Abstract
Mastectomy complications include infection, lymphedema, pain, deep venous thrombosis, and seroma. The chapter discusses the prevention and management of these complications.
Keywords
mastectomy, wound infection, lymphedema, seroma, complications
Rehabilitation of the postmastectomy patient produces problems of varying complexity. This chapter reviews commonly used approaches for the care of postmastectomy wounds and addresses the complications encountered in these patients. The goals of postoperative care are to anticipate and prevent adverse events and to accelerate recovery.
Care of the Postmastectomy Wound
The various operative techniques used in the treatment for breast carcinoma are described elsewhere in this text. Complications after any operation can be minimized with thorough preoperative evaluation, meticulous technique, hemostasis, and wound closure. In addition to the standard oncologic evaluation, preoperative evaluation includes assessment of the patient’s overall physiologic condition, with particular emphasis on tolerability of anesthesia, uncontrolled diabetes, hypertension, anemia, coagulopathy, or steroid dependency.
Technique at operation and wound closure is an essential part of wound repair. Meticulous hemostasis must be confirmed before closure. Closed-suction drains should be placed into the mastectomy wound site, because most patients will develop a seroma. We prefer closed-suction catheter drainage of the mastectomy wound, commercially available as Blake (Ethicon, Johnson & Johnson Health Care Systems, Piscataway, NJ) or Jackson-Pratt tubing (Baxter Healthcare, Deerfield, IL), and each system should be appropriately placed at operation to allow superomedial and inferolateral positioning to ensure thorough, dependent aspiration. After the wound is closed, the tubing is connected to a closed suction system to ensure removal of all wound contents (e.g., clots, serum). Suction catheter drainage, as a rule, is necessary for 5 to 10 days postoperatively. Earlier removal of the catheters is allowed only when the function of this closed-system technique is compromised. Routinely, catheters are removed only when less than 30 mL of serous or serosanguineous drainage is evident for two consecutive 24-hour intervals.
The skin is closed in two layers using absorbable suture. Thereafter, the skin margins may be covered with strips of surgical tape or wound adhesive. A light, dry gauze dressing is applied to the incision. Pressure dressings over the dissected skin flaps are unnecessary and do not decrease the amount and rate of seroma formation. Postoperatively, the wound is carefully inspected with regard to flap adherence, and the patient is encouraged to resume preoperative activity.
In most circumstances, the breast cancer patient is allowed to begin the gradual resumption of presurgical activities. Younger women usually regain full range of motion of the arm and the shoulder soon after drain removal, whereas some older patients may require intense (supervised) exercise for several months before attaining their former levels of activity. Visits from volunteers of the American Cancer Society or the Visiting Nurse Association are of particular value for psychosocial and physical recovery of the postmastectomy patient.
Complications of Mastectomy
Mastectomy has traditionally been a safe operation with low morbidity and mortality. Although the incidence of postoperative complications is low, physicians should be aware of the morbidity unique to mastectomy and axillary node dissection.
An analysis of National Surgical Quality Improvement Program data recently reported that return to the operating room was the most common morbidity after breast surgery, followed by superficial and deep surgical site infections. Complications can lead to readmissions after mastectomy. A recent report demonstrated a readmission rate of 5.59% after breast surgery with infections being the most common indication for readmission. A detailed review of complications after mastectomy are outlined in the following sections
Lymphedema
Lymphedema affects 6% to 30% of all patients who have had a modified radical mastectomy and is a lifelong risk after the procedure. Lymphedema occurs as a consequence of the en bloc ablation of lymphatic routes (nodes and channels) within the field of resection of the primary mammary tumor. The subsequent increase in plasma hydrostatic pressure that results with removal of these conduits may follow the surgical procedure, irradiation, or uncontrolled progression of neoplasm. Injury, capillary disruption, infection, obstruction to lymphatic or venous outflow, hyperthermia, or exercise will accelerate protein leakage into these tissues. The incidence of lymphedema may be significantly reduced by the use of the axillary reverse mapping procedure advocated by Klimberg and colleagues.
Previous attempts to evaluate the degree of arm lymphedema have been classified by Stillwell according to the percentage of volume increase. This methodology has been subsequently investigated and further refined. We grade an increase of less than 10% in arm volume as mild, whereas an increase of greater than 80% is classified as severe
Factors that have been identified as risk factors for the development or progression of lymphedema include the extent of axillary dissection, the use of axillary radiotherapy, pathologic nodal status, infection or injury, and obesity. Gilchrist stresses the importance of free and complete active range of motion of the arm and shoulder in the early postoperative period. Traditionally it has been believed and taught that avoidance of excessive sun exposure, injections, infections, or other potentially active or passive injury to the ipsilateral extremity is paramount to prevent lymphedema. Two recent studies, however, have challenged this notion. A study by Ferguson and coworkers involved prospective evaluation of a large cohort of breast cancer patients. Factors associated with lymphedema development include high body mass index, cellulitis, prior axillary node dissection, and regional radiation therapy. These authors found no association between injections in the arm, blood pressure measurements in the arm, air travel, or trauma and lymphedema. This is similar to the findings in the Physical Activity and Lymphedema (PAL) trial, which showed no association between blood draws, blood pressure measurements, and air travel on lymphedema risk. Further study is warranted to further define the optimal balance of lifestyle management, upper extremity precautions, and lymphedema risk.
Furthermore, early recognition of incipient edema by the patient and immediate physical therapy with compression massage often alleviates and augments the prophylaxis of further edema. When lymphedema is severe, the mechanical expression of tissue fluid, with application of an intermittent pneumatic compression device, may be helpful, although a recent small randomized trial has questioned its value compared with conservative measures. The physician may wish to prescribe antibiotics if there is evidence of supervening cellulitis. The arm should be elevated above heart level when the patient is inactive. A more thorough discussion of medical, mechanical, and surgical treatment of chronic lymphedema is provided in Chapter 36 .
Wound Infection
Wound infection after mastectomy have been reported to be between 1% and 20%. A large 2012 study from the Mayo Clinic reported infection rate between 2.7% and 8.0% depending on the definition of wound infection used. Infectious complications have been shown to be more common in patients having bilateral mastectomy compared with unilateral mastectomy.
Infection of the mastectomy wound or ipsilateral arm may represent serious morbidity in the postoperative patient and produces an immediate disability that may progress to late extensive tissue dissection that creates thin, devascularized skin flaps. Thereafter, progressive tissue necrosis provides a medium that supports bacterial proliferation with invasive tissue infection. Early debridement of obviously devascularized tissue is an important prophylactic adjunct to prevent progressive invasive infection. When abscess formation does occur, attempts should be made to culture the wound for aerobic and anaerobic organisms, with immediate Gram stain of identifiable strains to document the bacterial contaminant. The predominant organisms are Staphylococcus aureus and Staphylococcus epidermidis.
A meta-analysis examining five prospective randomized controlled trials of preoperative prophylactic antibiotics versus placebo demonstrates that prophylactic antibiotics in breast surgery substantially reduce the incidence of postoperative wound infections in breast surgery without any adverse sequelae from the antibiotic administration. We currently use a first-generation cephalosporin before the incision (given intravenously within 30 minutes before the incision) in patients undergoing a mastectomy. Furthermore, it is suggested that reducing postoperative infections is important to prevent delays in adjuvant therapy and reduce cost.
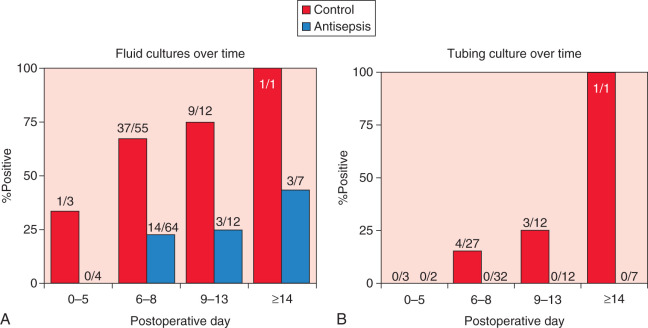
Recent data have suggested that drain antisepsis may have an important role in reducing wound infections after breast surgery. Degnim and colleagues demonstrated that drain bulb irrigation with a dilute sodium hypochlorite solution can significantly reduce bacterial colonization of the drain ( Fig. 34.1 ). Furthermore, in a randomized trial, the technique of drain bulb irrigation combined with the use of chlorhexidine disc dressing was associated with a significant reduction in postoperative infections in breast cancer patients.
Seroma
A seroma is a collection of serous fluid within a surgical cavity that is clinically evident. After a mastectomy, seromas occur in the dead space beneath the elevated skin flaps and represent the most frequent complication of mastectomy, developing in up to 30% of cases. With surgical ablation of the breast, the intervening lymphatics and fatty tissues are resected en bloc; thus, the vasculature and lymphatics of the gland are transected. Thereafter, transudation of lymph and the accumulation of blood in the operative field are expected. Furthermore, extensive dissection of the mastectomy flaps results in a large potential dead space beneath the flaps, as does the irregularity of the chest wall, especially in the deep axillary fossa. Continual chest wall respiratory excursions and motion in the shoulder initiate shearing forces that further delay flap adherence and wound repair. Operative technique should minimize lymphatic spillage and transudation of serum to allow rapid adherence of the skin flaps to deep structures without compromise of blood flow to skin flaps or the axilla.
Factors influencing the likelihood of seroma formation include physical activity after surgery, prior history of breast irradiation, obesity, technique at surgery, use of closed-suction drainage, and closure of anatomic dead space; these factors have been examined in many studies. Historically, various techniques for flap fixation and wound drainage have been used to enhance primary wound repair and to minimize seroma accumulation. Two types of external suture fixation have been advocated. In the study by Orr, tension sutures tied over a rubber tubing bolster to fixate the flaps to underlying intercostal muscles and the latissimus dorsi muscle were used. In the report by Keyes and colleagues, through-and-through flap sutures were tied directly to the skin surface to secure the breast flap to the chest wall. Penrose drains were used to drain excessive accumulation of lymph and blood. Thereafter Larsen and Hugan recommended the application of buried fixation sutures of silk or absorbable material to secure the flaps. These authors secured skin flaps with 30 to 50 subcutaneous cotton sutures and avoided the insertion of any type of drain when possible. Two recent studies have reported that quilting sutures are associated with lower rates of seroma formation compared with conventional closure with drains. However, in general, these flap fixation techniques using bolsters, through-and-through sutures from the flap to the underlying chest wall, and quilting sutures have fallen into disfavor because of additional time required to perform these procedures and the emergence of simple and effective suctioning devices.
The use of closed-system suction catheter drainage since the 1990s has greatly facilitated the reduction in protracted serum collections. Removal of serum accumulation was first accomplished by using static drains, such as Paul’s tubing, and inserting various soft Penrose drains. Both Paul’s tubing and Penrose drains required bulky gauze dressings and multiple dressing changes for the continuous serous soilage expected with wound discharge. In 1947 Murphy proposed continuous closed-suction drainage methods to prevent serum collection beneath extensive flaps. At present, the majority of surgeons use this technique of closed-suction drainage to aspirate excessive collections of serum, lymph, and blood from the mastectomy wound.
In the classic report by Maitland and Mathieson in 1970, 1193 wounds were drained during a 5-year period. Of 153 mastectomies, traditional drainage (i.e., wicks, Penrose) was used in 72, whereas suction drainage was used for the other 81. For operations at various sites, including the genitourinary, alimentary, and biliary tract and soft tissue areas (e.g., breast, thyroid), significant differences were not evident for the two techniques. However, in evaluation of the breast as a subset of the overall analysis, the incidence of wound infection with suction drainage (4.9%) versus traditional drainage (12.50%) was 1.7 times less frequent ( p = .045). For this subset of the patient population, the authors noted a diminished wound infection rate and increased primary healing with the application of closed-suction drainage techniques.
These results were confirmed by Morris after a controlled clinical trial performed to compare the effectiveness of suction drainage with that of static drainage. For radical mastectomy wounds, this trial established that the rate of wound repair was superior with suction drainage technique. Furthermore, the volume of aspirated drainage was greater with the closed-suction method, which also afforded a reduction in the infection, tissue necrosis, and wound disruption frequency.
Tadych and Donegan determined the daily wound drainage and total hospital drainage (THD) via a closed-suction system for 49 consecutive patients undergoing a mastectomy to evaluate the frequency of seroma and lymphedema formation. Of this series of patients undergoing modified radical mastectomies and who did not receive irradiation, the THD varied from 227 to 3607 mL and did not correlate with body weight. Ipsilateral edema of the arm directly correlated with THD. Of clinical and practical significance, no patient with less than 20 mL of drainage in the 24 hours before catheter removal developed a seroma.
The extent of surgery also affects seroma formation. A more extensive surgery increases the likelihood of seroma formation in addition to other surgical complications. Aitken and colleagues evaluated 204 consecutive mastectomies (radical and modified radical) in which the techniques used for flap closure and wound management were identical. All potential dead space was obliterated with absorbable sutures that incorporated the pectoralis major, serratus anterior, and latissimus dorsi muscles, as well as the subdermal skin of the axillary flap. Two closed-suction Hemovac drains, one placed in the axillary apex along the lateral part of the chest wall and the other placed over the anterior portion of the chest, were inserted via a separate lower flap stab incision. The average initial volume and total volume of the fluid aspirated from the wounds were similar in both radical and modified radical mastectomy groups (91.1 mL vs. 91.7 mL).
Table 34.1 summarizes the wound complications observed in this series. Postoperative fluid accumulation occurred in 9.31%, with greater frequency in the radical mastectomy group. Infected seroma was identified only in the radical mastectomy group, with an overall frequency of 0.98%. The magnitude of the radical mastectomy procedure perhaps also accounted for the frequency of superficial wound infections, which were more than four times as frequent in this group as in the modified radical mastectomy group. Aitken and Minton identified a decreased incidence of seroma accumulation in these less extensive operations on the breast (simple mastectomy had less incidence than modified radical, which in turn had less incidence than radical mastectomy).
| Complication | TYPE OF MASTECTOMY | |||
|---|---|---|---|---|
| Radical (n = 72) | Modified Radical (n = 117) | Simple (n = 15) | Total (n = 204) | |
| Hematoma or seroma | 14 (19.44%) | 5 (4.27%) | — | 19 (9.31%) |
| Infected seroma | 2 (2.78%) | — | — | 2 (0.98%) |
| Superficial wound infection | 5 (6.94%) | 2 (1.71%) | — | 7 (3.43%) |
Surgical technique also influences the likelihood of seroma formation. The use of electrocautery increased the rate of seroma formation in one trial in which patients were randomly assigned to dissection of the mastectomy flaps with either scalpel or electrocautery. Seromas developed in 38% of the electrocautery group versus 13% of the scalpel group ( p = .01; Table 34.2 ). Some patient characteristics also affect seroma formation. Three studies indicate that patients with a higher body mass index have an increased rate of seroma formation. The incidence of seroma formation also increases with age.
| Seroma (n = 21) | No Seroma (n = 59) | P b | |
|---|---|---|---|
| Dissection technique | |||
| Scalpel | 5 | 33 | .01 |
| Cautery | 16 | 26 | |
| Estimated blood loss (mL) | 90 ± 87 | 196 ± 165 | .006 |
| Drain type c | |||
| Blake | 4 | 29 | .006 |
| Jackson-Pratt | 15 | 30 | |
| Duration of drains (days) | 5 ± 2 | 6 ± 3 | .19 |
| Drainage last day (mL) | |||
| Drain 1 | 27 ± 47 | 17 ± 20 | .17 |
| Drain 2 | 22 ± 18 | 17 ± 13 | |
| Total drainage (mL) | |||
| Drain 1 | 320 ± 514 | 213 ± 135 | .15 |
| Drain 2 | 311 ± 493 | 241 ± 213 | .40 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








