Endometrial carcinoma is the most common malignancy of the female genital tract in developed countries, and the fourth most common cancer in women after breast, lung, and colorectum. Developing countries and Japan have incidence rates four to five times lower than Western industrialized nations, with the lowest rates being in India and South Asia (1).
In the United States, it was anticipated there would, not will be 53,630 new cases and 8,590 deaths from the disease in 2014 (2). African American women have a 40% lower risk of developing the disease but a 54% greater risk of dying from it, mainly because of late diagnosis (3).
Two different clinicopathologic subtypes of endometrial cancer are recognized: The estrogen related (Type I, endometrioid), and the nonestrogen related (Type II, nonendometrioid). Each subtype has specific genetic alterations, with endometrioid tumors commonly exhibiting microsatellite instability and mutations in PTEN, PIK3CA, K-ras, and CTNNBI (β-catenin), while nonendometrioid (predominantly serous and clear cell) tumors are characterized by having p53 mutations and chromosomal instability (4). Patients with Type II tumors are more likely to be older, nonwhite, multiparous, current smokers, nonobese, and to have had breast cancer treated with tamoxifen (5).
Approximately 80% of newly diagnosed endometrial carcinomas in the Western world are endometrioid in type (4). Any factor that increases exposure to unopposed estrogen (e.g., estrogen replacement therapy, obesity, anovulatory cycles, estrogen-secreting tumors) increases the risk of these tumors, whereas factors that decrease exposure to estrogens or increase progesterone levels (e.g., oral contraceptives or smoking) tend to be protective (1). A population-based Finnish study reported that use of continuous estradiol–progestin therapy for 3 years or more decreased the risk of Type I cancers by 76%, whereas sequential estradiol–progestin therapy for at least 5 years increased the risk by 69% if the progestin was added monthly, and by 76% if the progestin was added three monthly (6). Women with Type I cancers associated with obesity are more likely to die of cardiovascular disease than cancer (7), and lifestyle issues need to be addressed when treating these women (8).
The average age of patients with endometrioid cancer is approximately 63 years, and 70% or so are confined to the corpus at the time of diagnosis. Their 5-year survival is approximately 83% (9). By contrast, the average age of patients with nonendometrioid cancer is 67 years, and at least half have already spread beyond the corpus at the time of diagnosis. Their 5-year survival is approximately 62% for clear cell carcinomas and 53% for serous cancers (9).
Endometrial cancer may occasionally develop after radiation treatment for cervical or other pelvic cancers. Kumar et al. (10) reported 205 radiation-associated cancers and 1,001 sporadic second endometrial cancers in women with a primary cancer of pelvic organs (bladder, rectum, cervix, vulva, or vagina). The cases were identified from the Surveillance, Epidemiology, and End Results (SEER) database between 1973 and 2005. Lesions in the radiation-associated cohort were more likely to be nonendometrioid histology (76% vs. 51%; p < 0.001), poorly differentiated (58% vs. 28%; p < 0.001), and advanced stage (43% vs. 16%; p < 0.001). The 5-year survival for radiation-associated and sporadic endometrial cancers was 27.1% and 57.1%, respectively (p < 0.001) (10).
Screening of Asymptomatic Women
The ideal method for outpatient sampling of the endometrium has not yet been devised, and no blood test of sufficient sensitivity and specificity has been developed. Therefore, mass screening of the population is not practical. Screening for endometrial carcinoma or its precursors is justified for certain high-risk people, including those shown in Table 9.1.
Only about 50% of women with endometrial cancer have malignant cells on a Papanicolaou (Pap) smear, but compared with patients who have normal cervical cytology, patients with suspicious or malignant cells are more likely to have deeper myometrial invasion, higher tumor grade, positive peritoneal cytologic findings, and a more advanced stage of disease (11).
The appearance of normal-appearing endometrial cells in cervical smears taken in the second half of the menstrual cycle or in postmenopausal women is controversial. Montz reported endometrial histology from 93 asymptomatic postmenopausal women receiving hormone replacement therapy (HRT) with normal endometrial cells on a Pap smear. Eighteen patients (19%) had abnormalities identified, including seven endometrial polyps, seven cases of simple hyperplasia (one with atypia), three cases of complex hyperplasia (one with atypia), and one endometrial carcinoma (12). A Dutch study of 29,144 asymptomatic postmenopausal women found that when normal endometrial cells were reported in the cervical smear, the prevalence rate of premalignant uterine disease was significantly higher (6.5%) as compared to smears without these cells (0.2%), resulting in a relative risk of 40.2 (95% confidence interval [CI] 9.4 to 172.2) (13). If morphologically abnormal endometrial cells are present, then approximately 25% of women have endometrial carcinoma (14).
Guidelines from the Bethesda system recommended that benign endometrial cells should be reported in women of age 40 years and older. Beal et al. (15) reported that benign endometrial cells were rarely associated with significant endometrial pathology in asymptomatic premenopausal women and that these women may not need further evaluation. By contrast, Moroney et al. (16) using liquid-based cytology, reported that 2.1% of asymptomatic premenopausal women with benign endometrial cells had significant endometrial pathology.
The unsatisfactory results obtained with cervical cytology are the result of the indirect sampling of the endometrium, and several commercially available devices have been developed to allow direct sampling (e.g., Pipelle, Gyno Sampler, Vabra aspirator). A satisfactory endometrial biopsy specimen also may be obtained in the office with a small curette such as a Novak or Kevorkian. All of these office techniques for endometrial sampling cause the patient some discomfort, and in approximately 8% of patients it is not possible to obtain a specimen because of a stenotic os.
A meta-analysis reported that the Pipelle was the best device, with detection rates for endometrial cancer in postmenopausal and premenopausal women of 99.6% and 91%, respectively (17). The sensitivity for the detection of endometrial hyperplasia was 81%. The specificity for all devices was 98%.
Table 9.1 Patients for Whom Screening for Endometrial Cancer is Justified

Table 9.2 Patients in Whom a Diagnosis of Endometrial Cancer Should be Excluded

In the 1990s, transvaginal ultrasonography, with or without color flow imaging, was investigated as a screening technique. Mean thickness of the endometrial strip was measured as 3.4 ± 1.2 mm in women with atrophic endometrium, 9.7 ± 2.5 mm in women with hyperplasia, and 18.2 ± 6.2 mm in women with endometrial cancer (18). In a large, multi-institutional study of 1,168 women, all 114 women with endometrial cancer and 95% of the 112 women with endometrial hyperplasia had an endometrial thickness of 5 mm or more (19). An earlier meta-analysis reported that 4% of endometrial cancers would be missed using transvaginal ultrasonography for the investigation of postmenopausal bleeding, with a false-positive rate as high as 50% (20), while a more recent meta-analysis suggested the cut-off for endometrial thickness should be 3 mm (21).
Tamoxifen increases the risk of endometrial cancer twofold to threefold (22) and produces a sonographically unique picture of an irregularly echogenic endometrium that is attributed to cystic glandular dilatation, stromal edema, and edema and hyperplasia of the adjacent myometrium (23). Routine ultrasonic surveillance of asymptomatic women on tamoxifen is not useful because of its low specificity and positive predictive value. Canadian investigators studied 304 women on tamoxifen as therapy for breast cancer. Using an endometrial thickness cut-off of 9 mm, the positive predictive value for the detection of endometrial cancer was only 1.4% (24).
Patients taking tamoxifen should be informed of the increased risk of endometrial cancer and told to report any abnormal bleeding or spotting immediately. Any bleeding or spotting must be investigated by biopsy. A retrospective review of tamoxifen-treated women who underwent dilatation and curettage found that uterine cancer was found only in those with vaginal bleeding (23).
Clinical Features
Symptoms
Endometrial carcinoma should be excluded in all patients shown in Table 9.2. Ninety percent of patients with endometrial cancer will have abnormal vaginal bleeding, most commonly postmenopausal bleeding, and the bleeding usually occurs early in the course of the disease. The usual causes of postmenopausal bleeding are shown in Table 9.3.
Table 9.3 Etiology of Postmenopausal Bleeding

Intermenstrual bleeding or heavy prolonged bleeding in perimenopausal or anovulatory premenopausal women should arouse suspicion. The diagnosis may be delayed unnecessarily in these women because the bleeding is usually ascribed to “hormonal imbalance.”
Occasionally, vaginal bleeding does not occur because of cervical stenosis, particularly in thin, elderly, estrogen-deficient patients. In some patients with cervical stenosis, a hematometra develops, and a small percentage of patients have a purulent vaginal discharge resulting from a pyometra.
Signs
Physical examination commonly reveals an obese, hypertensive, postmenopausal woman, although approximately one-third of patients are not overweight. Abdominal examination is usually unremarkable, except in advanced cases when ascites may be present and hepatic or omental metastases may be palpable. Occasionally, a hematometra appears as a large, smooth midline mass arising from the pelvis.
On pelvic examination, it is important to inspect and palpate the vulva, vagina, and cervix to exclude metastatic spread or other causes of abnormal vaginal bleeding. The uterus may be bulky, but often it is not significantly enlarged. Rectovaginal examination should be performed to evaluate the fallopian tubes, ovaries, and cul-de-sac. Endometrial carcinoma may metastasize to these sites or, alternatively, a coexistent ovarian tumor such as a granulosa cell tumor, thecoma, or epithelial ovarian carcinoma may be noted.
Diagnosis
All patients suspected of having endometrial carcinoma should have an endocervical curettage and an office endometrial biopsy. A histologically positive endometrial biopsy allows the planning of definitive treatment. If insufficient tissue is obtained, light bleeding may be ascribed to atrophic endometritis without need for further curettage if the endometrial thickness on transvaginal ultrasound is 4 mm or less (25).
Because there is a false-negative rate of approximately 10%, a negative endometrial biopsy in a patient with heavy bleeding, or with an endometrial thickness greater than 4 mm, must be followed by a fractional curettage under anesthesia. Hysteroscopy is usually performed in conjunction with the curettage, and may identify some small bleeding polyps that would otherwise have been missed. The development of smaller diameter hysteroscopes has improved patient acceptance, and allowed hysteroscopy to be performed in an outpatient setting without anesthesia (26). There has been speculation that fluid hysteroscopy may facilitate the abdominal dissemination of malignant cells, but there is no evidence that it has any impact on the disease-free survival (27).
Fractional Curettage
While the patient is under anesthesia, careful bimanual rectovaginal examination is performed, a weighted speculum is placed in the vagina, and the cervix is grasped with a tenaculum. The endocervical canal is curetted before cervical dilatation, and the tissue placed in a specially labeled container. The uterus is sounded, the cervix dilated, and the endometrium systematically curetted. The tissue is placed in a separate container so that the histopathologic status of the endocervix and endometrium can be determined separately.
Preoperative Investigations
Routine preoperative hematology and biochemistry should be performed, and a chest, pelvic, and abdominal computed tomographic (CT) scan is usually performed. It has limited usefulness in determining the depth of myometrial invasion or the presence of nodal disease (28), but has been the traditional investigation to exclude liver or lung metastases, adnexal masses, or hydronephrosis in high-risk cases. If a fractional curettage has not been performed, an endocervical curettage should be performed to evaluate the endocervix.
Nonroutine tests are sometimes indicated, particularly for more advanced cases or high-risk histologies on curettage. A colonoscopy should be performed if there is occult blood in the stool or a recent change in bowel habits because concomitant colon cancer occasionally occurs, particularly if there is a family history of bowel cancer.
A magnetic resonance imaging (MRI) scan has been shown to have an 83.3% accuracy (100 of 120 cases) for differentiating deep from superficial myometrial invasion (29) and a positive predictive value of 89.8% for the detection of cervical involvement (30). MRI may help to differentiate between low- and high-risk patients, which may be useful in triaging cases to a gynecologic oncologist in some areas.
Positron emission tomography (PET) has an overall diagnostic accuracy of 89.5% for the detection of lymph node metastases in patients with untreated endometrial cancer (31), but it is not considered part of the routine work-up (32). Elevated CA125 levels have been demonstrated to correlate with advanced stage of disease, including positive lymph node status (33).
Staging
In 1988, the Cancer Committee of the International Federation of Gynecology and Obstetrics (FIGO) replaced the old clinical staging system for endometrial cancer (Table 9.4) with a surgical staging system (Table 9.5). This was done in response to the Gynecologic Oncology Group (GOG) staging studies of the 1970s and early 1980s, which demonstrated the high incidence of lymph node metastases in high-risk cases (34,35). This surgical staging was further refined in 2009 (Table 9.6), after a critical review of its accuracy, reproducibility, and utility, particularly from pathologists (36).
The major differences between the FIGO 1988 and FIGO 2009 staging systems are as follows:
1. Cancer confined to the endometrium is no longer a separate stage (i.e., stage IA). All tumors with inner half myometrial invasion are now stage IA.
2. Only cases with cervical stromal invasion are classified as stage II. Cervical mucosal involvement, formerly stage IIA, is now merged with stage I.
3. There is no longer a separate stage (i.e., stage IIIA) for patients with positive peritoneal cytology.
4. Cases with metastases to lymph nodes are subdivided into stages IIIC1 and IIIC2, specifying the presence of positive pelvic and para-aortic lymph nodes, respectively.
Two large studies of women with endometrial cancer identified from the SEER database both reported that the 2009 FIGO staging system was highly prognostic (37,38). Distribution by 1988 FIGO surgical stage is shown in Table 9.7.
Table 9.4 1971 FIGO Clinical Staging for Endometrial Carcinoma
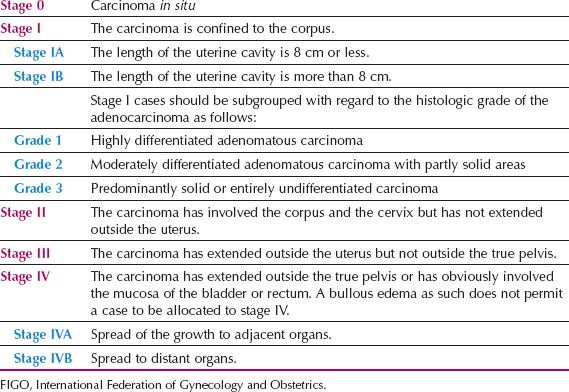
Table 9.5 1988 FIGO Surgical Staging for Endometrial Carcinoma

Spread Patterns
Endometrial carcinoma spreads by the following routes:
1. Direct extension to adjacent structures
2. Transtubal passage of exfoliated cells
3. Lymphatic dissemination
4. Hematogenous dissemination
Direct Extension
Direct extension is the most common route of spread, and it results in penetration of the myometrium and eventually the serosa of the uterus. The cervix, fallopian tubes, and ultimately the vagina and parametrium may be invaded. Tumors arising in the upper corpus may involve the tube or serosa before involving the cervix, whereas tumors arising from the lower segment of the uterus involve the cervix early. The exact anatomic route by which endometrial cancer involves the cervix has not been clearly defined, but it probably involves a combination of contiguous surface spread, invasion of deep tissue planes, and lymphatic dissemination (39).
Table 9.6 2009 FIGO Surgical Staging for Carcinoma of the Endometrium

Transtubal Dissemination
The presence of malignant cells in peritoneal washings and the development of widespread intra-abdominal metastases in some patients with early-stage endometrial cancer strongly suggest that cells may be exfoliated from the primary tumor and transported to the peritoneal cavity by retrograde flow along the fallopian tubes.
Lymphatic Dissemination
Lymphatic dissemination is clearly responsible for spread to pelvic and para-aortic lymph nodes. Although lymphatic channels pass directly from the fundus to the para-aortic nodes through the infundibulopelvic ligament, it is rare to find positive para-aortic nodes in the absence of positive pelvic nodes. It is quite common to find microscopic metastases in both pelvic and para-aortic nodes, suggesting simultaneous spread to pelvic and para-aortic nodes in some patients. This is in contrast to cervical cancer, where para-aortic nodal metastases are virtually always secondary to pelvic nodal metastases.
Table 9.7 Carcinoma of the Endometrium: Distribution by 1988 FIGO Surgical Stage Patients Treated in 1999 to 2001

It seems likely that vaginal metastases also result from lymph-vascular spread. They commonly occur in the absence of cervical involvement, excluding direct spread as the mechanism, and may occur despite preoperative sterilization of the uterus with intracavitary radiation, excluding implantation of cells at the time of surgery as the mechanism (40). In a study of 632 patients with stage I endometrial cancer managed with hysterectomy at the Mayo Clinic between 1984 and 1996, Mariani et al. (41) reported that grade 3 histology and lymphovascular invasion were significant predictors of vaginal relapse, whereas depth of myometrial invasion was not.
Hematogenous Spread
Hematogenous spread most commonly results in lung metastases; liver, brain, bone, and other sites are involved less commonly.
Prognostic Variables
Although stage of disease is the most significant prognostic variable, a number of factors have been shown to correlate with outcome within the same stage of disease. These prognostic variables are summarized in Table 9.8. Knowledge of them is essential if appropriate treatment programs are to be devised.
Age
Age appears to be an independent prognostic variable. The GOG reported 5-year relative survival rates of 96.3% for 28 patients 40 years of age or younger, 87.3% for 261 patients 51 to 60 years, 78% for 312 patients 61 to 70 years, 70.7% for 119 patients 71 to 80 years, and 53.6% for 23 patients older than 80 (p = 0.001) (42). All patients had clinical stage I or occult stage II disease. Using proportional hazards modeling of relative survival time, and taking 45 years of age as the arbitrary reference point, the relative risks for death from disease were as follows: 2 at 55 years, 3.4 at 65 years, and 4.7 at 75 years of age.
A study of 51,471 patients from the SEER database of the National Cancer Institute in the United States demonstrated that patients 40 years and younger had an overall survival advantage compared with women older than 40 years, independent of other clinicopathologic prognosticators (43).
Japanese researchers have reported menopausal status to be an independent prognostic variable for early endometrial cancer but not for patients with advanced disease (44).
Histologic Type
Zaino et al. (45) investigated the prognostic significance of squamous differentiation in 456 patients with typical adenocarcinomas and 175 women with areas of squamous differentiation who had been entered into a GOG clinicopathologic study of stage I and II disease. They reported that the biologic behavior of these tumors reflected the histologic grade and depth of invasion of the glandular component. Prognostically valuable information was provided by dividing these tumors into adenoacanthomas and adenosquamous carcinomas, and more information was gained when they were stratified by the histologic grade of the glandular component. They recommended that the terms adenoacanthoma and adenosquamous carcinoma be replaced by the simple term adenocarcinoma with squamous differentiation (45).
Table 9.8 Prognostic Variables in Endometrial Cancer Other than FIGO Stage

Serous carcinomas have a poor prognosis, even in the absence of deep myometrial invasion or lymph node metastasis (4,46–49). They disseminate widely, with a particular predilection for recurrence in the upper abdomen. The mechanisms that have been proposed to explain the characteristic intra-abdominal dissemination of these tumors include transtubal spread, vascular-lymphatic invasion, and multifocal disease. Sherman et al. (49) observed that “intraepithelial serous carcinoma” was present in the endocervix in 22% of their cases, in the fallopian tube in 5%, on the surface of the ovary in 10%, and on peritoneal surfaces or omentum in 25%. Serous elements are often admixed with endometrioid carcinomas, but a serous component of 25% will portend a poor prognosis (49).
In contrast to the slow, estrogen-driven pathway leading to the biologically more indolent endometrioid carcinoma, a rapid, p53-driven pathway appears to lead to the aggressive serous (4,49) and clear cell carcinomas (4).
Clear cell carcinomas represent fewer than 5% of endometrial carcinomas, although clear cell elements are commonly present in serous tumors (49). Vascular space invasion is more common in these lesions. In a review of 181 patients with clear cell endometrial carcinoma treated between 1970 and 1992 at the Norwegian Radium Hospital, 5- and 10-year actuarial disease-free survival rates were 43% and 39%, respectively. Two-thirds of the relapses were outside the pelvis, most frequently in the upper abdomen, liver, and lungs (50).
SEER data from 1988 to 2001 were used to compare uterine serous (n = 1,473), clear cell (n = 391), and grade 3 endometrioid carcinomas (n = 2,316) (48). Serous and clear cell carcinomas occurred in older patients, and were diagnosed at a more advanced stage. They represented 10%, 3%, and 15% of endometrial cancer, respectively, but accounted for 39%, 8%, and 27% of cancer deaths.
When serous or clear cell carcinomas are limited to the curettings, with no adverse features in the hysterectomy specimen, prognosis may not be impaired (51).
Squamous cell carcinomas of the endometrium are rare. In a review of the literature, Abeler and Kjorstad (52) estimated that the survival rate for patients with clinical stage I disease was 36%.
Histologic Grade and Myometrial Invasion
There is a strong correlation between histologic grade, myometrial invasion, and prognosis. The GOG reported the surgicopathologic features of 621 patients with stage I endometrial carcinoma (35). The frequency of positive pelvic and para-aortic nodal metastases in relation to histologic grade and depth of myometrial invasion is shown in Tables 9.9 and 9.10. When grade 1 carcinomas were confined to the inner third of the myometrium, the incidence of positive pelvic nodes was less than 3%, whereas when grade 3 lesions involved the outer third, the incidence of positive pelvic nodes was 34%. For aortic nodes, the corresponding figures were less than 1% and 23%, respectively.
Table 9.9 Grade, Depth of Invasion, and Pelvic Nodal Metastasis of Endometrial Carcinoma

Table 9.10 Grade, Depth of Invasion, and Aortic Nodal Metastasis of Endometrial Carcinoma

A combined European-Mayo review of 527 patients with grade 1 endometrioid endometrial cancer revealed 88 patients (16.8%) in whom the background endometrium was atrophic, rather than hyperplastic or proliferative (53). Atrophic endometrium correlated with older age, lower body mass index, advanced FIGO stage, lymph node metastases, vascular space invasion, and deep myometrial invasion. The authors concluded that atrophic endometrium was an independent prognostic factor for patients with grade 1 endometrioid carcinoma, and that these tumors may arise through unique carcinogenic pathways (53).
Recurrence at the vaginal vault can usually be prevented by prophylactic vault brachytherapy, and the risk of distant metastases in relation to histologic grade and myometrial invasion is shown in Table 9.11 (54).
Vascular Space Invasion
Vascular space invasion appears to be an independent risk factor for recurrence and death from endometrial carcinoma of all histologic types (42,55–61,62). In a GOG study, vascular space invasion carried a relative risk of death of 1.5 (42).
Table 9.11 Clinical Stage I Endometrial Carcinoma: Distant Metastases versus Histologic Grade and Myometrial Invasiona

Abeler et al. (55) reviewed 1,974 cases of endometrial carcinoma from the Norwegian Radium Hospital and reported an 83.5% 5-year survival rate for patients without demonstrable vascular invasion compared with 64.5% for those in whom vascular invasion was present. A Japanese study reported that lymph-vascular space invasion and the number of positive para-aortic lymph nodes were independent prognostic factors for patients with stage IIIC endometrial cancer (56).
The overall incidence of lymph-vascular invasion in stage I endometrial carcinoma is approximately 15%, although it increases with increasing myometrial invasion and decreasing tumor differentiation. Hanson et al. (58) reported vascular space invasion in 5% of patients with invasion limited to the inner one-third of the myometrium compared with 70% of those with invasion to the outer one-third. Similarly, it was present in 2% of grade 1 carcinomas and 42% of grade 3 lesions.
Lymphovascular space invasion is an independent risk factor for vaginal cuff recurrence (62), distant recurrence (59,62), lymph node metastases (60), and parametrial involvement (61) in patients with early-stage endometrial cancer.
Peritoneal Cytologic Results
The significance of positive peritoneal cytology is controversial (63), and it is no longer part of the FIGO staging system. About half the papers on early-stage endometrial cancer report increased recurrence rates and decreased survival in patients with positive cytology (64–68), while in the other half, no differences were detected (69–74). Positive washings are most common in patients with grade 3 histology, metastases to the adnexae, deep myometrial invasion, or positive pelvic or para-aortic nodes.
The GOG study reported by Morrow et al. (66) analyzed 697 patients with information on peritoneal cytology and adequate follow-up. Disease recurred in 25 of 86 patients (29.1%) with positive washings, compared to 64 of 611 patients (10.5%) with negative washings. The authors noted that 17 of the 25 recurrences were outside the peritoneal cavity. The GOG estimated that the relative risk of death for patients with positive cytologic washings was increased threefold (42).
In a review of the literature concerning patients with clinical stage I endometrial cancer, Milosevic et al. (64) reported positive peritoneal cytology in 8.3%, 12.1%, and 15.9% of patients with grades 1, 2, and 3 histologic types, respectively. Superficial and deep myometrial invasion were associated with positive washings in 7.6% and 17.2% of the cases, respectively. They concluded that the poor prognosis associated with malignant washings was largely a reflection of other adverse prognostic factors.
Takeshima et al. (70) studied 534 patients with endometrial cancer to assess the prognostic significance of positive peritoneal washings. They concluded that positive washings were not an independent negative prognostic factor, but potentiated other negative prognostic indicators. They felt that patients with positive peritoneal cytology, in the absence of other adverse prognostic factors, did not warrant upstaging.
These same researchers placed a tube in the abdomen to allow peritoneal irrigation in 50 patients with early-stage endometrial cancer and positive peritoneal smears detected at surgery (73). Washings were obtained via the tube 7 and 14 days postoperatively. Persistence of positive peritoneal cytology was observed in only 5 of 50 patients (10%), and 4 of these patients had adnexal metastases completely resected. They concluded that malignant cells found in the peritoneal cavity generally have a low malignant potential and that only malignant cells from special cases, such as patients with adnexal metastases, may be capable of independent growth.
Hormone Receptor Status
Estrogen receptor (ER) and progesterone receptor (PR) levels have been shown to be independent prognostic factors for endometrial cancer; that is, patients whose tumor is positive for one or both receptors have longer survival than patients whose carcinoma lacks the corresponding receptors (75–79). Liao et al. (76) reported that, even for patients with lymph node metastases, the prognosis was significantly improved if the tumor was receptor positive. PR appears to be a stronger predictor of survival than ER and, at least for the ER, the absolute level of the receptors may be important: the higher the level, the better the prognosis (79).
Nuclear Grade
Nuclear grade is a significant prognostic indicator (79). Christopherson et al. (80) found nuclear grading to be a more accurate prognosticator than histologic grade.
The FIGO grading system takes into account the nuclear grade of the tumor, and “nuclear atypia” inappropriate for the architectural grade raises the grade by 1. There is great variability in the literature regarding the criteria for nuclear grading, and intraobserver and interobserver reproducibility of nuclear grading are poor (81).
Tumor Size
In an analysis of 142 patients with clinical stage I endometrial carcinoma, Schink et al. (82) reported tumor size as an independent prognostic factor. Lymph node metastases occurred in 4% of the patients with tumors no more than 2 cm in diameter, 15% with tumors greater than 2 cm in diameter, and 35% with tumors involving the entire uterine cavity. Japanese researchers used tumor diameter in addition to intraoperative frozen section to identify low-risk cases (83). None of 55 patients with grade 1 or 2 tumors and less than 50% myometrial invasion had positive nodes if the tumor diameter was 3 cm or less. Mayo Clinic workers incorporated tumor diameter (2 cm or less vs. greater than 2 cm) into a Risk-scoring System for prediction of lymphatic dissemination in patients with endometrioid endometrial cancer (84).
DNA Ploidy and Other Biologic Markers
Only about 25% of patients with endometrial carcinomas have aneuploid tumors, but these patients are at significantly increased risk of early recurrence and death from disease (85–87).
In a prospective study of 174 patients, Susini et al. (86) reported a 10-year survival probability of 53.2% for patients with DNA aneuploid tumors, compared to 91% for patients with DNA diploid tumors. By multivariate analysis, DNA aneuploid type was the strongest independent predictor of poor outcome, followed by age and stage. The GOG estimated the relative risk to be 4.1 for disease-related death for patients with aneuploid tumors (87).
A number of genetic mutations have been shown to have prognostic significance in endometrial cancer. Greek investigators reported that loss of beta-catenin expression was a strong, independent predictor of a poor prognosis, whereas loss of PTEN was associated with a worse prognosis for patients with early-stage disease (88). The p53 mutation correlated with increased stage, lymph node metastases, and nonendometrioid histology in univariate analysis but was not an independent prognostic factor in multivariate analysis (88). Increasing expression of matrix metalloproteinases (MMPs) (89), nuclear Bcl-2 expression (90), and Ki-67 expression (91) also have prognostic significance. The clinical implications of these biologic markers are not yet clear.
Method of Treatment
In contrast to cervical cancer, patients with endometrial cancer treated with hysterectomy alone or hysterectomy and radiation do significantly better than those treated with radiation alone. This appears to be related to the inability of radiation therapy effectively to eliminate disease in the myometrium (92,93). Grigsby et al. (92) reported on 116 patients with stage II endometrial carcinoma. Ninety were treated with combined radiation and surgery, whereas 26 received radiation alone. The results of treatment are shown in Table 9.12.
Endometrial Hyperplasia
Classic teaching has been that endometrial hyperplasias represent a continuum of morphologic severity; the most severe form, termed atypical adenomatous hyperplasia or carcinoma in situ, was considered the immediate precursor of endometrial carcinoma (94). Since the mid-1980s, this continuum concept has been challenged. Independent studies by Kurman et al. (95) and Ferenczy et al. (96) have suggested the following:
1. Endometrial hyperplasia and endometrial neoplasia are two biologically different diseases.
2. The only important distinguishing feature is the presence or absence of cytologic atypia.
Table 9.12 Clinical Stage II Carcinoma of the Endometrium: Comparison of Treatment Methods

Table 9.13 World Health Organization Classification of Endometrial Hyperplasia

Ferenczy et al. (96) suggested a two-class classification, with endometrial hyperplasia being used for any degree of glandular proliferation devoid of cytologic atypia, and endometrial intraepithelial neoplasia (EIN) for lesions with cytologic atypia. Using similar criteria in a long-term follow-up study of 170 patients with endometrial hyperplasia, Kurman et al. (95) reported a 1.6% risk of progression to carcinoma in patients devoid of cytologic atypia, compared with a 23% risk in patients with cytologic atypia. A case-control study was reported among a cohort of 7,947 women diagnosed with endometrial hyperplasia at one prepaid health plan in the United States from 1970 to 2002. The cumulative 20-year progression risk among women who remained at risk for at least 1 year was less than 5% for women without atypia, and 28% for those with atypical hyperplasia (97).
The World Health Organization (WHO) still uses a four-class classification of endometrial hyperplasia, as shown in Table 9.13 (98). It is based on morphologic features of the architectural complexity of the glands, and the presence or absence of cytologic and nuclear atypia.
Although the studies of Kurman and Ferenczy are important, the reproducibility of the diagnosis has been questioned. In a GOG study, a panel of three expert gynecologic pathologists reviewed outside slides from 306 patients referred with a diagnosis of atypical endometrial hyperplasia. The majority panel diagnosis was adenocarcinoma in 29%, cycling endometrium in 7%, and nonatypical hyperplasia in 18% of cases (99).
Hysterectomy slides from the same GOG study were reviewed by the study pathologists, and 289 hysterectomy specimens were available for review (100). Concurrent endometrial carcinoma was present in 42.6% (123 of 289 specimens). Of these, 30.9% (38 of 123 specimens) had myometrial invasion, and 10.6% (13 of 123 specimens) had invasion to the outer half of the myometrium. Even when the study panel consensus diagnosis was less than atypical endometrial hyperplasia, 14 of 74 women (18.9%) still had carcinoma in the hysterectomy specimen.
Diagnosis
Complex atypical endometrial hyperplasia frequently coexists with endometrial carcinoma. Of 824 women with atypical hyperplasia on endometrial biopsy, 48% were found to have endometrial cancer on subsequent hysterectomy (101). If dilatation and curettage were performed, the risk of concurrent endometrial cancer was 30%. Increasing age was strongly correlated with the risk of cancer.
Immunohistochemical staining of complex atypical endometrial hyperplasia for PTEN has been reported to improve the prediction of a coexistent endometrial cancer, and the prediction may be better when MIB-1 and p53 expressions are added (102).
Treatment
Most women with endometrial hyperplasia without atypia respond to progestin therapy. A pooled analysis of the published literature revealed a 96% regression rate for these patients when treated with the levonorgestrel intrauterine device (103).
Patients with atypical endometrial hyperplasia should be advised to have a hysterectomy, but if a decision is made to retain the uterus because of medical comorbidities or fertility considerations, progestins should be tried. Those who do not respond are at a significantly increased risk of progressing to invasive cancer, although individual patient outcomes cannot be predicted (104).
Gunderson et al. (105) reviewed English language studies published from 2004 to 2011 to report oncologic and pregnancy outcomes in women with complex atypical endometrial hyperplasia or grade 1 adenocarcinoma treated with hormone therapy. Forty-five studies with 391 patients were identified, and the median age was 31.7 years. The major therapies were medroxyprogesterone (49%), megestrol acetate (25%), and the levonorgestrel intrauterine device (19%). Overall, 344 women (77.7%) responded to hormonal therapy. After a median follow-up of 39 months, a durable complete response was noted in 53.2%. The complete response rate was significantly higher for those with hyperplasia than carcinoma (65.8% vs. 48.2%; p = 0.002). The median time to complete response was 6 months (range 1 to 18 months). Recurrence after an initial response occurred in 23.2% with hyperplasia and 35.4% with carcinoma (p = 0.03). During the study period, 41.2% of those with hyperplasia and 34.8% with carcinoma became pregnant (p = 0.39), with 117 live births.
The type of progestin used does not appear to be important, the optimal dosage has not been investigated, and the regimens advocated are essentially arbitrary (104), because no clinical trials have been conducted (107). High doses of progestins are often better tolerated than lower doses. The main side effects are weight gain, edema, thrombophlebitis, and occasionally hypertension and depression. The incidence of venous thromboembolism may be slightly increased.
A suggested scheme of management is outlined in Figure 9.1.
Treatment of Endometrial Cancer
The cornerstone of treatment for endometrial cancer is total hysterectomy and bilateral salpingo-oophorectomy; this operation should be performed in all cases whenever feasible. Many patients require some type of adjuvant radiation therapy to help prevent vaginal vault recurrence and to sterilize disease in lymph nodes.
The surgery has traditionally been via laparotomy, but many of these patients are elderly and have comorbidities such as diabetes, hypertension, and obesity. Minimally invasive approaches are replacing open laparotomy for most patients with endometrial cancer. Laparoscopic-assisted vaginal hysterectomy, total laparoscopic hysterectomy, or robotic-assisted laparoscopic hysterectomy have all been compared to open laparotomy in randomized, prospective studies (108–110). These trials mainly focused on patients with Type I tumors, but when managed by expert laparoscopic or robotic surgeons, a high-risk histologic subtype does not seem to be a contraindication in patients with apparent early-stage disease (111).
Although long-term survival data are not yet available, the minimally invasive approach is associated with shorter hospital stay, less postoperative pain, and quicker resumption of normal daily activities. Minimally invasive operations do take longer, and the lymphadenectomy in particular has a steep learning curve; the former has implications for allocation of operating room resources, and the latter for the training of junior surgeons.
Stage I and Stage II Occult
Operative Technique
A recommended treatment plan is shown in Figure 9.2.
The initial approach for all medically fit patients should be total hysterectomy and bilateral salpingo-oophorectomy. Removal of a vaginal cuff is not necessary. The adnexa should be removed because they may be the site of microscopic metastases, and patients with endometrial carcinoma are at increased risk for ovarian cancer. Such tumors sometimes occur concurrently (112). Surgical staging, including lymphadenectomy, should be performed in those patients listed in Table 9.14. The use of minimally invasive surgery is addressed in detail in Chapters 21 and 22, but there will always be a minority of patients for whom an open laparotomy is more appropriate, for example, those with a large volume uterus, an adnexal mass, or extensive adhesions.
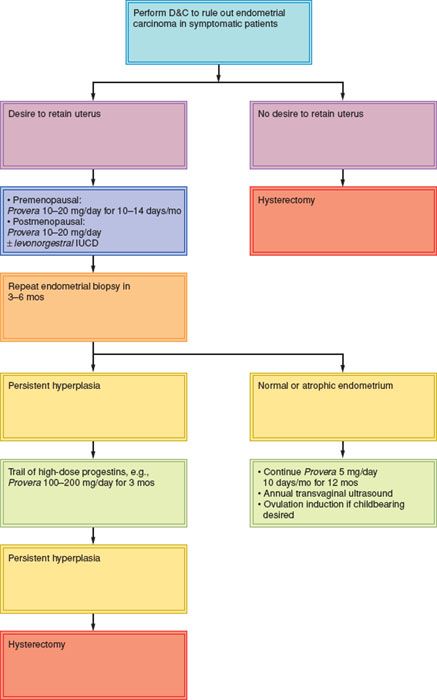
Figure 9.1 Management of endometrial hyperplasia. IUCD, intrauterine contraceptive device; mos, months.
The laparotomy is best performed through a lower midline abdominal incision, particularly in the obese patient. This incision allows easy access to the upper abdomen, including the omentum and para-aortic lymph nodes. A Pfannenstiel incision is commonly used for patients with grade 1 or 2 tumors and a normal-sized uterus. An alternative approach is to use a transverse, muscle-dividing incision (e.g., the Maylard or Cherney), as discussed in Chapter 20. This incision gives reasonable access to the upper abdomen.

Figure 9.2 Management of patients with stage I and occult stage II endometrial carcinoma. TAH, total abdominal hysterectomy; BSO, bilateral salpingo-oophorectomy; RT, radiation therapy.
Table 9.14 Endometrial Carcinoma Stages I and Occult II: Patients Requiring Surgical Staging

After the abdomen is opened, peritoneal washings are taken for cytology, and the abdomen and pelvis are explored, with particular attention to the liver, diaphragm, omentum, and retroperitoneal nodes. Any suspicious lesions are excised or biopsied, and any enlarged lymph nodes removed.
Technique for hysterectomy and bilateral salpingo-oophorectomy. The uterus is grasped with clamps that encompass the round and ovarian ligaments and the fallopian tube. After the round ligaments are divided, the incision is carried anteriorly around the vesicouterine fold of peritoneum, and posteriorly parallel and lateral to the infundibulopelvic ligaments. With a narrow Deaver retractor in the retroperitoneum providing gentle traction cephalad in the direction of the common iliac vessels, the iliac vessels and ureter are displayed. With the retroperitoneum displayed, the pelvic lymph nodes can be visualized and palpated, and any enlarged nodes can be removed and sent for frozen section.
With each ureter under direct vision, the infundibulopelvic ligaments are divided and tied. The bladder is dissected off the front of the cervix, and the uterine vessels are skeletonized and divided at the level of the isthmus. Straight Kocher clamps are used to secure the cardinal and uterosacral ligaments. A circumferential incision is made around the upper vagina, the uterus, tubes, and ovaries are removed, and the vaginal vault is closed. The pelvic peritoneum is not closed, and no drains are placed in the pelvis. The sigmoid colon is placed in the pelvis to help exclude loops of small bowel. A vertical abdominal wound is best closed with a continuous Smead–Jones type of internal retention suture, using a long-acting, absorbable suture such as Maxon or PDS.
Surgical Staging
The decision to undertake surgical staging is usually based on the histopathology from the uterine curettings, the gross findings on opening the uterus on the operating table, and possibly a frozen section of the resected uterus.
A relatively poor correlation has been reported between the grade of cancer on curettings or biopsy and the final grade in the resected uterus, presumably because of a sampling error in the diagnostic procedure. The poorest correlation is for grade 1 tumors, where 20–40% may be upgraded after evaluation of the hysterectomy specimen (113–115).
Our practice is to open the specimen on the operating table to determine the need for surgical staging in patients with grade 1 or 2 tumors (Figs. 9.3 and 9.4). All patients with grade 3 endometrioid, serous, or clear cell carcinomas are surgically staged.
For grade 1 tumors, gross examination fairly accurately predicts depth of myometrial invasion (115,116). A Greek study of 142 patients with apparent early-stage endometrial cancer reported a 93.5% accuracy for estimating depth of myometrial invasion for grade 1 tumors, 80.4% for grade 2, and 58.6% for grade 3. Cervical involvement was correctly determined in 138 of the 142 patients (97.2%) (116).
Tumor diameter should be taken into account when determining the need for surgical staging. Schink et al. (82) reported a 22% incidence of lymph node metastases for grade 2 tumors greater than 2 cm in diameter (7 of 32). None of 19 grade 2 tumors less than 2 cm in diameter had nodal metastases.
If doubt exists regarding the need for surgical staging, intraoperative frozen section can be obtained if sufficient expertise is available, but the pathologist still needs to use gross inspection to decide on the site(s) of deepest myometrial invasion, so accuracy rates of 77% (117) to 96% (118) have been reported. If the final histopathology is worse than was anticipated intraoperatively, the prognosis will not be impaired if external-beam pelvic radiation is given on the basis of histologic grade and depth of myometrial invasion, as long as any enlarged pelvic and/or para-aortic nodes have been resected as part of the standard management for all patients.
Other approaches that have been used to determine depth of myometrial invasion include preoperative MRI (115,119), transvaginal ultrasonography (120), or serum human epididymis protein 4 (HE4) titers (121). A pilot study of 96 patients with endometrioid adenocarcinomas revealed that serum HE4 levels greater than 70 pM had a sensitivity of 94% and a negative predictive value of 97% for distinguishing stage IA from stage IB disease. If these results can be duplicated, this could be a helpful way to triage high-risk patients to a gynecologic oncologist.
Pelvic Lymphadenectomy
No preoperative scan is able to detect micrometastases in lymph nodes, so if accurate surgical staging is to be obtained, full pelvic lymphadenectomy should be performed on all patients who meet the criteria in Table 9.14. Sampling will only lead to inaccurate information (122).

Figure 9.3 A small fundal grade 1 endometrial carcinoma. This patient does not require surgical staging.

Figure 9.4 A grade 2 endometrial carcinoma occupying most of the corpus. A patient such as this should undergo surgical staging.
In an analysis of 11,443 patients registered on the SEER database between 1990 and 2001, Chan et al. (123) reported that the removal of 21 to 25 lymph nodes significantly increased the probability of detecting at least one positive lymph node in endometrioid uterine cancer. Lutman et al. (124) reported that the pelvic lymph node count was an important prognostic variable for patients with FIGO stages I and II endometrial carcinoma and high-risk histology. In an analysis of 5,556 patients with low-risk endometrioid endometrial carcinoma from the SEER database, Chan et al. (125) were unable to find any survival advantage regardless of the extent of the lymphadenectomy (Fig. 9.5).
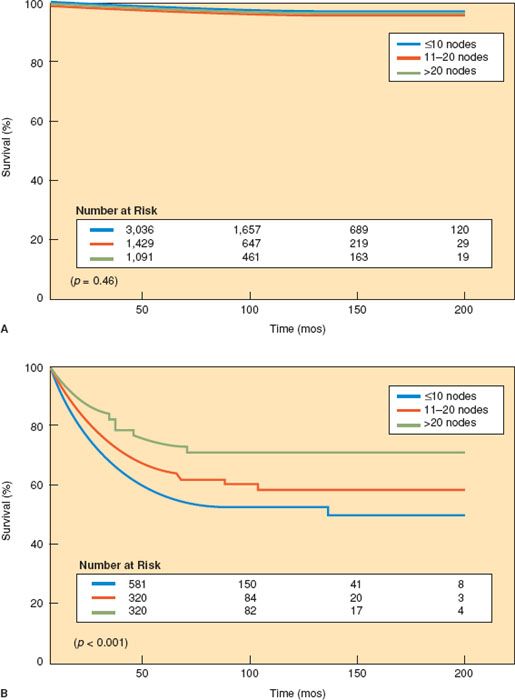
Figure 9.5 Survival for patients with (A) low-risk endometrial cancer and (B) high-risk endometrial cancer versus extent of lymphadenectomy. (Reproduced with permission from Chan J, Cheung MK, Huh WK, et al. Therapeutic role of lymph node resection in endometrioid corpus cancer: A study of 12,333 patients. Cancer. 2006;107:1823–1828.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree