19 Vaccines and Immunotherapy
Introduction
Colorectal cancer is the second leading cause of cancer death in the United States. The standard treatment options for colorectal cancer, as with many other gastrointestinal malignancies, include surgery, chemotherapy, and radiation, depending on the location and stage of disease at clinical presentation. Although early-stage colon cancer (stages I and II) has an excellent prognosis when treated with surgical therapy alone, recurrence-free and overall survival rates drop significantly with regional nodal metastases or distant metastases (stages III and IV disease, respectively). Even with standard adjuvant treatments such as chemotherapy and radiation, for patients with stage III disease, the overall 5-year survival rate is anywhere from 44% to 83%. Patients with the more advanced stage IV disease have a 5-year survival rate of about 10%. Given the fact that advanced colorectal cancer has a poor prognosis, it is even more unfortunate that in the United States only 39% of patients are diagnosed with early-stage disease. It is therefore critical to develop better adjuvant therapies for advanced colorectal cancer in addition to the standard modalities such as chemotherapy and radiation.1
Immunotherapy—An Overview
One of the most intriguing strategies that has been considered for adjuvant treatment of colorectal cancer is immunotherapy. Immunotherapy and the numerous strategies by which it can be used are broad topics of discussion. Immunotherapy has been investigated in the treatment of a number of cancers with varying degrees of success. To adequately discuss the subject, we must first define what immunotherapy is. Stated simply, immunotherapy is a therapeutic strategy that uses a person’s own immune system to eradicate malignant cancer cells. There are a number of ways in which the immune system can be primed to eradicate tumor cells, and it is important to understand the distinctions among the major classes of immunotherapy.2
Among the most famous historical examples of the active, nonspecific approach are the experiments of Dr. William B. Coley, a surgeon at the Memorial Hospital for Cancer and Allied Diseases in New York City during the 1890s. Coley noted that patients with incurable soft tissue sarcomas who developed wound infections had higher disease-free survival and overall survival rates than patients who did not develop infectious wound complications. Coley actually conducted experiments in which he administered live streptococcal culture to patients with sarcomas to generate systemic infections. Then, he observed regression of the primary tumors in a significant number of patients. Coley postulated that the immune responses against the bacterial infection somehow cross-reacted with the tumor tissue, resulting in eradication of the malignant disease. For his early experiments, Coley has often been referred to as the “Father of Cancer Immunotherapy.”3 In contemporary times, the active nonspecific approach to immunotherapy continues to be used. An example is the systemic administration of interferon-α (IFN-α) or high-dose interleukin-2 (IL-2) to patients with advanced melanoma. In contrast to the active nonspecific approach, the active specific approach focuses on selective activation of the immune system only against primary tumor antigens. These responses are most typically mediated by T cells and B cells. In addition to short-term antitumor responses, the active specific approach ideally generates tumor antigen-specific memory T cells that can respond to a future tumor challenge. Currently, the most promising method being investigated to generate long-term active specific immunity is the use of tumor vaccines.2,4
In considering immunotherapy to treat colorectal cancer, a number of unique challenges must be addressed. Not all cancers are equal, and some cancers are better than others at evading host immune responses. There are multiple reasons for this. Some cancers lack major antigens that can be the target of a host-derived immune response. Historically, many researchers have considered colon tumors as being relatively deficient in immunodominant antigens when compared with other cancers such as melanoma, in which more than 10 major antigens have been identified.5
Many research efforts have focused on identifying antigens that could be the targets of vaccine-induced immune responses. It is highly likely that most of the aberrant antigens that arise on colorectal cancer cells do so as normal colonic cells undergoing malignant transformation. The transformation from normal colonic tissue into cancer is a complex, multistep process in which multiple genes can undergo a variety of point mutations. Indeed, most colon cancers have several mutations rather than one discrete genetic anomaly. This results in a heterogeneous group of colon tumors with wide genotypic and histologic variation. Therefore, it is unlikely that an individual antigen will be isolated that can be the target of a single colon cancer vaccine.2,6,7
To date, a number of potential target antigens have been identified. These include, but are not limited to, the proto-oncogene K-ras, the tumor suppressor gene p53, carcinoembryonic antigen (CEA), transforming growth factor-β receptor type II (TGFβRII), squamous cell antigen recognized by T cells 3 (SART-3), and cyclophylin.4 In addition, even when a vaccine is active against a particular patient’s tumor antigen profile, the antigen profile can change as tumors continue to transform. This may result in some tumor cell clones “escaping” the vaccine. Therefore, it may become necessary to administer polyvalent vaccines that are active against an entire spectrum of cancer antigens or to administer vaccines for early-stage disease before tumors have a chance to grow and change their molecular profiles.8
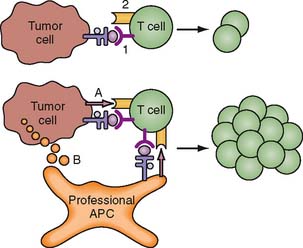
An additional problem is that even though vaccination may induce tumor antigen-specific responses in a host, this does not always result in sustained antitumor activity. These activated, tumor antigen-specific immune effectors often become attenuated over time. This may be due to secondary host immune responses that downregulate the activity of these cells, or it may be due to the lack of sufficient costimulatory signals that maintain host immune cell activation. This downregulation in activity of tumor antigenspecific immune effector cells is referred to as tolerance. One proposed mechanism for tolerance induction is illustrated in Figure 19-1.8 For vaccine strategies to become effective, these issues of tumor antigen identification, antigen escape, and tolerance are a few of many challenges that need to be resolved.
Passive Immunotherapy
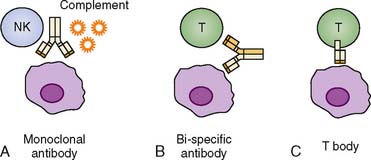
First, we address some of the passive immunotherapy approaches that have been used against colorectal cancer. A classic passive immunotherapy approach involves the administration of antitumor antibodies. Table 19-1 shows a synopsis of some of these modalities. Antibodies that are specific for tumor antigens can elicit antitumor activity in a number of ways. First, the Fc (nonbinding, free terminus) regions of tumor-specific antibodies might be able to bind complement and facilitate lysis of tumor cells, or they may result in opsonization and tumor cell destruction. Some antibodies are designed to cross-link tumor cells and native T cells in circulation to result in more efficient antigen encounter and possible activation by native T cells.
Table 19-1 Summary of Major Immunotherapy Modalities
| Approach | Examples |
|---|---|
| Passive Immunotherapy | |
| Antitumor antibodies27 | Edrecolomab (mAB 17-1A); antibody to Ep-CAM, an epithelial cell adhesion molecule |
| Passive and Possible Active | |
| Adoptive immunotherapy25,26 | Tumor-infiltrating lymphocyte (TIL) therapy involving transfer of activated CD8 and CD4 TILs |
| Active Immunotherapy | |
| Nonspecific79 | |
| Specific | |
A final class of antibodies, referred to as T bodies, have variable regions that are specific for tumor antigens and Fc regions that act as cytosolic T-cell activation domains. These T bodies can bind tumor antigens and then intercalate into the membranes of T cells, triggering a cascade that results in T-cell activation. Figure 19-2 depicts some of the different antibody-mediated, passive immunity strategies.4 In clinical practice, a few of these strategies have been used in human trials. Some of these human trials have involved the use of mAB 17-1A (edrecolomab), an antibody that targets Ep-CAM, an epithelial cell adhesion molecule. Edrecolomab causes cell death by complement-dependent cytotoxicity (CDC) and antibody-dependent cellular cytotoxicity (ADCC).9 Riethmüller and colleagues10 published the 7-year results of a prospective, randomized trial in which 189 patients with resected Dukes C colorectal cancer were assigned to receive either vaccination with mAB 17-1A or no additional therapy. At 7 years, administration of the antibody therapy reduced overall mortality and recurrence rates by 32% and 23%, respectively.
Not all studies with mAB 17-1A have been so promising, however. In contrast, in another large study reported by Punt and colleagues,11 2761 patients with stage III colon cancer were assigned to receive either adjuvant 5-fluorouracil (5-FU) and folinic acid alone or in combination with edrecolomab. The authors reported no difference in 3-year overall survival, but surprisingly they found that the patients who received the antibody therapy had a higher incidence of cancer recurrence. There have also been a number of other smaller studies investigating the use of edrecolomab alone or in combination with chemotherapy and/or cytokines such as granulocyte macrophage colony-stimulating factor (GM-CSF) or IL-2. Some of these studies showed no therapeutic benefit, whereas others showed partial regression of tumors in patients with advanced metastatic cancers. The data in these smaller studies are by no means definitive, and they have to be weighed against the potential toxicities of the drug including allergic infusion reactions to the antibody.12–16
An even more complex passive immunotherapy technique that has been tested in humans is adoptive immunotherapy. In adoptive immunotherapy, active immune cells are administered to host with cancer. Initial attempts at the treatment of cancer focused on the adoptive transfer of non–tumor antigen-specific T cells stimulated in vitro. Several human trials have been conducted using various immune effector cell lines and stimulatory agents. In some of the early experiments carried out by Rosenberg and colleagues,17,18 lymphocytes were stimulated in vitro with IL-2 and then transferred to patients with various gastrointestinal cancers. Initially, the data suggested that the transfer of these “lymphokine-activated killer cells” (LAKs) might confer a survival benefit to patients with malignancy when administered along with systemic doses of IL-2. Later experiments revealed that the benefit of administering non–tumor antigen-specific lymphokine-activated killer cells was no greater than that of administering IL-2 alone.17,18
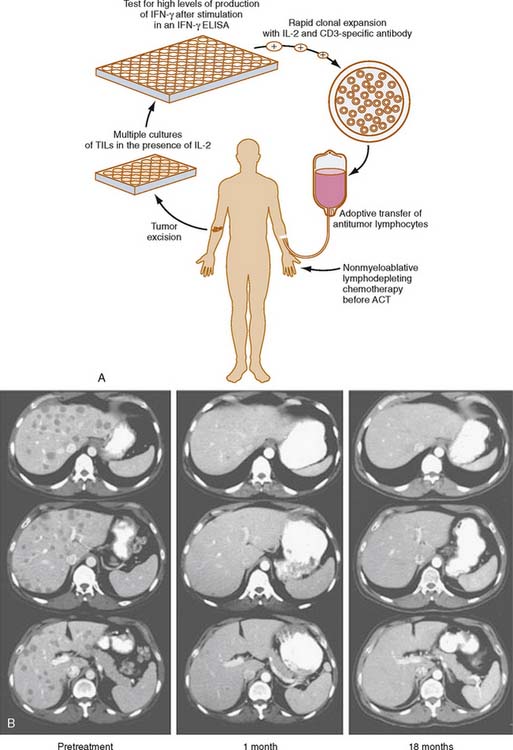
In one small phase I/II French series, monocytes from 15 patients with colorectal cancer were isolated, cultured, and stimulated in vitro with interferon-γ (IFN-γ). These activated monocytes were then readministered. In the 14 patients who were followed up, no significant clinical responses were noted.19 A similar treatment regimen was administered to nine patients with advanced colorectal cancer in a phase I German trial. These patients were treated with an infusion of autologous monocytes that had been cultured with IFN-γ and lipopolysaccharide in vitro. One of these nine patients had stabilization of previously progressive disease for 12 weeks.20 Given these rather disappointing results with adoptive transfer of nonspecific immune effectors, emphasis shifted toward the adoptive transfer of effector cells, which might be more specific for the tumors they were targeted against. One such strategy focuses on the adoptive transfer of activated tumor-infiltrating lymphocytes (TILs) isolated ex vivo from resected cancer specimens. Rosenberg and Dudley21 have demonstrated dramatic clinical responses in some patients with advanced metastatic melanoma who have been treated with adoptive transfer of TILs expanded in vitro with IL-2 and administered after myeloablative chemotherapy.
The adoptive transfer of TILs is well described for melanoma22 (Fig. 19-3) The data regarding its efficacy in the treatment of colorectal cancer are more limited. In a nonrandomized, prospective study published by Gardini and colleagues,23 47 patients with colorectal cancer hepatic metastases underwent surgical hepatic resection. Fourteen patients received adjuvant infusion of IL-2-activated TILs isolated from their surgical specimens, and 14 received adjuvant chemotherapy. The remaining 19 patients received no additional therapy. No difference in disease-free or overall survival was seen at 1, 3, and 5 years. In another Italian study, nine patients with advanced colorectal cancer were treated with adoptive immunotherapy of IL-2-stimulated TILs along an infusional course of systemic IL-2. An additional 19 patients were treated with the same treatment regimen after surgical resection of their metastases to increase the ratio of TILs to solid tumor cells. No major treatment effects were seen in patients who did not undergo surgical resection of their disease. In 8 of the 19 patients who underwent surgical resection, 8 remained disease-free at a median follow-up time of 21 months.24
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree