Chapter 5 Zidovudine
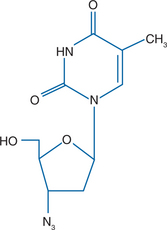
Zidovudine (39-azido-39-deoxythymidine, AZT) (ZDV) is a pyrimidine nucleoside analog of thymidine (Fig. 5-1). ZDV differs from thymidine in that the 39-hydroxyl group of thymidine has been replaced. ZDV (Retrovir) was first synthesized in 1964.1 It was the first nucleoside analog available for the treatment of human immunodeficiency virus (HIV) infection. It was approved by the United States Food and Drug Administration in 1987 based on demonstrable survival benefit in patients with acquired immunodeficiency syndrome (AIDS), and clinical benefit in those with symptomatic or asymptomatic HIV disease who had a CD4+ T-lymphocyte count of 500 cells/mm3 or less.
MECHANISM OF ACTION AND IN VITRO ACTIVITIES
In order to inhibit HIV-1 reverse transcriptase (RT) ZDV requires the addition of three phosphate groups by cellular kinases, forming the active agent zidovudine triphosphate (ZDV-TP).2,3 Once formed, ZDV-TP is then incorporated into the nascent DNA strand by HIV-1 RT. However, the lack of a 39-hydroxyl group in ZDV leads to termination of the elongating DNA chain. The initial phosphorylation of ZDV is performed by cellular thymidine kinase-1 (TK-1) and results in ZDV monophosphate (ZDV-MP). TK-1 is a cytosolic enzyme that is primarily active in proliferating cells.4 Cells from patients with HIV-1 infection have been shown to have higher median TK-1 activity levels, and more variability in activity, than cells from healthy matched controls.5,6 In a cross-sectional study, patients receiving ZDV therapy were found to have lower TK-1 activity levels when compared to treatment naïve patients, whereas TK-1 activity levels from stavudine-treated patients were not similarly reduced.5 The clinical importance of this finding is questionable since sequential measures of total cellular ZDV phosphates did not show a significant decline after 12 months of therapy.7 TK-1 mediated phosphorylation is inhibited by both ribavirin and by doxorubicin, reducing intracellular AMP levels.8,9 However, subsequent phosphorylation steps are not affected, and ribavirin does not reduce the intracellular activity of ZDV.10
Following the initial phosphorylation, ZDV-MP accumulates intracellularly since the conversion of ZDV-MP to ZDV-DP and ZDV-TP is less than 1% as efficient as the conversion of thymidine-MP to thymidine-TP.2 The accumulation of intracellular ZDV-MP may be responsible for some of the toxicity of ZDV.11 ZDV-MP binds to the ATP-binding site of TK, further decreasing its activity. It therefore appears that ZDV works as a chain terminator, also by competitively inhibiting the binding of TTP at the active site in RT, and potentially by depleting intracellular pools of phosphorylated nucleosides. ZDV-TP may also decrease RNAase activity, preventing the degradation of the viral RNA template and inhibiting the formation of the complete viral preintegration complex.12 The in vitro activity of ZDV against HIV-1 is dependent on the activation state of the cell. ZDV has its greatest activity in cells which are metabolically active, such as CD4+ T cells.13,14 In vitro, ZDV has ∼100–1000 times more antiviral activity in activated cells versus resting cells. ZDV is much less active in macrophages than is didanosine. ZDV is also less efficiently converted to its active triphosphate form in monocyte-derived dendritic or langerhans cells than is lamivudine or tenofovir.15 Synergistic activity against HIV-1 has been demonstrated by combinations of ZDV and either abacavir, didanosine, lamivudine, or zalcitabine.16,17 Since ZDV and stavudine utilize the same phosphorylation pathways, they are antagonistic in vivo, although some studies have indicated in vitro synergy.18
ZDV is active in vitro against human immunodeficiency type two (HIV-2) in certain experiments, but not in others.19 Isolated HIV-2 RT is fourfold less sensitive to ZDV than is HIV-1 RT.20 Studies from HIV-2 infected patients treated with ZDV have demonstrated the development of ZDV resistance, confirming that ZDV has some activity against this virus.21 ZDV is also active in vitro against human T-cell leukemia/lymphoma virus (HTLV-1). In a single cycle replication assay model for HTLV-1, the 50% inhibitory concentration for ZDV was 0.11 μM/L, versus 22.0 μM/L for lamivudine, 4.6 μM/L for abacavir and 0.005 μM/L for tenofovir.22 ZDV may also have activity as an antitumor agent for human herpes virus-8 (HHV-8) associated primary effusion lymphoma and for Epstein–Barr virus (EBV) associated Burkett’s lymphoma. In a mouse model ZDV was found to induce apoptosis of human-derived EBV-associated primary central nervous system lymphoma cells following induction of EBV-TK expression by ionizing radiation.23 In cell lines derived from HHV-8 and EBV-associated lymphomas ZDV inhibited the phosphorylation of IkB, reducing the antiapoptotic effects of NFκB and enhancing tumor cell apoptosis.24 Since interferon alpha (IFN-α) increases tumor cell apoptosis, the combination of IFN-α and ZDV may be a useful treatment for these viral-associated lymphomas.25,26
ZDV has activity in vitro against certain enteric bacteria, primarily Salmonella and Enterobacteriaceae species. The minimal inhibitory concentrations for ZDV against S. typhimurium and E. coli were 0.5 μg/mL (0.125–4 μg/mL) and 0.125 μg/mL (0.031–1.0 μg/mL) respectively. For the same organisms the inhibitory concentrations for both didanosine and stavudine were approximately 10-fold higher.27 In a macrophage cell line ZDV had greater inhibitory activity against S. typhimurium than did ceftriaxone. ZDV is not inhibitory in vitro for Pseudomonas sp., gram-positive bacteria, anaerobes, spirochetes, mycobacteria, Candida sp., Mycoplasma sp., or Ureaplasma sp.28,29 ZDV does not have antiviral activity against hepatitis B virus, hepatitis C virus, herpes simplex virus or cytomegalovirus.
CLINICAL PHARMACOLOGY
ZDV may be administered both orally and intravenously. Following a 2 mg/kg intravenous dose of ZDV plasma levels were found to peak (Tmax) within + h at 1.5–2 μM/L.30 The Tmax of ZDV following a 300 mg oral dose is 1.03 h, the Cmax is 2.59 ± 0.52 μM/L, the AUC02212 hours is 4.59 ± 0.79 μM/L, average bioavailability is 63 ± 13% and the mean volume of distribution is 3.0 + 0.6 L/kg.31–33 With decreasing CD4+ count levels there is decreasing bioavailability of ZDV in HIV-infected patients.34 There is conflicting data about the effect of food on ZDV absorption. In patients given a high-fat meal, there was decreased absorption, an increased maximal concentration and an increased half-life of ZDV.35 However, in another study, patients who were given a standard breakfast had little change in the bioavailability of ZDV.36 Since ZDV is lipophilic it is likely that its absorption may be influenced by high-fat meals. When ZDV is used as a fixed- dose combination with lamivudine, dose adjustment for meal content would be difficult. ZDV elixir administered rectally produced lower peak plasma levels than was observed when ZDV capsules were administered orally.37
ZDV is less than 25% protein bound, primarily to albumin.38 Intracellularly, the Cmax of ZDV-TP was 82 FMOL per 106 cells following a 300 mg dose.39 The intracellular half-life of ZDV-TP is ∼8–12 h, making ZDV compatible with twice-daily dosing.32,40 Women may have higher intracellular ZDV-TP levels than men. In AIDS Clinical Trials Group (ACTG) study 161 the AUC of total intracellular phosphorylates of ZDV was significantly higher for women than men.41 In a separate study, ZDV-TP concentrations were twofold higher in women than in men, and women had a shorter time to virologic suppression on ZDV therapy.42
Approximately 75% of ZDV is metabolized by the enzyme uridine diphosphoglucuronyltransferase to form 59-glucuronylzidovudine (GZDV).43 This enzyme has been shown to be induced by both rifampin and rifabutin. Of the remaining 25% of ZDV, 15–20% is excreted as the parent compound in urine, and ∼2% is metabolized to 39-amino-39-deoxythymidine (AMT).44,45 In a study of ZDV clearance in children, it was found that those less than 2 weeks of age had impaired glucuronidation.46 Infants therefore had higher levels of ZDV, and decreased ZDV clearance, compared to older children.
Since ZDV is excreted in the urine, patients with reduced glomerular filtration rates have impaired ZDV clearance. Clearance of ZDV has been shown to be decreased by ∼50% in those with severe renal insufficiency.47 Dose adjustment for ZDV is therefore necessary in patients who have a creatinine clearance of less than 10–20 mL/min. Dialysis has little effect on ZDV levels, since ZDV is highly lipophilic. Probenecid decreases excretion of GZDV.48 Renal excretion of GZDV is also reduced by the histamine H2 receptor antagonist cimetidine, but not by ranitidine.49 This effect is not sufficient to be clinically significant. ZDV clearance is also affected by a hepatic disease. In HIV uninfected patients with cirrhosis, the glucuronidation of ZDV has been found to be reduced by approximately threefold.50 Similar findings have been found in a small study of patients with AIDS and hepatic disease, in which clearance was reduced by 63%.51 In a study of patients with mild liver disease ZDV clearance was reduced by only 32%.52 Pregnancy does not appear to affect the pharmacokinetics of ZDV.53
As expected of a lipophilic compound, the penetration of ZDV into the central nervous system is relatively high. In several studies, the cerebrospinal fluid (CSF) to plasma ZDV ratio ranged from 0.15 to 1.53.33,54,55 In adults given ZDV at doses ranging from 600 to 1500 mg/day, the median CSF ZDV concentration equaled 0.153 μM/L (0.056–0.027 μM/L) and the CSF:plasma ratio varied from 0.09 to 1.2.54 In other studies, sequential sampling of individual patients has shown up to a 200% difference in CSF ZDV concentrations over time.54 This high degree of variability may be due, in part, to differences in adherence over the interval studied. Intravenous dosing of ZDV eliminates this potential effect. In children given intravenous ZDV, the CSF to plasma ratio was ∼0.25 ± 0.09.56 In adults treated intravenously the CSF to plasma ratio was ∼0.75 ± 0.26.57 Nucleoside analog levels in brain parenchyma may not be accurately predicted by CSF levels since brain endothelial transport proteins actively remove nucleosides from the central nervous system.57 However, ZDV is less of a substrate for these transporters than is didanosine. ZDV has also been shown to improve cognition in patients with AIDS dementia complex.58
ZDV levels in other bodily fluids and compartments have been extensively studied. ZDV levels in salivary fluid have been found to be correlated with those in plasma.59 ZDV levels in semen have varied, depending on the fraction of the semen studied. In one study, the semen to serum ratio varied from 0.35 to 5.5.60 It is likely that lipid-rich components of semen, and cells in semen, will have therapeutic levels of ZDV. ZDV treatment in nonpregnant women has been shown to lead to a significantly faster decay in viral RNA levels in cervix and vaginal secretions than that observed for plasma.61 Approximately 70% of ZDV crosses the placenta.62 In newborns, umbilical and venous ZDV levels have been found to be approximately equivalent.53 In aborted fetuses from 26 HIV-infected women given a 200 mg dose of ZDV, the fetal plasma level was 92% of the maternal plasma level.63 In two studies of a total of 30 lactating women who were receiving treatment with ZDV, drug levels in breast milk were found to exceed those in plasma by 1.48–3.21-fold.64,65 This result is in agreement with the lipophilic properties of ZDV.
ADVERSE REACTIONS
Common symptoms and laboratory abnormalities reported with ZDV include headache, fatigue, malaise, myalgia, anorexia, nausea, anemia, and neutropenia (Table 5-1).66–68 Of the symptom side effects, only vomiting was statistically significantly greater with ZDV use in randomized trials comparing ZDV to either stavudine or abacavir.69,70 Initial symptoms, particularly headache, insomnia, fatigue, and gastrointestinal problems, are mild and can be managed symptomatically. In a small percentage of patients, particularly those receiving three- and four-drug regimens, persistent symptoms may require modification of the dose of ZDV or substitution by another nucleoside analog. Rare cases of rash and fever have been described that necessitated discontinuing ZDV.
Table 5-1 Side Effects Associated with ZDV
| Headache | Vomiting | Myopathya |
| Fatigue | Anorexia | Feverb |
| Myalgia | Anemia | Rashb |
| Insomnia | Neutropenia | Hepatitisb |
| Nausea | Nail pigmentationa | Steatosis/lactic acidosisb |
| Lipoatrophya |
Atlas of Infectious Diseases. Philadelphia: Current Medicine, with permission.
a Associated with long-term use.
Anemia is more prominent in advanced disease than in early disease (7% vs 1%, respectively). Serum folate levels are normal or elevated, and vitamin B12 levels are normal or slightly decreased. The reticulocyte count is frequently depressed and may be the first sign of bone marrow toxicity. Bone marrow examinations in cases of severe anemia show a decrease or absence of red blood cell precursors. Erythropoietin levels are commonly elevated and in some cases are quite high (>500 IU/L), suggesting that ZDV-induced erythroid hypoplasia is not due to interference with erythropoietin production but is more likely due to inhibition of cell commitment to the erythroid line or direct toxic effects on committed erythroid stem cells.71 Anemia can be seen as early as 4–6 weeks after initiation of ZDV. Progressive declines in the hemoglobin concentration should signal the potential for severe anemia, particularly if the hemoglobin concentration declines to 7.5–8.0 g/dL. In cases of severe anemia, ZDV (whenever possible) should be temporarily interrupted until the hemoglobin concentration increases, which typically takes 7–14 days. Recombinant human erythropoietin has been shown to be safe and to decrease blood transfusion requirements in patients without marked elevation in endogenous erythropoietin levels who develop severe anemia while receiving ZDV.72 Alternatively, the dose of ZDV can be decreased or another antiretroviral agent substituted for ZDV. Abacavir, stavudine, and tenofovir have all been shown to cause less anemia than ZDV in comparative trials.69,70,73 Macrocytosis with an elevation in the mean corpuscular volume of 25–40 units is common and is typically not associated with anemia. Increases in mean corpuscular volume can occur within 6–8 weeks of starting ZDV and are most prominent after 16–24 weeks.
Neutropenia also occurs more frequently during advanced HIV disease than in the early stages of the disease (37% vs 8%, respectively) and is noted within 12–24 weeks of initiating ZDV. ZDV suppresses the receptor for granulocyte-monocyte colony stimulating factor (GM-CSF) on murine bone marrow cells.74 It is not known whether ZDV causes neutropenia in humans by a similar mechanism. Mild to moderate neutro-penia, manifested by neutrophil counts of 750–1000 cells/mm3, may be more frequent and does not require any adjustments in the ZDV dose. Declines in the neutrophil count to less than 750 cells/mm3 suggest a need to decrease the dose of ZDV or temporarily interrupt the drug (when possible) until the neutrophil count increases. If persistent or recurrent neutropenia occurs, another antiretroviral agent can be substituted for ZDV. In an open label trial comparing indinavir plus lamivudine, in combination with either ZDV or stavudine, there was no significant difference in the occurrence of neutropenia between ZDV and stavudine.70 In two double-blind trials ZDV was shown to cause neutropenia more frequently than does tenofovir, but not more frequently than abacavir.69,73
Long-term use of ZDV may be associated with several toxicities, including nail hyperpigmentation, hepatic toxicity, muscle toxicity and lipoatrophy. The nail hyperpigmentation consists of multiple longitudinal streaks and diffuse pigment changes ranging from shades of blue to brown-black.75 Nails on both the hands and feet can be involved, and pigment changes appear to move distally from the base of the nail. Hyperpigmentation changes in the nail appear to occur more frequently among those of the black African population. Associated skin pigment changes have not been described.
Muscle toxicity manifested by myalgia, progressive muscle wasting, weakness, and elevation of the serum creatine kinase concentration has been described, as well as rare cases of cardiomyopathy. The lower extremities and gluteal muscles appear to be preferentially involved. Muscle biopsy histology is consistent with a destructive mitochondrial myopathy with ragged-red fibers and proliferation of abnormal mitochondria.76 Reduction of mitochondrial DNA has been noted and is likely due to inhibition of mitochondrial DNA replication by DNA polymerase-γ.77 Asymptomatic elevations in creatine kinase may also be seen.
Increased liver enzyme levels have been noted with ZDV use, although in a comparative trial stavudine was more likely to cause such abnormalities than was ZDV.70 In comparative trials ZDV was not more likely to cause liver enzyme elevations than either tenofovir or abacavir.69,73 In a subset of patients ZDV use may be associated with severe steatosis, lactic acidosis, and death. This syndrome is typically characterized by progressive increases in liver aminotransferase levels and moderate to severe hepatomegaly. Scans and biopsy of the liver are consistent with steatosis. Associated symptoms of tachypnea, dsypnea, and severe acidosis with progressive liver and renal failure and death have been described and are mostly due to dysfunction of hepatic mitochondria. This syndrome has now been described with most nucleoside RT inhibitors (NRTIs). Most of these cases have been in women. Obesity and prolonged administration may be risk factors. In a case series of eight patients, and a review of 50 other reported cases, use of ZDV was associated with higher mortality than that observed with stavudine or lamivudine.78 However, fatalities associated with ZDV may have been more frequent due to failure to recognize and treat this syndrome in the 1980s and early 1990s. If this syndrome is suspected, antiretroviral therapy should be interrupted. Uridine has shown in vitro beneficial effects on reducing the toxic effects of ZDV on hepatocytes.79 A sugarcane extract, mitocnol, increases uridine levels in humans and is being tested for other nucleoside-associated toxicities.
Changes in body fat composition, particularly lipoatrophy, have been reported in patients receiving ZDV. The incidence of lipoatrophy appears to be less for ZDV-treated patients than for stavudine-treated patients. In two prospective studies that compared stavudine to ZDV, both in combination with lamivudine and a third agent, the incidence of lipoatrophy at 30–36 weeks was 48–57% in the stavudine arm and 19–22% in the ZDV arm.80,81 The combination of ZDV and lamivudine also appears to produce less lipoatrophy than a stavudine/didanosine combination. In a substudy of ACTG Study 384, in which these two nucleoside combinations were compared in treatment naive patients, 157 patients underwent body fat measurements and dual-energy X-ray absorptiometry (DEXA) scanning at 16 week intervals.82 After the first 16 weeks of treatment, limb fat increased by 9.4% in the stavudine-containing arm and by 11.6% in the ZDV-containing arm. Limb fat subsequently decreased in both groups after week 16, but to a greater extent in the stavudine-containing arm. By week 64 total limb fat in the ZDV group had decreased nearly to baseline, whereas the stavudine group had a net loss of 16.8% of limb fat compared to baseline. In contrast to stavudine, tenofovir is less likely to result in lipoatrophy than ZDV. In a randomized study comparing tenofovir and ZDV, both in combination with emtricitabine and efavirenz, week-48 total limb fat was significantly less in a subgroup of patients in the ZDV–lamivudine group who underwent DEXA scanning than in a subgroup of patients in the tenofovir–emtricitabine group (mean, 6.9 kg vs 8.9 kg respectively; P = 0.03).73 In a separate study, 12 patients who replaced ZDV with tenofovir had approximately a 4% increase in limb fat over 24 weeks.83 This result indicates that ZDV-induced lipoatrophy is partially reversible after discontinuation of ZDV.
The mechanisms by which ZDV therapy causes lipoatrophy in humans have not been completely determined. In 20 HIV-uninfected patients who were given either stavudine or ZDV for 2 weeks, and who then had a biopsy of adipose tissue performed, both nucleoside analogs produced a decrease in adipose mitochondrial DNA transcription compared to baseline.84 Another group compared 12 HIV-uninfected controls to 18 lipoatrophic patients who had been treated with stavudine (12) or ZDV (6) containing regimens for ∼30 months.85 Compared to the controls, the nucleoside-treated patients had no difference in adipose mitochondrial DNA content and no difference in tumor necrosis factor alpha levels in plasma or in adipose tissue. There was a decrease in plasma adiponectin levels of 50% in those receiving ZDV and of 58% in those receiving stavudine. Both groups had equal reductions in mitochondrial respiratory chain enzymes. These results suggest that ZDV may cause dysfunction of adipose mitochondria and of adiponectin production.
CLINICAL EFFICACY
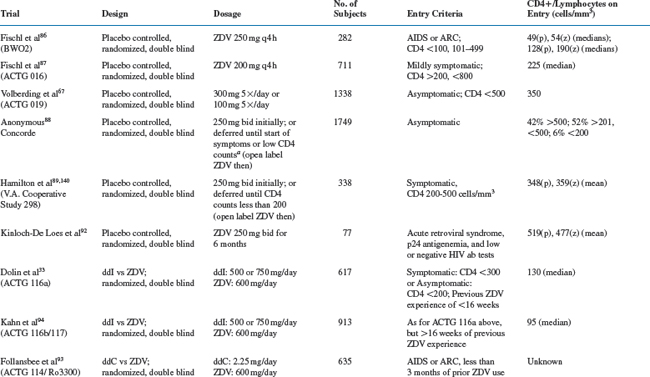
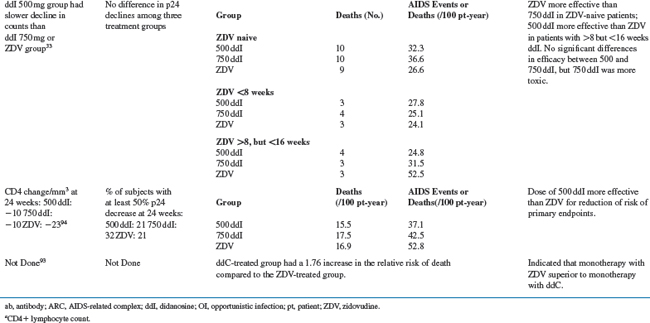
ZDV in combination with other antiretroviral agents is discussed in subsequent chapters of this book. Monotherapy trials and comparative trials of ZDV are detailed in Table 5-2 and discussed below.
ZDV Placebo-Controlled Trials
ZDV was the first antiretroviral agent to indicate that treatment intervention can improve the outcome in HIV disease. For advanced HIV disease, the probability of surviving more than 24 weeks was significantly better for ZDV recipients than for placebo recipients (0.98 vs 0.78; P < 0.001).86 Similarly, the risk of disease progression over 24 weeks was significantly lower for ZDV recipients than for placebo recipients (0.23 vs 0.43; P < 0.001). Subsequent placebo-controlled studies showed that the risk of disease progression was decreased more than threefold by 18 months for patients with symptomatic disease (3.23; P = 0.0002) and by 12 months for patients with asymptomatic disease (3.1; P = 0.005).67,87 No differences in survival were demonstrable in either study. Early versus delayed use of ZDV therapy has also been evaluated in patients with asymptomatic and with symptomatic HIV infection. The estimated 3-year survival probabilities were 92% for immediate therapy and 94% for delayed therapy for asymptomatic patients. Similar findings were noted for progression to AIDS or death.88 Another study compared ZDV therapy in symptomatic patients who started treatment at CD4+ counts between 200 and 500/mm3 versus ZDV begun when the CD4+ counts fell below 200/mm3.89 There were 23 deaths in the early-therapy group (n = 170) and 20 deaths in the late-therapy group (n = 168) (P = 0.48). In the early-therapy group fewer patients developed AIDS (P = 0.02; relative risk, 1.76); and early therapy prolonged the time to a CD4+ count below 200/mm3. These data confirmed the benefit of ZDV but pointed out the limited duration of the benefit.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree