Women’s Health Issues in Diabetes Mellitus
Julie Lund Sharpless
Several aspects of the female reproductive life cycle are influenced by insulin and the metabolic effects of diabetes. From puberty through childbearing to menopause, women with diabetes may need to make special adjustments in their regimens to maintain optimal control as the reproductive hormones change. Conversely, poor diabetic control can impair normal reproductive function. The waning of reproductive hormones at menopause raises special issues in decisions about hormone replacement therapy in women with diabetes, as well as additional risks for osteoporosis and endometrial cancer.
Most investigations into the effects of diabetes on reproductive function have focused on type 1 insulin-dependent diabetes because this is the most prevalent form in women of reproductive age. Insulin resistance also has reproductive consequences, both in severe forms such as type A insulin resistance and in milder forms as in polycystic ovary syndrome. The changing epidemiology of diabetes in the United States, with an increasing prevalence of obesity and an earlier onset of type 2 diabetes, makes the reproductive consequences of insulin-resistant diabetes more significant. Studies of diabetes in postmenopausal women have focused largely on women with insulin-resistant diabetes. Although issues of cardiovascular disease, hyperlipidemia, and endometrial cancer are important in this population, another classic postmenopausal issue, osteoporosis, is of greater concern for women with type 1 diabetes. Thus, this chapter will review both type 1 and type 2 diabetes and their metabolic effects in women. In many studies, the type of diabetes has not been defined by strict standards such as C-peptide, autoimmune antibodies, or insulin levels; thus, subjects described as insulin-dependent may include persons with either type 1 or type 2 diabetes. In these situations the descriptions “insulin-dependent diabetes mellitus” or “non-insulin-dependent diabetes mellitus” are used as specified in those studies, as the heterogeneity of the populations may help reconcile the results.
MENSTRUAL CYCLES
Menarche
In 1925, Dr. Eliot Joslin observed that “unaided by insulin, no girl in our series developed menstruation after the onset of diabetes” (1). By the mid-20th century, a major series by Bergqvist reported delay in menarche of 15 months associated with diabetes (2). More recently, this observation has been contested by studies showing normal menarche in series of clinic patients (3,4,5,6). However, subanalyses in several of these studies showed
that those girls with the onset of diabetes before 10 years of age or before menarche still had a delay in menarche, as well as more irregular menses (3,6). The major complications of diabetes have now been shown to correlate with glucose control, as these studies would suggest for age of menarche. Most of these studies, however, did not specifically investigate degree of control in relation to onset of menarche. One indirect clue is available from a study by Yeshaya et al. (6), who found that, among women with diabetes, those with diabetic complications were older at menarche and more likely to have amenorrhea than those without complications, but a direct relationship between menarche and glycemic control has not been established.
that those girls with the onset of diabetes before 10 years of age or before menarche still had a delay in menarche, as well as more irregular menses (3,6). The major complications of diabetes have now been shown to correlate with glucose control, as these studies would suggest for age of menarche. Most of these studies, however, did not specifically investigate degree of control in relation to onset of menarche. One indirect clue is available from a study by Yeshaya et al. (6), who found that, among women with diabetes, those with diabetic complications were older at menarche and more likely to have amenorrhea than those without complications, but a direct relationship between menarche and glycemic control has not been established.
Menstrual Dysfunction
Beyond menarche, menstrual periods are often disrupted by diabetes. The major problems are absent menses (amenorrhea) or infrequent menses (oligomenorrhea), but overly frequent menses (polymenorrhea, or more commonly called “dysfunctional uterine bleeding”) have also been described. Some type of irregular menses has been reported in 22% to 47% of women with diabetes (3,7). These high prevalence rates likely reflect various definitions of irregularity, as the corresponding incidence in controls ranged from 11% to 35%. The lower figures come from one of the most thorough studies of menstrual cycles in insulin-dependent diabetes by Kjaer et al. (3), using an epidemiologic study of an entire county in Denmark. Kjaer and others (3,8) also have found that the incidence of irregularity correlated inversely with diabetic control and with body weight. Specifically, two groups have shown that the incidence of menstrual disturbances increases with the hemoglobin A1c concentration (HbA1c) and becomes statistically significant with HbA1c values above 10% (3,9).
Regular menstrual function requires the integration of neuronal and hormonal signals between the hypothalamus, the pituitary, the ovaries, and the uterus. Neuronal signals in the hypothalamus trigger the pulsatile release of gonadotropin-releasing hormone (GnRH), which causes the pituitary to release follicle-stimulating hormone (FSH) and luteinizing hormone (LH), which in turn stimulate the development of eggs, estrogen, and progesterone in the ovary. The estrogen fosters proliferation of the endometrium and feeds back to the hypothalamus and pituitary to control its own production. No single mechanism of impairment in diabetes has been established, but abnormalities from the hypothalamus to the ovary have been described.
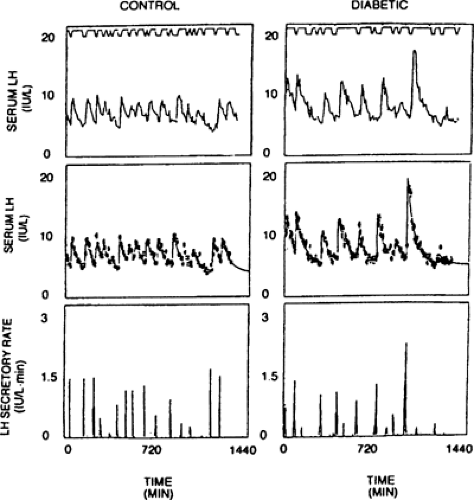
The most clearly described syndrome of menstrual dysfunction in diabetes is a form of hypothalamic amenorrhea. Hypothalamic amenorrhea (HA) results from lack of stimulation by the hypothalamic GnRH to the pituitary LH and FSH. In HA, serum levels of LH and FSH are low, and studies using frequent sampling show that LH and FSH pulses are decreased or absent and insufficient to stimulate ovulation; thus menses do not occur (10). Women with insulin-dependent diabetes and amenorrhea have been found to have low LH and FSH levels, and decreased LH pulses (8,11) (Fig. 44.1). Various investigators have tried to assess GnRH responsiveness of the pituitary gonadotroph cells by administering a single dose of GnRH to women with diabetes, but results are conflicting and not as informative as those from pulse profiles of LH (12). However, a study by South et al. (11) that showed decreased LH pulses in amenorrheic, diabetic women (as compared with normal cycling nondiabetic women), also noted an increased LH response to GnRH. Both decreased pulses and an increased LH response to GnRH are seen in other forms of hypothalamic amenorrhea. The clinical features of HA in diabetic women are similar to those in the general population. As in women with poorly controlled diabetes, hypothalamic amenorrhea also occurs in nondiabetic women who have inadequate nutrition, frequently as a result of excessive exercise or anorexia nervosa (13). Severe illness can also suppress levels of LH and FSH, as seen in patients hospitalized for other diseases (14). In diabetes, HA may reflect a combination of these factors. Diabetic women with hypothalamic amenorrhea tend to be underweight and/or to have poor glucose control. In the series of HA in diabetes by South et al. (11) and O’Hare et al. (15), women were selected for normal body weight but had elevated mean glycosylated hemoglobin of 12.8% and HbA1c of 11.8%. Djursing et al. (8) included both normal and underweight subjects but did not find a correlation between amenorrhea and glycemic control. An obvious approach to these patients is to improve diabetes control. Unfortunately, after 6 months of improved metabolic control decreasing HbA1c levels from 11.8% to 8.5%, with a concomitant mean weight gain of 4.2 kg, none of six amenorrheic subjects in the series of O’Hare et al. (15) resumed menses, suggesting additional processes contributed to the observed amenorrhea. Women with hypothalamic amenorrhea have an increased risk for estrogen-deficiency osteoporosis (16) and need treatment to restore or replace (i.e., oral contraceptive pills) menstrual cycles to ensure adequate estrogen supplies (17). Although the bone-density response to oral contraceptive pills has not yet been specifically evaluated in diabetes, treatment must be considered because of the additional increased risk of osteoporosis in type 1 diabetes (see discussion below). Other causes of HA, such as eating disorders, which have an increased prevalence in women with diabetes (18), must also be addressed. Even without meeting the full psychiatric DSM III-R criteria for anorexia nervosa or bulimia, 15% to 40% of young women with diabetes disclose
disordered eating and underdosing their insulin as a tool for weight loss (19,20). In addition to worsened glycemic control, diabetic patients with eating disorders have an increased incidence of diabetic complications (21,22).
disordered eating and underdosing their insulin as a tool for weight loss (19,20). In addition to worsened glycemic control, diabetic patients with eating disorders have an increased incidence of diabetic complications (21,22).
Oligomenorrhea has also been associated with poor glucose control and low body weight in insulin-dependent diabetes (3,8). Some cases of oligomenorrhea in women with diabetes may represent a form fruste of hypothalamic amenorrhea. For research purposes, these studies define hypothalamic amenorrhea as 3 or 6 consecutive months of missed periods and oligomenorrhea as irregular periods occurring nine or fewer times per year. In diabetic women, as in nondiabetic women, the causes of oligomenorrhea are diverse. Oligomenorrhea specific to diabetes has received very little study. Most investigations of oligomenorrhea and diabetes have focused on polycystic ovary syndrome (PCOS) and type 2 diabetes rather than type 1 diabetes. PCOS is defined by oligomenorrhea and hyperandrogenism (such as hirsutism or acne) or hyperandrogenemia (such as elevated serum testosterone concentration or DHEAS). It is often associated with obesity and insulin resistance and carries a high incidence (30%–50%) of subsequent type 2 diabetes (23,24). PCOS is rare in type 1 diabetes with negative C-peptide levels (25). Prelevic et al. (26) found that oligomenorrheic patients with insulin-dependent diabetes who were negative for C-peptide had low androgens and low LH/FSH ratios, while those who were positive for C-peptide had more classic features of PCOS, with elevated androgen levels and LH/FSH ratio and histories of obesity and oligomenorrhea before the diagnosis of diabetes. One recent paper has contested these views, finding a 39% prevalence of PCOS in a group of Spanish patients with insulin-dependent diabetes (27). However, this group did not test C-peptide levels, and controls were excluded if they had signs of hyperandrogenism (hirsutism or acne)—important because normal body hair varies by ethnicity, so higher hirsutism scores are normal in Hispanic women with or without diabetes. Treatment with insulin-sensitizing medications ameliorates the hyperandrogenism of PCOS and has been shown to restore ovulatory cycles (28). Weight loss has also been found to restore regular menses and ovulation, as well as insulin sensitivity in PCOS (29). Because insulin augments LH-driven ovarian androgen synthesis, an elevation in androgens that induces insulin resistance (30) is difficult to distinguish from the elevated insulin levels of insulin resistance that induce hyperandrogenism (31). Blockade of this loop in either direction, by treating the hyperandrogenism (i.e., with the antiandrogens estrogen, flutamide, or spironolactone) or treating the insulin resistance (i.e., with metformin or troglitazone) is effective for PCOS (32).
Oligomenorrhea also is seen in the syndromes of severe insulin resistance. Type A insulin resistance, which is mediated by insulin-receptor defects, and type B insulin resistance, which is mediated by antibodies, share symptom patterns including hirsutism, oligomenorrhea, and hyperandrogenism. Treatment of these syndromes includes the use of antiandrogens, as well as GnRH analogues, to block LH production (33). A defect in the insulin receptor has also been found in some women with PCOS (34).
Ovarian dysfunction is suggested by increased levels of the androgens, including testosterone and androstenedione, in women with insulin-dependent diabetes (35). However, these levels are increased in normally cycling women with insulin-dependent diabetes and are not usually associated with clinical signs of hyperandrogenism such as hirsutism or acne, and the free androgen levels are normal (36). Rather than an ovarian problem, these androgen levels are likely due to the elevated levels of sex hormone-binding globulin (SHBG) stimulated by insulin. This is further supported in a study by Djursing et al. (36) that found that SHBG levels were decreased in the amenorrheic women both with and without diabetes. Thus, hypothalamic, pituitary, and ovarian factors may contribute to menstrual disruption in diabetes.
Carbohydrate Metabolism
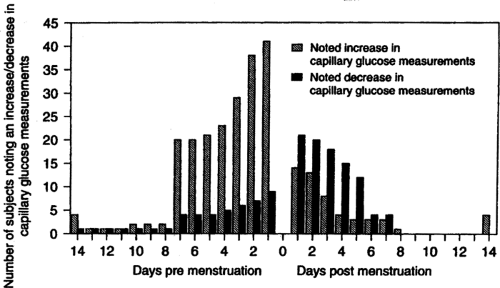
Even with regular menstrual cycles, many women with diabetes note changes in their home monitoring of blood glucose results during the cycle. In observational series of women with diabetes, 61% to 70% of patients reported cyclic glucose changes (7,37). Three fourths of these women described higher glucose levels in the luteal phase, especially during the week before menses. A consistent but smaller set report hypoglycemia at the beginning of menses (Fig. 44.2). In Lunt and Brown’s study (37), 36% of the women adjusted their insulin to accommodate perimenstrual glucose changes, but this subset did not have better control and even showed a trend toward slightly higher levels of glycosylated hemoglobin. This study did not assess carbohydrate intake or exercise levels but did note that the insulin-adjusting group reported more perimenstrual changes in appetite.
The mechanism behind menstrual glucose changes has been a source of controversy, perhaps because of the heterogeneity of
individual responses. In normal cycling, nonobese, nondiabetic women, some (38,39) but not all (40,41,42), studies of oral glucose tolerance have shown a decline in glucose tolerance during the luteal phase of the menstrual cycle. Progesterone, which increases during the luteal phase, has been implicated as the cause of worsened glucose tolerance because of its ability to induce insulin resistance (43). Intravenous glucose tolerance tests have not, however, shown consistent changes across the menstrual cycle (44). Euglycemic, hyperinsulinemic clamp studies in normal subjects showed no differences in basal levels of glucose, insulin, or glucose turnover during the follicular or luteal phase (45,46). In contrast, in hyperglycemic clamp studies, impaired glucose metabolism is seen in the luteal phase (47). When these same investigators performed hyperglycemic clamp studies in women with insulin-dependent diabetes, they found a heterogeneous response, with no cycle phase differences in some women, and a decrease in luteal-phase insulin sensitivity in others (48). The women who showed cyclic decreases in insulin sensitivity in clamp studies were those who noted premenstrual hyperglycemia; however, they were not different from women without cyclic differences with regard to duration or control of diabetes, age, or body weight. The worsened premenstrual insulin sensitivity was associated with a greater increase in estrogen from the follicular to luteal phase.
individual responses. In normal cycling, nonobese, nondiabetic women, some (38,39) but not all (40,41,42), studies of oral glucose tolerance have shown a decline in glucose tolerance during the luteal phase of the menstrual cycle. Progesterone, which increases during the luteal phase, has been implicated as the cause of worsened glucose tolerance because of its ability to induce insulin resistance (43). Intravenous glucose tolerance tests have not, however, shown consistent changes across the menstrual cycle (44). Euglycemic, hyperinsulinemic clamp studies in normal subjects showed no differences in basal levels of glucose, insulin, or glucose turnover during the follicular or luteal phase (45,46). In contrast, in hyperglycemic clamp studies, impaired glucose metabolism is seen in the luteal phase (47). When these same investigators performed hyperglycemic clamp studies in women with insulin-dependent diabetes, they found a heterogeneous response, with no cycle phase differences in some women, and a decrease in luteal-phase insulin sensitivity in others (48). The women who showed cyclic decreases in insulin sensitivity in clamp studies were those who noted premenstrual hyperglycemia; however, they were not different from women without cyclic differences with regard to duration or control of diabetes, age, or body weight. The worsened premenstrual insulin sensitivity was associated with a greater increase in estrogen from the follicular to luteal phase.
The presence or absence of premenstrual syndrome did not affect carbohydrate metabolism assessed by oral glucose tolerance testing in normal subjects across the cycle (41). Premenstrual symptoms in women with diabetes have been correlated only with depression (specifically not hypoglycemia) and do not differ (except the perception by women with diabetes that they were less severe) from those in women without diabetes (7).
SEXUAL DYSFUNCTION
Some surveys have found a high incidence of complaints of sexual dysfunction in women with diabetes, whereas others have reported a similar incidence in women with and without diabetes [reviewed in reference (49)]. The specific components of sexual dysfunction in women are poorly understood. In women as compared with men, sexual function is more variable within an individual and across the life cycle, has a less predictable response to hormones, and is more susceptible to social influences (50). These issues and others have limited research on sexual dysfunction in women, particularly in women with diabetes (51). Extrapolating from research on male sexual dysfunction is not necessarily relevant because of the gender differences in sexual function. For instance, in contrast to studies in men, the first study of sildenafil in women showed no improvement in sexual function (52), although one later study did show some improvement in sexual fantasy and satisfaction (53). Also in contrast to the findings in men, two studies of sexual function in women with diabetes found no correlation between sexual function and glycemic control (with the caveat that the sexual dysfunction predated the diabetes in many of the subjects) (54,55). One study of xerostomia noted that complaints of vaginal dryness were more common in diabetic women who had abnormally low salivary flow rates than in other diabetic women (56). The low salivary flow correlated with higher HbA1c but not with cardiovagal autonomic dysfunction. Thus, although poor diabetic control and extensive vascular and neurologic complications are known to contribute to sexual dysfunction in men (57), few data exist to support parallels in women.
It is important to exclude other comorbid illnesses that can inhibit sexual function. Vulvovaginal candidiasis, which can cause dyspareunia, occurs more frequently in women with poorly controlled diabetes (58,59). Depression, which has an increased prevalence in people with diabetes (60), is also a major cause of sexual dysfunction (61). Treatments for comorbid conditions, particularly depression and hypertension, also can have an impact on sexual function. Treatments for depression, especially selective serotonin reuptake inhibitors, may inhibit sexual function as a side effect but also improve sexual function as the depression is treated (62). Many antihypertensive medications have been implicated in male sexual dysfunction. In women, thiazide diuretics and spironolactone have been noted to decrease vaginal lubrication (49). In summary, diabetes has not yet been shown to be a direct cause of sexual dysfunction in women, but knowledge of the common issues associated with diabetes offers potential therapeutic approaches.
FERTILITY
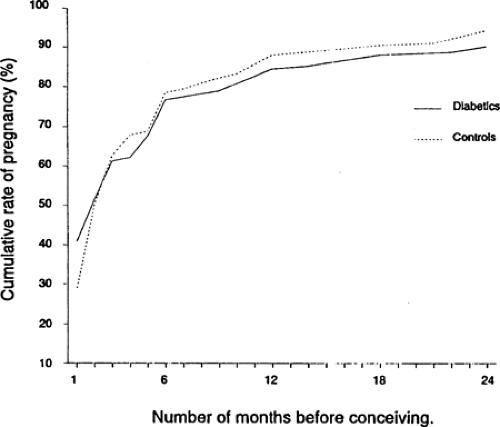
In women with diabetes who have regular menstrual cycles, the ability to conceive is not affected. Among women with insulin-dependent diabetes diagnosed prior to pregnancy, the cumulative rates of pregnancy (85% in the first year) and of involuntary infertility (17%) were the same as in the control Danish population (63) (Fig. 44.3). In women with menstrual irregularity such as amenorrhea or oligomenorrhea, missed periods represent missed ovulations and therefore decreased opportunity for fertilization. Thus, women with PCOS and diabetes may have decreased fertility (32). Fertility in women with PCOS (studied in nondiabetic patients) is enhanced by weight loss (29) and insulin sensitizers (28). The results of in vitro fertilization in a small series of women with insulin-dependent diabetes, in good control as part of the therapy, were not different from the results in women without diabetes (64). Poor glycemic control does not impair the ability to conceive but does impair fertility because of an increase in spontaneous abortion in proportion to the
increase in HbA1c (65). For women seeking fertility, the first approach should be optimization of glucose control, not only to improve ovulation and decrease the risk of spontaneous abortion but also to decrease the risk of birth defects. The prevalence of fetal malformations is increased when glycemic control is poor during early pregnancy (66) (see Chapter 61).
increase in HbA1c (65). For women seeking fertility, the first approach should be optimization of glucose control, not only to improve ovulation and decrease the risk of spontaneous abortion but also to decrease the risk of birth defects. The prevalence of fetal malformations is increased when glycemic control is poor during early pregnancy (66) (see Chapter 61).
CONTRACEPTION
Contraception is an essential issue in the care of women with diabetes. Pregnancies must be planned because poor glycemic control during pregnancy leads to an increase in maternal and fetal complications, and good glycemic control reduces that risk (65,66). Nevertheless, most pregnancies in women with diabetes are still unplanned (63). Because of the medical importance of good compliance with the contraceptive regimen in this population, logistical issues are as important as medical effects and side effects associated with various contraceptives (Table 44.1).
TABLE 44.1. Effectiveness of Family-Planning Methods | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
For some women with advanced complications of diabetes, contraceptives are necessary for the protection of their own health. Pregnancy may worsen retinopathy (67) and nephropathy (68) and is associated with increased maternal mortality, especially if nephropathy or coronary artery disease is present. For women with these complications who have completed their families, surgical sterilization by tubal ligation should be considered. Women with diabetes need careful counseling on the risks of planned and unplanned pregnancy (see Chapter 61).
Combination Oral Contraceptive Pills
Oral contraceptive pills (OCPs) are the most popular form of reversible contraception and are associated with one of the lowest rates of unintended pregnancy. OCPs combine supraphysiologic doses of estrogen and progesterone and work by several mechanisms, principally by suppressing the hypothalamic and pituitary stimulation of the ovary. A common concern is the potential to worsen glycemic control with OCPs. This stems from reports in which early OCP formulations were used at much higher doses than are currently used. Worsened glucose tolerance was first reported by Waine et al. in 1963 (69), who noted that nondiabetic women treated with a high-dose OCP (containing 100 μg of mestranol) developed impaired glucose tolerance. Women with a history of gestational diabetes mellitus (GDM), a first-degree relative with diabetes, or who are obese or older are at higher risk for the development of impaired glucose tolerance while receiving higher-dose OCPs (70). The impaired glucose tolerance usually reverses within 6 months of discontinuation, except in women with a history of GDM.
Most OCPs currently in use are “low dose” (<50 μg of estradiol) OCPs, which also contain up to a 25-fold lower dose of progesterone and have minimal to no effect on glucose tolerance, even in women with a history of GDM (71,72,73). In women with pre-existing insulin-dependent diabetes, OCPs can also decrease glucose tolerance, but significant adverse effects on glucose control are very unusual (74). The low-dose OCPs maintain the same contraceptive efficacy as the high-dose OCPs but are associated with a lower risk of some of the other dose-related side effects, such as stroke and cardiovascular disease, effects that have deterred physicians from using OCPs in women with diabetes (75). Not only do changes in the estrogen and progestin doses impact glucose tolerance, so do differences in the particular progestin. The gonane-derived progestins (e.g., norgestrel) produce more hyperinsulinemia (71).
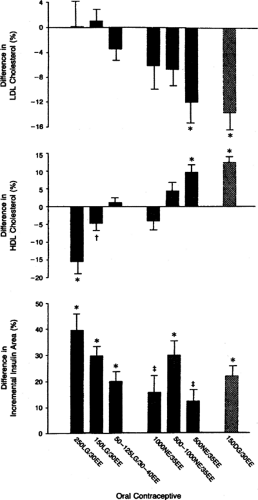
Various OCP formulations can have minor or marked effects on lipids, changes particularly important in women with diabetes who already are at increased cardiovascular risk. In a large study of nine different OCPs used by 1,040 women (without diabetes), compounds with desogestrel increased high-density lipoprotein (HDL) cholesterol and decreased low-density lipoprotein (LDL) cholesterol, compounds with levonorgestrel decreased HDL and increased LDL, and norethindrone had an
intermediate effect (76) (Fig. 44.4). Petersen et al. (77) examined the effects of OCPs with norethisterone, levonorgestrel, and gestodene in 30 diabetic women and found no adverse changes in plasma lipids from any of the compounds. In rare cases, OCPs can induce severe hypertriglyceridemia. This is mostly a risk for women with baseline triglycerides greater than 600 mg/dL (78). Estrogens are contraindicated in patients with any of the familial hypertriglyceridemia syndromes because of the increased risk of pancreatitis. Women with diabetes who are receiving estrogen therapies should have their lipid profiles monitored because poor glycemic control also results in elevated triglycerides. This effect is secondary to the decrease in lipoprotein lipase activity caused by relative insulin deficiency.
intermediate effect (76) (Fig. 44.4). Petersen et al. (77) examined the effects of OCPs with norethisterone, levonorgestrel, and gestodene in 30 diabetic women and found no adverse changes in plasma lipids from any of the compounds. In rare cases, OCPs can induce severe hypertriglyceridemia. This is mostly a risk for women with baseline triglycerides greater than 600 mg/dL (78). Estrogens are contraindicated in patients with any of the familial hypertriglyceridemia syndromes because of the increased risk of pancreatitis. Women with diabetes who are receiving estrogen therapies should have their lipid profiles monitored because poor glycemic control also results in elevated triglycerides. This effect is secondary to the decrease in lipoprotein lipase activity caused by relative insulin deficiency.
Hypertension, another infrequent side effect of OCPs in normal women (79), merits careful attention in the diabetic population. Studies of the use of high-dose OCPs in healthy women demonstrated new hypertension in 4% to 5% and worsening in 9% to 16% of women with pre-existing hypertension (80). This effect may be due to both the estrogen and the progestin components and is reversible with cessation of the OCP. Angiotensinogen production is increased by ethinyl estradiol (81), and progestins may have mineralocorticoid agonist or antagonist effects. Sudden development or worsening of hypertension after starting OCPs should be considered a complication of the medication and mandates discontinuation of the OCP. Few data exist for women with diabetes, except for the lack of problems noted in studies looking at other aspects of OCPs in carefully selected populations. A large (384 women with type 1 diabetes) and reassuring cross-sectional study by Klein et al. (82) showed no association between current or past use of OCPs and severity of hypertension or retinopathy or level of current glycemic control. Another smaller retrospective study also showed that the use of OCPs in women with a mean age of 22.7 years but a mean duration of diabetes of 13.8 years did not increase the risk of their developing early retinopathy or nephropathy (83).
Concern about increased risk of macrovascular complications stems in part from early retrospective observations in a series of diabetic women who were taking high-dose formulations, among whom cerebrovascular thromboses developed in three and myocardial infarction in one of 120 women who used OCPs compared with no such events in the control group who used nonhormonal contraception (84). This increased risk has been supported by subsequent studies demonstrating relative risks from 1.8 to 6.9 in women with diabetes versus nondiabetic OCP users (85,86). However, neither of these studies assessed the relative risk of cerebral or myocardial infarction in women with diabetes not taking OCPs. Another study showed an increased relative risk of stroke in women with diabetes that was the same in users and nonusers of OCPs (87). The risk for cardiovascular events is likely based on thrombotic events, which were increased in women taking OCPs (88), and may be related to the increased clotting factors (factor X, factor II, plasminogen, PAI-1) and decreased platelet aggregation that occur with OCPs (89) and with hyperinsulinemia and hyperglycemia (90,91). Older studies also showed some increased risk among nondiabetic OCP users as compared with nonusers, but the newer (low-dose estrogen and second- and third-generation progestins) OCPs are actually associated with the same or lower risk of cardiovascular disease in nonsmoking, nondiabetic OCP users (88,92,93). It is controversial whether these studies can appropriately account for the excess risk of cardiovascular disease due to smoking, which is high and accounts for the majority of myocardial infarctions in young women (94,95). In nonsmoking women with well-controlled, uncomplicated diabetes, there is probably not an excess risk of cardiovascular disease associated with OCP use, but this has not yet been confirmed in prospective studies. A large retrospective study by Klein et al. (96) did not show any excess mortality in diabetic OCP users. Thus, in otherwise healthy women with diabetes, OCPs decrease health risks by decreasing the risks associated with unintended pregnancy. For women with complications of diabetes, especially vascular disease, or who smoke, these additional risks of estrogen-progestin-based OCPs must be weighed in comparison to the additional risks of pregnancy.
Newly available transdermal forms of estrogen-progestin contraceptives offer the possibility of a lower thrombotic risk based on extrapolation from lower-dose transdermal hormone replacement therapy (97). Another recent therapy is the use of
OCP tablets taken in extra doses as emergency postcoital contraception. This approach provides higher acute but less chronic exposure to estrogen and progesterone and is more convenient. Unfortunately, it is often poorly tolerated and not as effective as regular OCP use (98). Data are not yet available on the use of these newer contraceptive options in women with diabetes.
OCP tablets taken in extra doses as emergency postcoital contraception. This approach provides higher acute but less chronic exposure to estrogen and progesterone and is more convenient. Unfortunately, it is often poorly tolerated and not as effective as regular OCP use (98). Data are not yet available on the use of these newer contraceptive options in women with diabetes.
Progesterone
A major advantage of progesterone-based contraceptives is the lack of thrombotic or hypertensive effects (75,81). Weight gain and irregular bleeding are, however, common side effects. Progesterone contraceptive options include daily pills, short-term depot injections lasting 3 months (e.g., Depo-Provera), and long-term implants in silastic capsules lasting 5 years (Norplant). Intrauterine devices also may be coated with progesterone. Progesterone-only “mini-pills” are not as effective at suppressing ovulation as are combined OCPs, but they effectively decrease the volume of cervical mucus and increase its viscosity to block implantation (98). Progesterone-only pills have not been studied for their effects on glucose control in women with diabetes, but their high risk of failure is a concern (98). Depot formulations of progesterone do not fail as frequently, but they may increase insulin resistance slightly (99) and are associated with weight gain in some individuals (98). Subdermal levonorgestrel (Norplant) offers the advantage of long-lasting contraception (up to 5 years), which is suitable for many younger women, but requires a minor surgical procedure for insertion. Long-acting progesterone formulations offer good contraception for patients who are unable to comply with daily pills. Emergency contraception can also be accomplished with progesterone alone, using two doses of levonorgestrel (“Plan B”), which would have little metabolic impact on diabetes but is less effective than chronic use (98).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree