis and panophthalmitis have also been described, especially in drug addicts. Other cranial nerve disorders can occur and pseudobulbar palsy has been described.
Abnormalities seen on funduscopic examination have been reported in 10% to 25% of patients with IE (86) and they have been observed in 35% of those with neurologic complications (143). These lesions are nonspecific. Papilledema was observed in 9 of 39 patients examined in a series of 110 cases of IE with CNS complications (143). It was probably related to various degrees of intracranial hypertension due to space-occupying lesions (148). Retinal hemorrhages are found in 10% to 25% of IE cases. They are thought to be the consequence of small emboli. Those with a white center are described as Roth spots, which occur in 2% to 9% of IE cases (149). They are probably due to a hypersensitivity reaction and not embolic. Microscopically, they consist of lymphocytes surrounded by edema and hemorrhage in the nerve fiber layer of the retina (150). In candidemia with or without associated endocarditis, funduscopic examination may reveal multiple white, cotton-like, circumscribed exudates with filamentous borders located in the chorioretina and extending into the vitreous cavity. They may initially be confused with Roth spots, but they may proceed to vitreous abscess and endophthalmitis (151).
Spinal cord involvement, mainly in relation with ischemic lesions but also secondary to extramedullary compression by metastatic abscesses, can be observed and may result in girdle pain and paraplegia.
Peripheral nerve involvement as a result of embolic or immunologic lesions may account for cases of localized pain or mononeuropathy (152,153).
Diagnostic Procedures
Imaging Studies
CT scan is of utmost importance for the diagnosis and management of CNS disorders associated with IE (88,154). Of 51 CT scans performed in 64 patients with neurologic complications of IE, 25 (39%) were abnormal. Focal lesions were discovered in 5 patients who presented with encephalopathy or headache but no focal deficits (88).
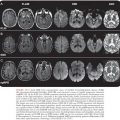
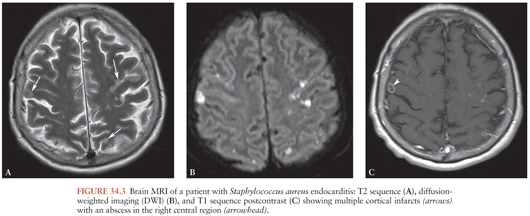
Brain MRI findings in IE play a major role in the diagnosis of multiple ischemic or hemorrhagic strokes, brain abscesses, and infectious aneurysms (Fig. 34.3)(155,156). MRI is very useful for the diagnosis of encephalopathy due to multiple microinfarcts and microabscesses that cannot be visualized by CT scan (155). MRI is more sensitive than the CT scan for most lesions including microhemorrhage. Magnetic resonance angiography (MRA) offers a noninvasive diagnostic method for intracranial aneurysms. Nevertheless, four-vessel cerebral angiography remains the method of reference (157). Diffusion-weighted imaging (DWI) allows the detection of ischemic lesions within minutes of symptom onset (158). DWI may be useful in differentiating cardioembolic stroke patterns originating from IE or NBTE (159). Patients with NBTE embolic events have multiple, widely distributed, small and large strokes, whereas patients with IE-associated embolic events exhibit a panoply of stroke patterns (159).
Asymptomatic lesions are frequently found among patients with IE, if MRI is systematically applied. For example, radiologic evidence of cerebral emboli can be shown among 65% to 80% of patients with IE, half of these lesions are asymptomatic (102,103). The clinical significance of asymptomatic emboli is unclear (160), and routine screening of patients with IE is not recommended. Asymptomatic microbleeds (i.e., cerebral microhemorrhages detected by T2 sequences) were identified among 57% of patients with IE in a French study (161). Given the strong association with IE in the case-control study (OR 6.12, CI 2.09 to 17.94), the authors suggest that this might be a new diagnostic criterion of IE. In a study of 26 patients with IE who underwent T2-weighted MRI, cerebral microbleeds were found in 14 patients (54%) and were strongly associated with subsequent ICH (162).
A French prospective study evaluated the impact of cerebral MRI on the management of IE. Among 130 patients, cerebral lesions were found in 82% and included ischemic lesions (52%), microbleeds (58%), and asymptomatic aneurysm (8%) (163). The systematic use of imaging modified the diagnostic or therapeutic management in 28% of patients. However, the authors did not study the impact of imaging on clinical outcome.
Cerebrospinal Fluid Examination
Despite similar rates of neurologic complications, the percentage of patients undergoing a lumbar puncture (LP) varied from 32% to 82% in four large series (83,88,92,145), with the most recent studies reporting the lowest rates. This is probably related to the recent availability of CT scan, which obviates the necessity for invasive procedures in many patients. Hence, there has been a decreasing number of LPs performed to investigate focal deficits in more recent studies (97).
The largest and most detailed study on CSF parameters in patients with neurologic complications of IE is from the Massachusetts General Hospital (83). CSF examination was performed in 69 (82%) of 84 patients. Neither the clinical setting nor any of the neurologic events were associated with a specific CSF formula, except for finding a purulent CSF more often in patients with meningeal signs. However, there was a good correlation between the CSF findings and the nature of the infecting microorganisms in that virulent bacteria such as S. aureus, enteric gram-negative bacilli, and S. pneumoniae were frequently associated with purulent CSF, whereas relatively avirulent bacteria such as viridans streptococci were usually associated with a normal or aseptic CSF. Positive culture of the CSF was found in only 11 of these 69 patients, and these were always associated with neutrophils in the CSF. There were eight cases of S. aureus and one case each of S. pneumoniae, Proteus mirabilis, and viridans streptococcal meningitis. The findings of this series are similar to those of other studies (88,92,143). Occasionally, a CSF analysis with an aseptic pattern will yield a pathogen such as a viridans streptococcus (164).
Beside diagnosis of meningitis, LP has little role in the diagnostic workup of IE. The clinical features and careful interpretation of the CT scan or MRI studies are better guides to patient management than the CSF findings alone (165).
Outcome
The overall mortality of patients with IE has decreased over the past 30 years, probably due to improved management (e.g., more widespread use of echocardiography, access to surgery, interdisciplinary care) (166). However, the mortality of patients with IE and neurologic complications has not changed appreciably, ranging from 34% to 74% (29,83,95,101), although some studies report rates as low as 20% (88,97). A mortality rate of 58% has been reported in patients with neurologic complications IE referred to the ICU (106). CNS complications were associated with higher mortality in several studies (24,83,92,101,145). For example, stroke was independently associated with in-hospital mortality (OR 3.67, CI 1.94 to 6.94) among patients with S. aureus endocarditis in the ICE-PCS (24). However, in a study of patients with IE admitted to the ICU, mortality was similar among patients with and without neurologic complications (106), whereas Glasgow Coma Scale (GCS) score less than 10 was a strong predictor of mortality. This suggests that severity of neurologic complications might be a more appropriate predictor of mortality than the occurrence of CNS complications per se, as confirmed by a study of 513 patients with complicated IE, where abnormal mental status at baseline was independently associated with 6-month mortality (9). Surgical treatment of IE does not appear to increase the CNS-related mortality of IE; among 55 patients with neurologic complications of IE, mortality did not differ between surgically and medically treated patients (97). In patients with PVE, the overall mortality was affected by the presence of neurologic complications in some studies (46,70) but not in others (167).
When only major cerebral events are considered, they are found to be the direct cause and often the only cause of death in more than 50% of fatal cases, both in NVE and in PVE. In many cases, the cause of death is multifactorial. Neurologic complications were found to be directly responsible for 8% to 20% of the deaths in patients with NVE (29,83) and for 10% to 40% in those with PVE (117). In a recent series of 55 cases, the mortality among patients with neurologic complications was not different between those with NVE and PVE (23% and 25%), and there was no difference in mortality between the episodes caused by various microorganisms (97).
The type of microorganism appears to play an important role in the outcome of patients with IE and neurologic complications, with S. aureus, Enterobacteriaceae, and fungi being associated with a higher mortality (83,88). Obviously, some neurologic complications such as major emboli, ICH, or purulent meningitis are associated with higher mortality rates than the less severe manifestations. Sequelae have been noted in up to 34% of patients with neurologic complications who survived (87).
Cerebral Emboli
Incidence
Emboli arising from the heart are responsible for 30% of strokes occurring in the general population (168–170). Emboli secondary to IE account for fewer than 1% of these episodes (171). The rate of CNS embolism due to IE is higher in autopsy series; for example, IE was found in 69 (1.5%) of 4,558 autopsies but was present in 32 (25%) of the 126 patients with cardiogenic cerebral emboli (104).
Occlusion of cerebral arteries by emboli is the most common neurologic complication of IE, comprising approximately half of the CNS complications (Table 34.3). Among 2,781 patients of the ICE-PCS, stroke complicated IE in 17% of cases (16). Lower incidence (9.6%) was reported in a recent study that used more stringent diagnostic criteria for stroke and the Duke criteria for IE (114). Stroke was present in up to 40% of patients with IE admitted to the ICU (106).
Approximately half the patients who present with major or minor cerebral embolism have clinically identifiable emboli to other organs as well (83). In contrast, evidence of systemic emboli was found in only 2% of unselected patients with stroke (172). In patients with IE and systemic emboli, cerebral emboli were found in 40% to 69% (83,154). It has been observed that the incidence of cerebral emboli associated with IE of the mitral valve was higher than when the aortic valve was involved despite a similar rate of peripheral emboli elsewhere (83,114,173). Except for the rare occurrence of paradoxical embolism, emboli are usually associated with lesions on the left side of the heart. In recent studies that compared NVE with PVE, no significant difference in the incidence of cerebral emboli was noted (46,88,97).
Pathogenesis
Most emboli related to IE are a result of dislodgment or disruption of cardiac vegetations into fragments, followed by lodgment of these fragments into peripheral vessels of various diameters, depending on size. Occasionally, emboli may be related to other concurrent disorders such as atrial arrhythmias. Vegetations are the result of complex interactions between various host components including serum, fibrin, platelets, fibroblasts, and inflammatory cells, as well as microbial factors including growth rate, adhesion, and production of extracellular proteases. These interactions influence the growth of the vegetation and the frequency of embolic episodes (174). Experimentally, the proteolytic capacity of the infecting organism was shown to influence the size of the vegetations and the course of the disease. In rabbits with IE induced by one of ten different strains of Enterococcus faecalis, proteolytic strains caused smaller vegetations with a soft and friable appearance, as well as an increased frequency of renal emboli, when compared to nonproteolytic strains (175). In humans, IE due to virulent microorganisms, particularly S. aureus, is associated with an increased frequency of systemic and cerebral emboli as compared to less virulent bacteria such as viridans streptococci. In a study of 52 patients with PVE, the calculated rate of stroke during uncontrolled infection ranged from 1% per day for nonvirulent streptococci to 9% per day for S. aureus (121). Pathologic examination provides some explanation for this difference: The vegetations of IE due to virulent bacteria are friable with little histologic evidence of healing, whereas in subacute disease, lesions progress more slowly with evidence of fibrotic reaction and early healing (176). In addition, certain microorganisms have been noted to produce large and mobile vegetations and are associated with an increased propensity to be complicated by major systemic and cerebral embolism. This has been described in IE due to microorganisms such as Haemophilus species or other slow-growing fastidious gram-negative rods, Abiotrophia, group B β-hemolytic streptococci, and fungi (Candida and Aspergillus species) (177,178). Occlusion of vessels by fragments of vegetations results in various degrees of ischemia and infarction depending on the vessels involved and the collateral blood flow. In addition, emboli occurring before the initiation or completion of successful antibiotic treatment may contain microorganisms capable of producing secondary infectious complications, such as abscesses of various sizes, meningitis, arteritis, or infectious aneurysms. In about 20% of the cerebrovascular episodes, hemorrhagic complications are observed (145). They may be due to the infarction itself or the erosion of the artery by the bacteria present in the emboli, with or without formation of a detectable infectious aneurysm (138). Concomitant anticoagulation appears to increase the risk of developing major hemorrhages at the sites of infarction (83,92,127,138). However, in patients with PVE, withholding anticoagulation has resulted in high rates of thromboembolism, and adequate anticoagulation has been shown to reduce the incidence of major CNS events (116).
As previously mentioned, macroscopic and microscopic cerebral emboli have been observed with greater frequency in patients with mitral valve infection as opposed to those with aortic valve involvement in most studies (55,83,106,179). Pathologic examination of operative or autopsy material shows that vegetations are not always found in patients who had an embolic episode; of 76 valves examined, a valvular vegetation was found in only 57% of patients with neurologic complications. Moreover, vegetations were found in 61% of the patients without CNS complications (88).
Clinical Presentation
Cerebral embolism associated with IE may present with protean clinical manifestations. Important determinants of the symptomatology are the number, location, and size of the emboli. Some patients may present with embolic occlusion of a single major cerebral artery, with symptomatology related to the territory involved. Other patients may have multiple microemboli with more diverse clinical presentations. In many patients, these two forms of emboli coexist (83,101).
By combining CT and clinical criteria, Hart et al. (154) found that 62% of 37 ischemic events involved the cortex or the cerebellar hemispheres, 16% were exclusively subcortical, 11% involved the retina, and 11% were in an uncertain location. Most infarcts were either small (58%) or moderately sized (33%). All three large hemispheric infarcts occurred in patients with S. aureus endocarditis (154).
Major cerebral embolism was the presenting symptom or first overt manifestation of IE in 22 (10%) of 218 patients (83) and in 12 (14%) of 86 patients (113) in two large series. The majority of the cerebral emboli occur before the initiation of antibiotic treatment or during the first 2 weeks of therapy (55,83,97,154,173,179–181). Among 109 episodes of stroke observed in 496 patients with IE, 31 (28%) occurred after the initiation of antibiotic treatment; the median time interval from onset of antibiotic treatment to the development of neurologic complications was 4 days, with a range of 1 to 21 days (179). In another study, 87% of ischemic events occurred either at the time of diagnosis (74%) or within 48 hours thereafter (13%) (154). Among the 25 initial ischemic events, 56% were simple and unifocal, 28% were multifocal, and 16% were associated with toxic encephalopathy. Embolization after the completion of successful antibiotic treatment is uncommon (55), but it has been described and can occur up to 2 years later (83). Recurrences of emboli events are rare. In one study of 55 neurologic events among 218 patients with IE, only one recurrent episode of cerebral embolism was reported (97). In another series of 64 patients with neurologic complications of IE, two recurrent embolic events occurred before initiation of antibiotic treatment (88).
More than 90% of large cerebral emboli affect the middle cerebral arteries and their branches, leading mainly to contralateral hemiparesis and/or hemisensory deficits. This localization may also produce parietal lobe signs including sensory loss, neglect, dyspraxia, hemianopia, and when the dominant hemisphere is involved, aphasia. Occlusion of anterior or posterior cerebral arteries may also produce similar symptoms, especially those involving the lower extremities. Posterior cerebral artery occlusion may produce homonymous hemianopia. The vertebrobasilar system was affected in 6 of 10 and 4 of 84 patients with CNS complications of IE in two series (83,143). Emboli affecting the vascular supply of the spinal cord or peripheral nerves may also occur (182).
TIAs are occasionally noted, sometimes as the presenting manifestation of IE. Such episodes were recorded in 15 (27%) of 55 patients with strokes associated with IE (143). Among 25 episodes of ischemic strokes reported in a study by Hart et al. (139), 3 were TIAs, all causing amaurosis fugax. In a patient with a TIA, the presence of fever or any other nonspecific signs of infection should raise the possibility of IE. Some of these patients may present with fluctuating neurologic signs, presumably from emboli that disintegrate after initial lodgment; autopsy may reveal multiple small or microscopic infarcts (137).
Multiple cerebral microemboli are common, causing about one third of all ischemic events (154). Of 45 patients autopsied because of a fatal neurologic complication, 38 had major embolism, but 23 were found to also have microscopic cerebral infarcts, presumably due to embolic occlusion of small vessels. Six also had microabscesses. These findings correlated with seizures or fluctuating neurologic signs in four patients and were clinically silent in four others. In 14 patients, multiple microscopic infarcts manifested as an altered level of consciousness not adequately explained by other abnormalities (83). Thus, encephalopathy among patients with IE, may partly be explained by multiple microemboli, resulting in multifocal microinfarcts, sometimes associated with cerebritis or microabscesses (145). Microabscesses were found in 6 of 23 patients with multiple microinfarcts at autopsy (83), demonstrating that a continuum exists between the ischemic cerebrovascular lesions and overtly infected CNS lesions complicating IE (145).
In summary, emboli may cause a wide variety of CNS symptoms and signs, ranging from hemiplegia to diffuse and fluctuating neurologic dysfunction, depending on their size, location, and number. Most emboli affect the middle cerebral artery and occur before or soon after the initiation of therapy.
Diagnostic Procedures
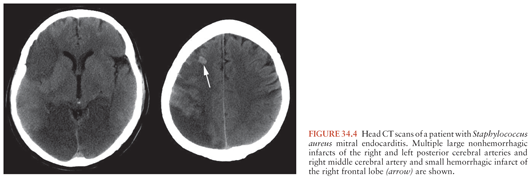
Cerebral CT scan and MRI are the most useful diagnostic procedures when emboli are suspected. They are helpful both in the differential diagnosis and in the distinction between nonhemorrhagic and hemorrhagic infarcts (Fig. 34.4). When acute hemorrhage is suspected, CT scan is preferred. MRI and MRA also provide a baseline in monitoring for the development of ICIA, which may arise at the site of a previous embolus. Follow-up imaging studies are also indicated when the secondary formation of an abscess is suspected.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree