Virology
Hepatitis B virus is a partially double-stranded DNA virus that is a member of the family Hepadnaviridae. This family includes several other animal viruses, including woodchuck hepatitis virus, duck hepatitis
virus, and ground squirrel hepatitis virus
74. Humans are the only natural host for HBV infection. The chimpanzee is the primary experimental model for infection, but the disease has also been studied in gibbons, marmosets, and other primates.
74 HBV can retain infectivity for at least 1 month at room temperature and much longer when frozen. Heating to 90°C for 1 hour renders HBV noninfectious.
The HBV genome has approximately 3200 nucleotides and replicates through an RNA intermediate that is transcribed by a gene product with reverse transcriptase activity. The proteins encoded by the HBV genome are the envelope, the nucleocapsid, the X protein, and a DNA polymerase. The envelope proteins encoded by the HBV genome includes the surface proteins, pre-S, pre-S2, and the HBsAg. HBsAg is a glycosylated lipoprotein that contains the major site for binding of neutralizing antibody. It can circulate as a part of the complete virion (called the Dane particle) or independently of viral particles. Four subtypes of HBsAg have been identified, which are designated adw, ayw, adr, and ayr. The “a” epitope, which is common to all HBsAg subtypes, is the binding epitope for neutralizing antibodies. Therefore, antibodies to HBsAg are protective for all subtypes.
75 Nonlethal mutations can also occur in the S gene, sufficiently altering the expression of this protein to permit viral escape from neutralizing antibodies. The occurrence of the other subtype determinants varies geographically, such that HBsAg subtyping has been used in epidemiology studies to establish patterns of transmission.
76 The subtypes do not appear to differ in infectivity or virulence.
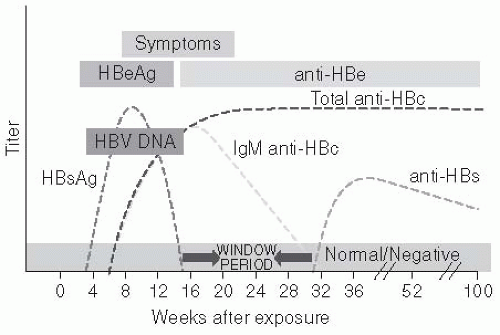
The HBV nucleocapsid proteins—HBeAg and HBV core antigen (HBcoreAg)—are also used to delineate between important disease states. HBeAg is a marker for current active viral replication. However, mutations can occur that truncate expression of “e” antigen without substantially altering virion production. The resultant viral phenotype results in clinical infection with HBV DNA and HBsAg but no detectable HBeAg in the blood. Some individuals infected with these HBeAg-negative mutant viruses have developed fulminant hepatitis.
77,
78,
79 and
80 The HBcoreAg is the major nucleocapsid protein and is not detected in the serum but is present in the liver. The cellular immune response to HBcoreAg in the liver is believed by some investigators to be responsible for hepatic necrosis associated with chronic liver disease in HBV carriers.
80,
81,
82,
83 and
84 Persons infected with HBV form antibodies to HBcoreAg that are persistent, making them useful in the diagnosis of current or previous infection.
Transmission Routes
HBV can be transmitted by percutaneous blood exposure, sexual intercourse, and from a mother to her infant (
Figure 23-9). The risk of transfusiontransmitted HBV infections in the United States declined substantially starting with HBsAg screening of blood donors in 1973, followed by exclusion of high-risk populations for HBV, HCV, and HIV
infection as blood donors and in 2010 routine utilization of nucleic acid testing by PCR to detect HBV DNA among blood donors. Also, blood centers in the United States and Europe routinely test donated blood for anti-HBc. These testing procedures have virtually eliminated transfusion-transmitted HBV in the countries adopting this policy. However, screening donated blood for anti-HBc is not feasible in many countries in Asia or Africa because the rate of anti-HBc is so high that 40% to 70% of possible donors would be excluded. Therefore, transfusiontransmitted HBV is more frequent in these areas.
105 Also, parenteral transmission still occurs in some situations. Persons who inject illicit drugs commonly share injection equipment and have a very high prevalence and incidence of HBV infection.
108,
109 Persons receiving pooled blood products may likewise be at risk high rates for HBV infection because very large pools may include a rare donor who was in the seronegative window period or had a false-negative test for HBsAg or very low levels of HBV DNA at the time of donation.
In many developing countries, where the HBsAg carrier rate is very high (i.e., 10% or higher) in the general population and disposable injection equipment is not available, HBV transmission by medical injections continues to be common. Also, parenteral exposures, such as acupuncture, tattoos, and body piercing, are risks for HBV transmission. Because of the very high concentration of virus in some persons with acute or chronic HBV infection (as much as 10
10 virions/mL), exposures to minute amounts of blood that occur from activities such as sharing of toothbrushes, razors, wash cloths, or towels and the presence of eczematous skin lesions that exude serum can result in transmission in the household setting.
110,
111 and
112 HBV transmission has also been demonstrated to occur between children and teachers in a classroom.
113 Other populations who are at high risk of HBV infection include clients of institutions for the developmentally disabled and prisoners.
HBV is also transmitted commonly by sexual intercourse. Numerous studies in various populations have shown that the prevalence of HBV increases with the number of sex partners.
114,
115 and
116 Populations with large numbers of sex partners, such as commercial sex workers and both homosexuals and heterosexuals with multiple partners, can have a high prevalence of HBV infection.
114,
115 and
116 In households, the sexual partners of an HBsAg carrier are at greater risk of HBV infection than are others in the household.
114,
115 and
116 Virologic studies have shown that HBV is present in semen and other secretions, although at levels 100- to 1000-fold lower than in the blood.
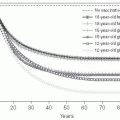
In many parts of the world, HBV infection is acquired during the perinatal period or in early childhood. Infants born to a mother who is an HBsAg carrier and is HBeAg positive have a 90% risk of acquiring an HBV infection if they are not given hepatitis B immunoglobulin (HBIG) and HBV vaccine soon after birth.
86,
87 However, if HBIG is given within a few hours of birth along with an initial dose of HBV vaccine, the transmission rate is reduced by as much as 90%. HBV vaccination alone reduces the risk of transmission by 70%. Limited data also suggest that antiviral therapy of the pregnant woman with high HBV DNA levels (more than 10
8 IU/mL) may further reduce transmission to infants. Infants of women who are HBsAg positive but HBeAg negative have a lower risk.
Worldwide Epidemiology
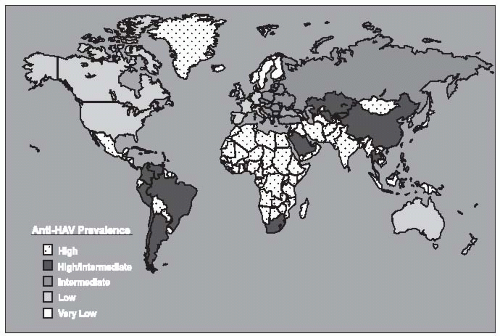
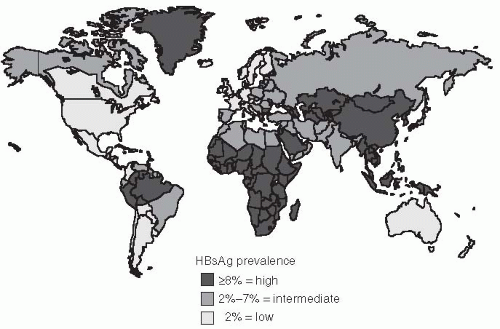
The prevalence of HBV infection varies greatly worldwide. In some areas of the world, HBV infections are highly endemic, and 8% or more of the total population are chronic carriers of HBsAg. These areas encompass 45% of the global population and include China and Southeast Asian countries, sub-Saharan Africa, and several areas in the Arctic, including Alaska, northern Canada, and Greenland (
Figure 23-6). The First Nation people of Canada and the United States have high rates of HBV. In most of these areas, primary liver cancer is also very common and is often the most frequent cancer in adult males. Nearly all HBV infections occur during the perinatal period or in early childhood in these areas. Because acute HBV infections in infancy and early childhood result in a high rate of chronic carriage, the high endemicity is perpetuated from one generation to the next. The perinatal transmission of HBV among Alaska natives has been reduced dramatically in the last several years by the implementation of an effective program to vaccinate newborns immediately after they were born.
117In developed countries of North America, Western Europe, Australia, and some areas of South America, the rates of HBV infection are much lower. Less than 2% of the population are chronic carriers, and the overall infection rate, measured by the prevalence of anti-HBc, is 5% to 20%. In these areas, which constitute 12% of the global population, the frequency of perinatal or early childhood infection is low; however, they may account for a disproportionately high number of chronic HBV infections. Some subgroups also have higher rates, such as those living in the Amazon
basin, and immigrants from areas with higher population prevalence. These immigrant populations may contribute substantially to the subset of HBV infections that are transmitted during the perinatal and early childhood period.
118,
119 and
120 In these areas, most infections occur among high-risk adult populations, including injection-drug users; homosexual men; persons with multiple sex partners; patients with multiple exposures to pooled blood products, such as hemophiliacs; and healthcare workers.
115,
119,
121The remaining parts of the world, which constitutes 43% of the global population, have an intermediate rate of HBV infection. The prevalence of HBsAg positivity ranges from 2% to 8%, in these regions and serologic evidence of past infection is found in 20% to 60% of the population. In these areas, there are mixed patterns of infant, early childhood, and adult transmission. In many countries in sub-Saharan Africa, for example, transmission of HBV commonly occurs during childhood rather than the perinatal period. Another risk for populations in developing countries is that posed by contaminated blood products or medical equipment. Nosocomial transmission in developing countries where HBV is highly endemic remains common.
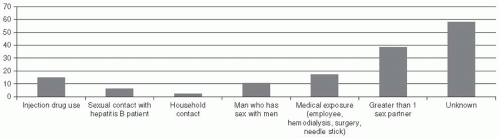
In the United States, the CDC has conducted a detailed assessment of behaviors in the Sentinel Counties Study of viral hepatitis to estimated the risk factors for acute HBV infection. The CDC estimate of the proportion of persons with acute HBV infections associated with known risk factors in 1992-1993 was as follows: heterosexual contact, 41%; injectiondrug use, 15%; homosexual contact, 9%; household contact, 2%; healthcare employment, 1%; other, 1%; and unknown, 31% (
Table 23-4,
Figure 23-9). It is likely that the relative contribution of injection-drug
use to HBV infections may be higher in populations with a high burden of drug use.
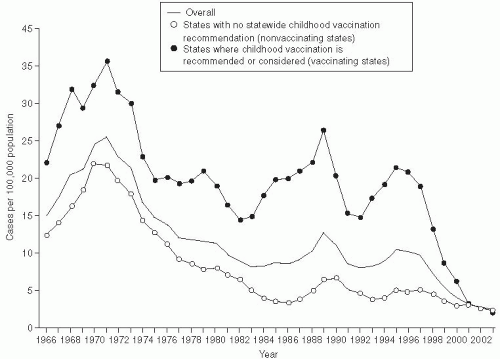
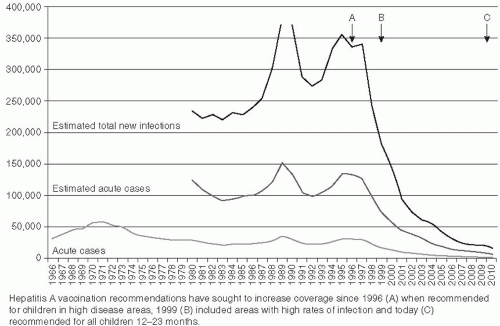
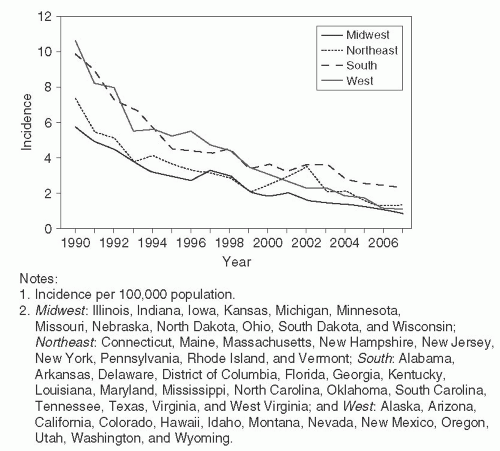
The incidence of hepatitis B infection has declined in the United States since the mid-1980s. In 2007, it was estimated to be 1.5 cases per 100,000 population, which was the lowest rate since active surveillance began in 1996 and represents an estimated decline of more than 80% from the rate in 1990, when a national strategy was implemented to reduce the incidence of HBV infections.
118 (
Figure 23-10) CDC has estimated that 38,000 new HBV infections occurred in the U.S. population in 2008, which represents a sharp decline from an estimated 73,000 cases in 2003. The greatest decline in incidence has occurred among the cohort of children in whom the recommendations for routine infant and adolescent vaccination have been implemented. Vaccination coverage among young adults (19-35 years of age) is estimated at 93%. The incidence among children younger than age 15 years declined 98% from 1.2 cases per 100,000 population in 1990 to 0.02 cases per 100,000 in 2007.
118The incidence of HBV among adults has also declined, albeit to a lesser extent than among infants and children. Few cases are now reported in certain populations that previously were considered to be at high risk, e.g. dialysis patients and health care workers. These declines are a result of improvements in infection control and routine use of hepatitis B vaccination in these populations. A high proportion of the new HBV infections occur among injection drug users, MSM, and persons with multiple sex partners
117,
118,
119,
120,
121,
122 and
123In recent years, however, a new high-risk group has been identified. During 1999-2007, 16 outbreaks of hepatitis B were detected and investigated in the United States, involving 157 persons. In nearly all of these outbreaks, the cases involved diabetics in nursing or assisted care facilities sharing glucosemonitoring equipment.
124 and
125 CDC has estimated that 38,000 new HBV infections occurred in the U.S. population in 2008, which declined from an estimated 73,000 cases in 2003. Overall, 4.35% to 5.6% of the U.S. population is estimated to have been infected with HBV, which gives an estimated 800,000 to 1.4 million persons with HBV in the United States
118The risk of transfusion-transmitted HBV infections in the Untied States has declined substantially in the last few decades. Screening of blood donors for HBsAg was instituted in 1972. More effective screening of blood donors for drug use or sexual risk behavior and exclusion of men who have sex with men was implemented to reduce the risk of HIV transmission in the early 1980s. Most blood collection centers in the United States implemented routine nucleic acid testing to detect HBV DNA among blood donors in 2010. Also, blood
centers in the United States and Europe routinely test donated blood for antibodies to anti-HBc. Anti-HBc is a very sensitive test for HBV as any persons ever infected would be removed from the blood supply, but a less specific test as not all persons with anti-HBc are viremic for HBV. These testing procedures virtually eliminated transmission-transmitted HBV. However, screening donated blood for anti-HBc is not feasible in many countries in Asia or Africa because the rate of anti-HBc is so high that 40% to 70% of possible donors would be excluded. Therefore, transfusion-transmitted HBV is more frequent in these areas.
114,
121,
122Although the incidence of HBV has declined in the United States, the prevalence of chronic HBV infection has not changed; this steady state is largely related to immigration of individuals from countries with endemic HBV.
117 Chronic HBV infections are recognized especially among several populations in the United States—namely, First Nations people from Canada and the United States, injection-drug users, persons with multiple sex partners, immigrants from Asia
118 and Africa , and immunocompromised patients with AIDS and other causes of immunosuppression whose HBV infection has reactivated.
114,
118,
121A recent study of the incidence of hepatocellular carcinoma in the United States estimated that the number of cases increased by 46% between 1976-1980 and 1991-1995.
126 This increased incidence of liver cancer probably reflects the effects of an increased rate of chronic HBV and HCV infections, combined with the effects of alcohol use among carriers of these viruses. The incidence of hepatocellular carcinoma tripled in the United States between 1975 and 2005, increasing from 1.6 to 4.9 cases per 100,000 population.
129 Worldwide, primary cancer of the liver is the third leading cause of cancer mortality among men and the sixth leading cause among women.
126
Screening Hepatitis B Virus Carriers for Cirrhosis and Hepatocellular Carcinoma
It is particularly important to periodically screen persons who are chronic HBV carriers for hepatocellular carcinoma (HCC). In 2007, an expert committee of the American Association for the Study of Liver Disease (AASLD) published recommendations for screeing of HBV carriers, i.e., persons who are HBsAg positive.
127 The committee recommended screening the following HBsAg positive patients at 6 month intervals with ulstrasound to detect early liver cancer: Asian over 40 years of age, African patients over 20 years of age, all patients with cirrhosis, and patients with a family history of HCC. These recommendations were based, in part, on a randomized control trail in Shanghai which found a reduction of mortality from liver cancer by 37% from 131.5 to 83.2 per 100,000 population in a population of 18,816 HBsAg carriers whose HCC was detected at an earlier stage by screening.
128 Most of these patients, in addition to screening would be candidates for treatment of their chronic HBV infection with anti-viral drugs to suppress their viral replication. More recent studies have found that pre-core mutations in the X gene of HBV may precede the occurrence of HCC by several years.
129,
130 These data suggest that it might be possible to prevent or delay the onset of HCC by screening chronic carriers of HBV to detect high-risk mutations and treating such patients to reduce their viral load.
Although these screening recommendations may appear straightforward, it is sometimes difficult to determine whether a patient with chronic HBV infection might have cirrhosis. Many would argue that liver biopsy is the gold standard for diagnosis, and although histologic examination of a liver biopsy may be the best method to detect cirrhosis, a biopsy is invasive and occasionally causes complications. Moreover, it represents only a small sample of the liver, so it may not always detect early cirrhosis. Recently, other noninvasive diagnostic methods have become available, including fibroscan, which allows for more frequent assessment. Whereas fibroscan abnormalities reliably detect advanced fibrosis or cirrhosis they are less sensitive in detecting early stages of fibrosis. In addition, biochemical methods have been used to diagnose cirrhosis; these include measurements of elevated liver enzymes, AST/platelet ratio, haptoglobin, apolipoprotein-A1 and other methods that evaluate the functional status of the liver.
129
Risk Factors and Biomarkers for Hepatocellular Carcinoma
Several viral, host, and environmental factors have been found to be associated with an increased risk of hepatocellular carcinoma among persons with chronic HBV infection. Host factors include older age, male sex, the presence of cirrhosis, a family history of liver cancer, and coinfection with HIV, HDV, or HCV. Virus factors include high levels of HBV replication, infection with HBV genotype C versus genotype B or A, a common double mutation in the core-promoter region of the virus (nucleotides 1762 A to T and 1764 G to A mutation), and several other mutations in the x gene, or pre-core genome of HBV. Environmental factors include frequent high alcohol
consumption, aflatoxin exposure, cigarette smoking, diabetes, and obesity.
130Among these risk factors, the association between the acquired double mutation in the HBV genome 1762
T/1764
A may be especially useful as a biomarker for the prediction of increased risk of subsequent liver cancer, as prospective studies have detected such mutations 5 or more years prior to the appearance of a cancer.
131Although the presence of cirrhosis is an important risk factor for the subsequent development of liver cancer, it is important to recognize that HCC may sometimes develop among HBV chronic carriers in the absence of cirrhosis. Only 40% to 50% of liver cancer among chronically HBV-infected patients in Africa have cirrhosis, whereas cirrhosis precedes liver cancer in 90% of such patients in Asia, the United States, and Europe.
132An increased rate of HCC among males has been found in every population. In a study of more than 2 million pregnancies between 1983 and 2000 in Taiwan, 17% of the women were HBsAg positive at the time of delivery. By 2005, 294 of these women had died from HCC.
133 The relative risk (RR) of HCC among HBsAg carriers was 42% lower (RR = 0.58; 95% confidence interval [CI], 0.42-0.74) in women having two pregnancies and 52% lower (RR = 0.48; 95% CI, 0.34-0.72) in women having three or more pregnancies.
136 In addition to these data among women, a nested case-control study among men with chronic HBV infection who subsequently developed HCC found higher levels of androgenic hormones among the men several years prior to the diagnosis of HCC.
134 These data lend support to the hypothesis that the levels of female and male hormones may be cofactors in the pathogenesis of HCC among persons with HBV infections and may partially explain the sex difference in HCC.
Staging of Liver Disease
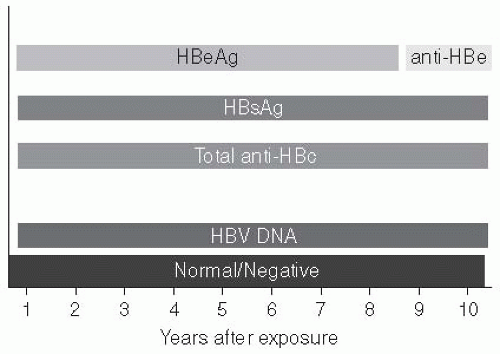
The natural history of chronic HBV infection is now recognized to have four phases, although not all patients go through all phases. These phases of HBV infection are most prominent when infection is acquired in infancy or childhood.
The first phase is the “immune tolerant phase,” which is characterized by the presence of HBeAg, high levels of serum DNA, normal serum transaminases, and minimal or no inflammation on liver biopsy. During this phase, which may last several decades in patients with infection acquired perinatally, spontaneous or treatment-induced HBeAg clearance is unusual and patients rarely progress to cirrhosis or liver cancer.
130 In patients who acquire HBV infection during late childhood or as adults, this phase may be short-lived.
The second phase, immune clearance, is characterized by the presence of HBeAg, elevated HBV DNA levels, persistent or intermittent elevation in serum liver enzymes, and active inflammation in the liver. The elevated liver enzymes reflect immunecytolysis of infected hepatocytes. Some of the flares in liver enzymes may lead to hepatic decompensation. The immune response in this phase may lead to clearance of HBeAg from the serum.
The third phase, known as the “immune control” phase, is characterized by the absence of HBeAg, presence of anti-HBe, normal aminotransferase levels, and low levels of serum HBV DNA (i.e., less than 10
5 IU/mL). Liver biopsy at this stage may show mild hepatitis and minimal fibrosis. However, some patients may have cirrhosis if the liver inflammation during the immune clearance phase was severe or persistent. The use of sensitive assays to detect HBV DNA has revealed that replication is ongoing,
138 but when this immune control stage persists, the patient is at minimal risk of progression to severe liver disease or cirrhosis.
Some inactive carriers have “reactivation” of their HBV infection, a fourth stage. This may occur spontaneously or as a result of immunosuppression.
A study of 283 patients in Taiwan who were followed for approximately 9 years after they developed antibodies to HBeAg (the third phase) found that 67% had a sustained remission, 4% had HBeAg revision to positivity, and 24% had HBeAgnegative chronic hepatitis.
135 Cirrhosis developed in 8% and HCC in 25% of these patients; these conditions occurred more frequently in those patients with active hepatitis after their loss of HBeAg. In fact, the core-promoter double mutation
131 and a stop codon mutation in the pre-core region of the HBV genome, NT/896,
131 will turn off production of HBeAg. The double mutation commonly precedes the onset of hepatocellular carcinoma in patients with HBeAg-negative chronic HBV infection.
131The data from follow-up of patients after HBV infection indicate that the virus is not generally directly cytopathic, but that the sequelae of cirrhosis and liver cancer are primarily immune mediated. Furthermore, HBV infections are not curable. Thus the goal of antiviral therapy of chronic HBV infection is the prevention of liver fibrosis, cirrhosis, and liver cancer. The main stages of the infection when inflammation, fibrosis, and cirrhosis develop are during the immune clearance and reactivation stages. Antiviral therapy should be used in patients during the immune clearance or reactivation stages of this infection when
inflammation and fibrosis of the liver may occur. The goal of treatment during these stages is to suppress viral replication and liver inflammation and fibrosis.