Chapter 33 Acute HIV Infection
EPIDEMIOLOGY
The HIV/AIDS pandemic continues unabated. According to estimates in 2006, there are ∼40 million individuals infected with HIV-1 worldwide. This includes 17.5 million women and 2.3 million children. Approximately five million individuals acquired HIV-1 infection and an additional three million HIV-1 related deaths occurred in the year 2005 alone. Since its recognition in 1981, the AIDS epidemic has resulted in more than 25 million deaths worldwide.1
In the United States, the estimated number of people living with HIV-1 infection exceeds one million with ∼40 000 new infections every year.1 Men having sex with men remains the dominant mode of HIV transmission in the United States, accounting for 65% of newly diagnosed infections in 2003. However, for women living with HIV-1, heterosexual sex was the main mode of transmission. A striking feature of the HIV-1 epidemic in the United States is the high incidence of infection among African-Americans. Despite constituting 12.5% of the country’s population, African-Americans accounted for ∼50% of new cases as well as HIV-1-related deaths in the United States in 2003.2
As many as a quarter of HIV-infected individuals in the United States are unaware of their diagnosis.3 Establishing the diagnosis, particularly during acute infection has important implications. There is ample evidence to suggest that acute HIV-1 infection is associated with a high probability of transmission.4–8 In addition, early diagnosis provides a unique opportunity to study the natural history of this disease, with direct implications on vaccine and microbicide research. Finally, early diagnosis enables the study of the potential benefits of immediate antiretroviral therapy.9–12
TRANSMISSION
The majority of HIV transmissions worldwide occur across a mucosal surface13 such as the anorectal mucosa, vaginal mucosa and less frequently, the oral mucosa.14 HIV-receptive cells have been found in the lamina propria of rectal, cervicovaginal, foreskin, urethral, and oral epithelia in primate models.15 Intravenous drug abuse and mother-to-child transmission also remain important routes of HIV transmission worldwide. Quite early in the AIDS epidemic, HIV was isolated from seminal secretions in infected, asymptomatic men16 and genital secretions of infected women.17,18 In fact, despite prolonged treatment with highly active antiretroviral therapy (HAART), HIV-1 may persist in the male and female genital tracts.19,20
It has been shown that host infectiousness increases as a function of the concentration of virus in the genital tract.13,21 During acute HIV-1 infection, increased viral transmission correlates with the dramatically elevated plasma viral loads in such patients.22,23 Higher viral loads in the blood have been associated with the transmission of HIV-1 to sexual partners of individuals with transfusion-acquired infections.24 Acquisition of HIV-1 during pregnancy and high maternal viral load are associated with increased risk of perinatal transmission.25 Individuals with viral loads greater than 50 000 copies/mL of plasma have a more than 50-fold increased risk of transmitting the virus per sexual act, and transmission is rare among persons with levels of less than 1500 copies of HIV-1 RNA per mL of plasma.26 Further, individuals on potent antiretroviral therapy have a significant decrease in the risk of HIV transmission to their sexual partners.27–29
Reproductive tract infections and other sexually transmitted diseases are strongly associated with HIV-1 transmission even when adjusted for sexual behavior.30 Ulcerative sexually transmitted diseases such as chancroid, syphilis and herpes are associated with a significant increase in the risk of HIV for men31 and women.32 Other sexually transmitted diseases such as gonorrhea and chlamydia,31–34 bacterial vaginosis,33 urethritis,35,36 and cytomegalovirus infection37 are associated with increased seminal levels of HIV and hence increase the risk of transmission. Consequently, treatment of sexually transmitted diseases in the population at risk has been shown to significantly decrease the incidence of HIV-1 transmission.38 Cervical ectopy in women13,39 and lack of circumcision in men33 are associated with increased risk of HIV-1 transmission.
The choice of contraceptive methods impacts on HIV transmission. Condoms are protective for both sexes.27,40–42 In animal models, the use of hormonal contraceptives containing progesterone also increases HIV-1 transmission by mucosal thinning.43
Epidemiologic studies suggest that host factors may influence susceptibility to HIV-1 infection.44–46 Individuals homozygous for a 32-bp deletion in the CCR5 molecule are relatively resistant to infection by CCR5-tropic strains of HIV-1 but not by CXCR4-tropic strains.47 Heterozygosity for the 32-bp deletion in CCR5 results in retardation of disease progression but does not protect against infection.48 Recent data demonstrates interindividual variation in the copy number of segmental duplication within the gene encoding for CCL3L1. CCL3L1 is the most potent known natural ligand for the CCR5 receptor, and it is a dominant HIV-1-suppressive chemokine.49 Possession of a CCL3L1 copy number lower than the population average is associated with markedly enhanced susceptibility to HIV-1 infection.45
The properties of HIV-1 itself may influence transmission. To date, the two major viral types characterized are: HIV-1, the predominant HIV type throughout the world; and HIV-2, first reported and primarily found among individuals from West Africa.50,51 Although HIV-2 shares the same modes of transmission as HIV-1, the distribution of HIV-2 remains restricted primarily to West Africa where the rates have been stableover time in contrast to the rising rates of HIV-1 infection worldwide.52 Prospective epidemiologic studies demonstrate a large difference in transmissibility between the two types with significantly lower rates of sexual and maternal-to-infant transmission for HIV-2 than for HIV-1.53 Within HIV-1, whether one subtype has greater transmissibility than the other remains controversial and beset by multiple confounding factors that have not yet been resolved.54–56
Finally, HIV-1 transmission is closely linked to complex social factors.57 Sexual practices such as ano-receptive intercourse, frequency of sexual contact, number of sexual partners, level of education and awareness, use of recreation drugs such as crystal methamphetamine,58 use of HAART and urologic heterogeneity all impact on HIV-1 transmission in complex and interrelated manners.59
IMMUNOPATHOGENESIS
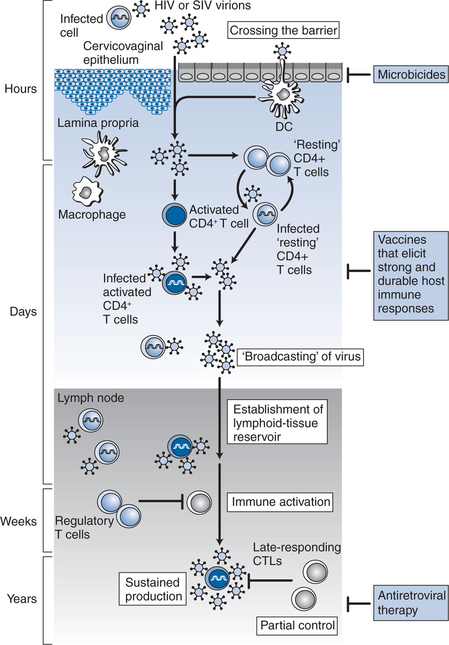
Studies derived from animal models60 and humans have provided insights into the earliest events of transmission (Fig. 33-1). The probability of transmission with each encounter is quite small (<0.001) and is related in part to the dose21 as described above. HIV-1 can cross the mucosal barrier by a breach in the intact mucosal epithelium. Mechanisms by which HIV-1 can access susceptible cells without disruption of the mucosa have also been described. For example, transcytosis by a vesicular pathway may enable HIV-1 to cross an intact mucosal barrier and infect susceptible host T cells.61 Another mode of entry is selective capture of CCR5-tropic HIV-1 by epithelial cells, and transfer of infection to subepithelial CCR5 expressing target T cells.62 More recently, it has been suggested that dendritic cells could capture intraluminal antigens across intact epithelium through transepithelial dendrites using CX3CR1, a chemokine receptor.63 It is believed that once HIV-1 enters the tissue, it is captured initially by the resident dendritic cells. These dendritic cells express CD4, CCR5, an integrin termed DC-SIGN64,65 and other C-type lectin receptors (CLRs).66 Recent evidence suggests these dendritic cell associated CLRs such as DC-SIGN facilitate the formation of an “infectious synapse” between dendritic cells and T cells.67 In the regions of these synapses, dendritic cells may concentrate HIV-1, resulting in efficient transfer of infection to CD4+ T cells.67 Based on SIV-infected macaque studies68,69 and studies of female genital organ cultures ex vivo,70 the first HIV-infected cells that could be detected were intraepithelial memory CD4+ T cells. In performing their physiological function of transferring antigens to generate an immune response, virus carrying antigen-presenting cells can disseminate infection to a large number of CD4+ T cells and serve to disseminate infection.
In the gut-associated lymphoid tissue (GALT), the largest immune organ in the body,71 the virus finds access to a substantial pool of densely packed CCR5-expressing memory CD4+ T cells and within days of infection, culminates in a profound depletion of these cells from the lamina propria.72–75 In addition, it has been observed that during acute HIV-1 infection gastrointestinal CD4+ T cells harbor significantly greater levels of proviral DNA and RNA compared to peripheral blood CD4+ T cells.76 Therefore, it may be hypothesized that mucosal sites such as the gastrointestinal tract play a critical role in the pathogenesis of acute HIV-1 infection.77 Substantial depletion of memory CD4+ T cells from mucosal sites within days of infection also suggests that the immunological injury due to HIV-1 is early and profound. Animal studies indicate that failure to repopulate mucosal sites with memory CD4+ T cells is associated with rapid progression.78 However, whether this is a cause or an effect of rapid disease progression remains to be resolved.
Virology
During acute HIV-1 infection there is an initial burst of viral replication.79 Once the basic reproductive rate or R0 becomes greater than or equal to 1, self propagating infection is established.80 Virus disseminates throughout the body and quickly establishes a pool of latently infected cells81,82 that represent a major obstacle to curing HIV-1 infection despite the availability of potent antiretroviral therapy.83 Between 93% and 99% of virus is produced by rapid rounds of infection and destruction of activated T cells, with the rest produced by long-lived chronically infected cells such as tissue macrophages, dendritic cells, latently infected lymphocytes and release of trapped virions from lymphoid tissue.84 During this phase of exponential viral growth, cytokines are released locally and systemically and infected individuals develop symptoms of “acute retroviral syndrome” as described below. Levels of plasma viremia at this stage are known to be in millions of copies per mL of plasma, often with an absent or evolving serologic response. The viral load then decreases substantially in temporal association with the development of HIV-1 specific immune response.85 In a cohort of 74 individuals studied during acute and early HIV-1 infection, Schacker et al showed that inflection point in the reduction of plasma HIV-1 occurred, on average at ∼117 days after infection.86 In the majority of cases, a quasi-steady state or “setpoint” is established 3–9 months after the initial infection, predictive of long term clinical outcome86–88 when HIV-1 production and clearance are in approximate balance.
There is conflicting information regarding HIV-1 transmission and viral variability. Early reports suggested that HIV-1 transmission was a clonal event and that an evolutionary bottleneck occurred at the level of transmission, with a single homogenous clone establishing infection in the new host.89 Long and Overbaugh reported in a study of heterosexually acquired HIV-1 that African men (n = 10) and women (n = 26) exhibit differences in transmission events. HIV-1 infection in men was always clonal (10/10) whereas in 15 of 26 women, multiple transmitted viruses were identified.90 Subsequent studies by this group found that women who acquired multiple viral genotypes were more likely to exhibit faster disease progression.91 However, there is no data regarding transmissibility, that is, whether women with multiple viruses are more likely to subsequently transmit.
Studies in homosexual men have been somewhat contradictory. Again, early studies suggested that early infection was indeed clonal, based on studies of HIV-1 envelope genetic variation. Yet, Zhang et al reported that despite homogeneity in envelope, higher levels of diversity in the gag region were observed in primary HIV-1 infection.92 Learn and Mullins found that eight homosexual men presenting with acute HIV-1 infection harbored more divergent envelope sequences (1.08% difference on average) than gag (0.81%).93 As infection progressed envelope diversity was reduced and gag diversity increased. These findings support the hypothesis that in homosexual men, multiple variants can be transmitted, and perhaps, this may relate to susceptibility, subsequent transmission and perhaps clinical course. Frost and coworkers analyzed eight recently infected individuals, an estimated mean 30 days (range: 21–85) after infection and the six men who infected them. Of note, three sources were chronically infected with HIV-1 while three were newly infected. Analyses of 11–15 clones per recipient found the diversity of envelope sequences was low, a mean of 0.25% with a range of 0.18–0.34%.94 Clinical correlations were not described. The viruses in the Frost study were much more homogenous than that published by Learn. Missing from these studies are clinical correlations. We believe that routes of transmission (oral, anal receptive versus anal insertive) and behaviors (substance use) are likely to affect these observed virologic factors.
Humoral Immunity
Humoral immunity plays a central role in clearing many viral infections. However, during acute HIV-1 infection, the neutralizing antibody response is variable,95 weak96–98 and frequently delayed.85 Antibodies are often non-neutralizing,99 being directed in part at virion debris.100 Furthermore, the timing of neutralizing antibody detection does not correlate with peak viremia,85,96 although this could be a function of the sensitivity of the assay used.95 Using a sensitive recombinant viral assay, it was shown recently that host-derived neutralizing antibodies do appear early in the course of HIV-1 infection and may exert immune pressure for rapid escape. This was supported by the finding that on longitudinal assessment of viral isolates initially identified during acute and early HIV-1 infection, sequence variation developed within the viral envelope resulting in escape from neutralization.101 Further in support of antibody induced immune pressure, Wei et al have proposed that HIV-1 may escape neutralization by modifying the carbohydrate residues associated with viral envelope.102 Changes within this “glycan shield” impact on viral binding to neutralizing antibodies but not to host cellular receptors. Preferential transmission of neutralization sensitive virus, containing fewer N-linked glycosylation sites, has been reported by Derdeyn et al103 in a study of subtype C HIV-1, although this may depend on subtype and mode of transmission.94,104
Induction of neutralizing antibodies in the context of established infection may be slow and inadequate to match viral diversity. In addition, it is clear that the virus has evolved many strategies to escape neutralization such as sequestration of critical domains, high replicative rate, and enormous sequence variation to name a few. As a result, enthusiasm for the potential of inducing neutralizing antibodies as an effective vaccine strategy waned early during the epidemic. However, many studies demonstrating that the presence of preexisting neutralizing antibodies may prevent HIV-1 or SIV infection or ameliorate the clinical course105–109 and have reemphasized the induction of neutralizing antibodies as an effective vaccine strategy.
Cellular Immunity
There is compelling evidence to suggest that cytotoxic T Lymphocytes (CTLs) play a crucial role in the control of HIV-1 replication (see Fig. 33-1). During acute HIV-1 infection, a drop in plasma viremia is temporarilly associated with the appearance of virus specific CTLs.85,110–112 In addition, there is marked expansion of oligoclonal populations of T lymphocytes in the peripheral blood of infected individuals at the time of virus containment in the early weeks after HIV-1 infection.113 Studies done in SIV-infected macaques show a clear temporal association between the expansion of CTLs and the clearance of virus.114–116 Perhaps the most compelling evidence demonstrating a role of virus specific CTLs arises from experiments during which antiCD81 antibodies were used to transiently deplete CD8+ T cells from SIV-infected macaques in vivo.117,118 When the duration of depletion was greater than 28 days, primary viremia was never cleared after acute infection and the SIV-infected monkeys died with a rapidly progressive AIDS-like syndrome. During chronic infection transient depletion of CD8+ T lymphocytes was associated with a substantial rise in levels of plasma viremia that returned to baseline levels coincident with the reemergence of the CD8+ T cell population. Robust CTL responses have also been shown to confer significant protection against simian immunodeficiency virus (SIV) and SHIV challenge in monkeys.119–121
Multiple mechanisms have been attributed to the antiviral effects of CTLs such as direct lysis of HIV-1 infected cells122 and production of multiple soluble factors.122,123 CTL recognition of an infected cell is based on the detection of processed viral proteins at the cell surface in the context of a major histocompatibility complex (MHC) class I molecule. Individual expression of class I molecules (grouped as A, B, and C) is genetically determined and inherited. Certain MHC class I haplotypes have predictive value for the rate of clinical disease progression. For example, heterozygosity at class I alleles, as well as the expression of the MHC class I molecules HLA-B27 and HLA-B57, in infected individuals are associated with better clinical outcomes.124–126 On the other hand, homozygosity for class alleles and HLA-B35 is associated with a morerapid disease progression124,127 Specific human leucocyte antigen (HLA) alleles have now also been associated with vaccine responsiveness in HIV vaccine trials128 suggesting that certain alleles may be associated with better antigen presentation than the others. These observations underscore the importance of CTLs in containing HIV-1 replication and highlight the genetic constraints on immune control, though the mechanisms by which these interact remain obscure. CTL function is critically dependent on the presence of virus-specific T-helper cells.129 These cells, which recognize viral protein presented in the context of an MHC class II molecule, are generated after initial antigen exposure by dendritic cells that serve to initiate this immune response.130,131 Using sensitive assays to detect cytokine production in response to HIV-1 antigens, HIV-1 specific CD4+ T cells have been observed in infected individuals.132,133 However, one of the hallmarks of chronic progressive infection is a dramatic reduction in the frequency of these T-helper, HIV-1 specific immune responses.134 Long term nonprogressors on the other hand, have preserved CD4+ T-helper responses.11 This could be explained by the finding that HIV-1 preferentially infects HIV-1 specific CD4+ T cells early in the course of infection.135 Numerous animal and human studies demonstrate that reduction of viral load during acute infection, through antiretroviral therapy or immunization is associated with the generation of strong virus-specific T-helper responses.11,136–139 However, some of the recent studies have shown that these responses are not durable once antiretroviral therapy is discontinued.140
As discussed above, virus specific CTL responses are detected in early HIV-1 infection. However, due to high replicative rate and lack of proofreading ability in the reverse transcriptase enzyme, viral escape from CTL control is well documented during acute infection.111,112,141 Escape occurs even through single amino acid mutations in an epitope, at sites essential for MHC binding or T-cell-receptor recognition, but may also be influenced by mutations in flanking regions that affect antigen processing. Recent studies in macaques immunized with SHIV provide the most direct link between immune escape from CTLs and disease progression.142 Immunized animals were not protected from infection but seemed to be protected from disease progression. During prolonged follow-up, one animal developed an increasing viral load which was temporally related to the emergence of a CTL escape mutation within a dominant epitope.
Innate Immunity
A growing body of evidence suggests that the function of Natural Killer (NK) cells is impaired in HIV-1 infection including reduced CC-chemokine production,143,144 antibody-dependent cellular cytotoxicity (ADCC),145 and changes in cytokine secretion.146,147 A recent report where 10 subjects with acute HIV-1 infection were followed longitudinally suggests that distinct changes in the NK compartment start during acute infection.148 There is an overall increase in NK cell number during acute HIV-1 infection with expansion of the CD3negCD56dimCD16pos NK subset, an early depletion of CD3negCD56brightCD16neg NK cells and a concomitant increase in the functionally anergic CD3negCD56negCD16pos cells. Numeric changes in NK cells are accompanied by reduced functional activity as measured by CD107a expression and cytokine secretion.
Gamma-delta T cells comprise 1–15% of peripheral blood lymphocytes.149 In HIV-uninfected individuals, Vg9Vd2 subset predominates in the peripheral blood while the Vd1 subset constitutes a minority of the total γd T cell population. During acute HIV-1 infection, there is a significant expansion of Vd1 and contraction of Vd2 subsets of γd T cells.150 Inversion of the Vd2:Vd1 ratio persists during chronic HIV-1 infection. However, the clinical significance of this observation remains unclear.
NK T cells are a subset of lymphocytes that share features of both NK cells and T cells. Recent reports have indicated that during acute and early HIV-1 infection, there is a decrease in the percentage of total NK T cells as well as CD4+ NK T cells.151 In addition, defects in NK T cell function have been observed during acute HIV-1 infection.152
CLINICAL MANIFESTATIONS
The recognition of signs and symptoms of acute infection with HIV-1 remains problematic. They are varied, inconsistent, and similar to other viral syndromes. It is estimated that 40–90% of people manifest symptoms at 2–4 weeks after infection.153,154 The clinical features have been described in all populations at risk of HIV-1 infection, including intravenous drug users155 and recipients of infected organs.156 They have been described in many different countries, and in all ages. There seems to be little effect of viral clade or host ethnicity on these symptoms. It is thought that the symptoms are related to the burst of viremia that accompanies acute infection, either directly or secondarily to the immune response.79,157,158
The first descriptions of acute HIV-1 seroconversion occurred in 1985.159 A prospective study in Australia identified 11 men with a specific illness at the time of acute HIV-1 infection. This illness was similar to infectious mononucleosis; it was comprised of fevers, sweats, malaise, anorexia, nausea, myalgias, arthralgias, headaches, sore throat, diarrhea, generalized lymphadenopathy, a macular erythematous truncal eruption, and thrombocytopenia. In 1996, a study of 46 adults revealed that 89% of acute seroconverters developed an acute retroviral syndrome similar in nature to the above. The most common symptoms were fever, seen in 77%, sore throat, fatigue, weight loss and myalgia.14 Seven of the patients had symptoms so severe that they required hospitalization. Fever was also a common symptom of seroconversion in studies of non-Caucasians, although the frequency was less.160,161 A prospective study of Kenyan female sex workers reported that only 53% were symptomatic during seroconversion,161 whereas, another study in India showed that 81% of patients with primary HIV-1 infection had fever, adenopathy, arthralgias, rash, or diarrhea.160 The other most common symptoms are fatigue, seen in 70–90% of cases, and rash, seen in 40–80% (Table 33-1).162 Pharyngitis, myalgias, and anorexia have also been recognized as frequent symptoms of seroconversion.162,163 Most symptoms do not resolve until serum antibodies to HIV-1 develop.161
Table 33-1 Symptoms Associated with Acute HIV Infection
| Symptom | Incidence (%) |
|---|---|
| Fever | 96 |
| Lymphadenopathy | 74 |
| Pharyngitis | 70 |
| Rasha | 70 |
| Myalgias or arthralgias | 54 |
| Diarrhea | 32 |
| Headache | 32 |
| Nausea and vomiting | 27 |
| Hepatosplenomegaly | 14 |
| Weight loss | 13 |
| Thrush | 12 |
| Neurologic disordersb | 12 |
a Including erythematous maculopapular, vesiculopapular, vasculitis, and oral/genital ulcers.
b Including meningoencephalitis, aseptic meningitis, peripheral neuropathy, facial palsy, Guillain–Barre syndrome, brachial neuritis, cognitive impairment, and psychosis.
Adapted from Niu MT, Stein DS, Schnittman SM. Primary human immunodeficiency virus type 1 infection: review of pathogenesis and early treatment intervention in humans and animal retrovirus infections. J Infect Dis 1993; 168:1490–501.
The differential diagnosis for acute HIV-1 infection is primarily comprised of infectious mononucleosis, acute hepatitis, disseminated gonorrhea, roseola, influenza, secondary syphilis, toxoplasmosis and CMV. Certain of these illnesses can be co-infections,164 and therefore anyone presenting with a sexually transmitted disease should be evaluated for possible acute HIV infection; it should also be considered in any patient with an unexplained severe febrile illness.
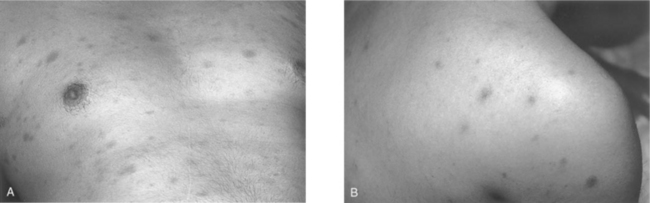
One of the more suggestive and specific symptoms of the acute retroviral syndrome is the central maculopapular rash found on the trunk and face, and occasionally involving the limbs including the palms and soles (Fig. 33-2A).162,163 The rash is painless, nonpruritic, and erythematous, with individual lesions typically 5–10 mm in diameter.156,162–164 There are also descriptions of vesicular or pustular rashes resembling measles (Fig. 33-2B).165,166 Histopathological evaluation of these rashes reveals a mononuclear cell infiltration mainly in the upper dermis, surrounding blood vessels and sweat ducts. Earlier studies have shown CD4+ T cells as the predominant cell type167; a more recent case demonstrated more CD8+ than CD4+ T cells, and CD1a + dendritic cells were seen infiltrating the perivascular space.166 Prior studies have demonstrated HIV-1 p24 antigens in the infiltrate, localizing to dendritic cells.168 There is evidence of focal lymphocytic vasculitis in these biopsies, and occasional reports of palpable purpura as a presenting sign of acute infection.169 It is thought that the interaction of CD8 + T cells with dendritic cells might be the cause of the resulting exanthem.
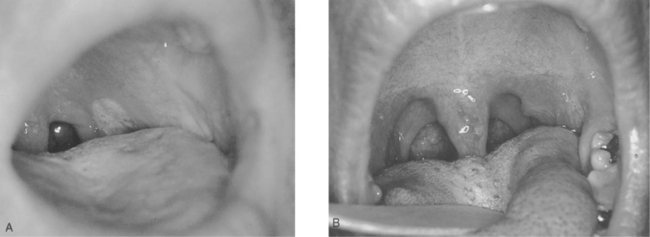
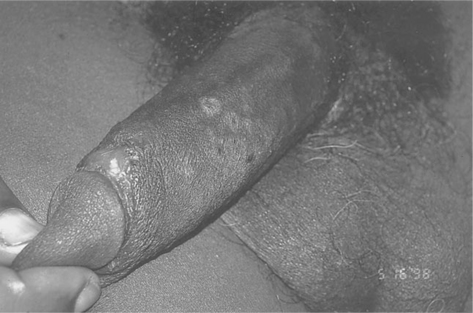
Mucosal involvement, in the form of ulcerations, is also fairly common and tends to be a more specific sign for acute HIV-1 infection than other signs.170 It can involve the genitalia and oral mucosa, including the palate (Figs 33-3 and 33-4).163 It can be associated with oral candidiasis. One study correlating symptoms with mode of transmission demonstrated that genital ulcerations are found only in those who contract the virus sexually.171
Acute HIV-1 infection often presents with neurological syndromes, reflecting the neurotropism of HIV-1 and the early dissemination of the virus to the central nervous system.172–174 Facial nerve palsy,174–176 ascending inflammatory demyelinating polyneuropathy,177 severe myelopathy172 and sensory ganglioneuritis178,179 have all been reported shortly after infection. The most common neurological manifestation is aseptic meningitis, associated in some cases with a lymphocytic pleocytosis in the CSF. It has also been associated with meningoencephalitis,180 acute psychosis,181 and myeloradiculoneuritis.182 The severity of neurological symptoms correlates with the amount of HIV RNA isolated from the CSF.174 These syndromes are usually self-limiting, although there are reports of persistent impairment months later.165,172
Abdominal manifestations of acute HIV infection are most commonly diarrhea, nausea and vomiting, and hepatosplenomegaly.163 There have been many reports of acute hepatitis as the presenting symptom.183,184 Less common manifestations include an inflammatory pseudotumor of the testis,185 pancreatitis,186 and ulcerative esophagitis.187
Infants also manifest symptoms of acute seroconversion. A retrospective case-control study done in Cote d’Ivoire described the acute retroviral syndrome in infants who were infected by breastfeeding.188 There were three dominant syndromes associated with infection; a mononucleosis-likesyndrome with fevers and pharyngitis, dermatitis, and generalized lymphadenopathy. Conversely, infants who are infected perinatally can be asymptomatic for prolonged periods, or may present with fever, failure to thrive, and oral candidiasis.
The most common laboratory abnormalities associated with acute HIV-1 infection are, like the signs and symptoms, quite nonspecific and may be transient. They include thrombocytopenia, lymphopenia, neutropenia, and elevated liver transaminases.156,162,169,180 Levels of CD4+ T lymphocytes can also decrease, with inversion of the CD41/CD81 lymphocyte ratio as the CD8+ cytotoxic T cell population expands, sometimes dramatically.158,180,189 Certain opportunistic infections have been associated with this immunosuppression. Herpes zoster is well described,175 as are oral and esophageal candidiasis.190–192
Immune activation during acute infection, as measured by CD38 expression on T cells, has been correlated with the level of viremia.193 In particular, the level of CD8+ T-cell activation is a strong predictor of the rate of CD4+ T-cell decline, indicating that the pathogenicity of HIV-1 in an individual host might be determined by the ability of the virus to activate the immune system.193 Moreover, lower nadir CD4+ T-cell counts and smaller decreases in HIV-1 RNA during the first 30 days after seroconversion also predict a more rapid disease progression.194
Studies have demonstrated that both the duration and severity of symptoms can predict HIV-1 disease progression.195,196 The incubation period of symptoms is also a predictor of a faster progression to AIDS. In one study, shorter incubations of fever, fatigue and myalgias were all independently associated with the rate of progression, as was a longer duration of myaglias, fever, arthralgias, fatigue, and headache.197 It is theorized that a shorter incubation period for fever is a marker for the secretion of high levels of cytokines and thus indicates a lower degree of control of HIV replication, and a higher viral burden.197 Further investigations into the correlation between symptoms, immunity and viral pathogenicity are warranted.
Overall, these clinical features differ greatly between individuals, and the identification of risk factors is difficult as they are often overlooked. Unfortunately, there is no combination of symptoms or physical findings that reliably distinguishes the acute HIV syndrome from other viral illnesses. As the study by Schacker et al demonstrated, only 26% of patients presenting with symptoms of seroconversion were correctly diagnosed.14 However, the recognition of this syndrome is very important for public health, as the risk of transmission is much higher due to extremely high viral loads and continuing high-risk behavior. A recent retrospective analysis of discordant couples in Uganda demonstrated that the highest rate of HIV transmission (0.0082/coital act) was during the first 2.5 months after seroconversion.8 Other recent studies have focused on the benefit of screening certain populations for the acute HIV syndrome. A recent study analyzed the prevalence of primary HIV infection in patients presenting with fever or other symptoms. In an urban population in the Northeastern US, 1.0% of those presenting with symptoms of a viral illness and with at least one risk factor for HIV were diagnosed with acute HIV infection.198 Most recently, a large-scale analysis of the 2000 National Ambulatory Medical Care Survey and the 2000 National Hospital Ambulatory Medical Survey of the United States demonstrated a prevalence of acute HIV-1 infection of 0.66% in all those who presented to an ambulatory setting with fever, 0.50% in those with rash, and 0.16% in those with pharyngitis.199 These numbers argue for increased use of nucleic acid testing (NAT) to identify the newly infected as rapidly as possible, and to improve prevention efforts in all affected populations.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree