Urothelial cancer
Derek Raghavan, MD, PhD, FACP, FRACP, FASCO  Richard Cote, MD, FRCPath, FCAP
Richard Cote, MD, FRCPath, FCAP  Earle F. Burgess, MD
Earle F. Burgess, MD  Stephen B. Riggs, MD
Stephen B. Riggs, MD  Michael Haake, MD
Michael Haake, MD
Overview
Urothelial malignancy is one of the most common cancers in Western society and involves the bladder, urethra, ureters, and renal calyces. It is predominantly associated with smoking, industrial dyes, schistomiasis, radiation exposure, and certain geographical locations. Well-defined molecular prognosticators have been identified and, in combination with improved staging techniques, have led to improved outcomes. Patients with nonmuscle invasive urothelial malignancy are best managed by surgical resection, often in combination with intravesical immunotherapy or chemotherapy. Muscle invasive disease is best managed by neoadjuvant cisplatin-based chemotherapy followed by cystectomy; less robust patients are often effectively treated by cisplatin-based chemoradiation. Patients with metastatic disease achieve response rates of up to 70% with MVAC (methotrexate, vinblastine, doxorubicin, and cisplatin) or GC combination chemotherapy but are infrequently cured. New approaches to the management of systemic disease are predicated on recent data reflecting the importance of unblocking checkpoints for immune function and correlate with expression of PD-L1 (programmed death-ligand 1).
Introduction and epidemiology
Bladder cancer is one of the most common malignancies in Western society, with an annual incidence of about 16 cases/100,000 males per year and 5 cases/100,000 females.1 In the United States, this translates into about 75,000 new cases per year, with approximately 16,000 deaths per year.2 An additional 3000 cases of upper tract malignancy present each year, and additional cases are found throughout the urothelial lining. This is one of the malignancies for which the incidence and mortality figures have not changed significantly in the past 50 years, although possibly the incidence figures in males are beginning to plateau, reflecting the reduction in cigarette smoking. This is predominantly a disease of older aged males, with a median age at presentation of 60–65 years. There are geographical variations in incidence with increased rates in the Great Lakes region of the United States, in the littoral basin of the Middle East, and in regions with an increased incidence of schistosomiasis (most often squamous carcinoma). In the Balkan region, endemic familial interstitial nephropathy is associated with a 100- to 200-fold increase in upper tract tumors. Urothelial cancer occurs more often in Caucasians than in Asian or African American populations.2
The etiology is well-defined, with the most common association being cigarette smoking, and other factors including exposure to dyes and industrial reagents, motor exhaust, reduced intake of fluids (controversial), and analgesic (phenacetin) abuse.1 Other associations include prior treatment with cyclophosphamide and other oxazophosphorine cytotoxics, high fat diet, chronic urinary infection, paraplegia, and prior pelvic irradiation. Family history is also relevant, especially for patients with Lynch syndrome, and for upper tract tumors.
Pathobiology and molecular determinants
Bladder cancer consists predominantly of urothelial carcinoma (UC), formerly known as transitional cell cancer.1, 3 This type of cancer can occur anywhere along the urothelial tract and may be multifocal in origin, with identical tumor histology irrespective of site of origin. About 90% of incident cases are UC, with about 5–10% being squamous cell carcinoma, 4–5% being adenocarcinoma, and the remainder consisting of rare cancers, such as small cell anaplastic cancer, sarcoma, melanoma, or lymphoma. Occasionally, other tumors metastasize to the bladder.
Increasingly, bladder cancer is believed to arise from cancer stem cells4 and that the cancer stem cells have the ability to differentiate along different pathways. Thus, not surprisingly, intermixed histological patterns will be found, although usually UC predominates in such situations. These tumors are associated with a field defect of the urinary mucosa, probably due to antecedent carcinogenic stimuli, and thus can occur at multiple sites.
UC presents as either noninvasive or invasive disease. In the former pattern, two distinct histological subtypes are known, papillary versus flat carcinoma in situ (CIS). Noninvasive papillary carcinoma is the single most common presentation for bladder cancer, comprising more than 60% of incident cases. This can be classified according to grade of disease, ranging from tumors generally considered benign (papilloma) to high-grade tumors with a high risk of developing invasion (grades 3 and 4). Grading systems are generally restricted to noninvasive papillary neoplasms, as CIS is high grade by definition, and virtually all invasive tumors are high grade as well.3
Grade 1 (well-differentiated) papillary neoplasms show a well-organized pattern similar to the organization of normal urothelium, including polarity of the urothelial cells and the presence of an umbrella cell layer. A fibrovascular stalk is often seen. In grade 2 tumors, there is a higher nuclear to cytoplasmic ratio, and prominent nucleoli, with loss of urothelial orientation and at least partial loss of the umbrella cell layer. Grade 3 (high grade) disease is characterized by poorly differentiated or undifferentiated tissues that are increasingly disorganized and manifest a high mitotic index, with complete loss of the umbrella cell layer. The latest WHO (World Health Organization) nomenclature combines tumor differentiation into only low and high grades, based on the finding that tumor behavior is more accurately reflected in a dichotomized system.3, 5 Although the bladder is heavily invested by fat and muscle, this is not the case in the upper tracts, and thus, the barriers to spread, and patterns of spread, are somewhat different.
Because the different morphologic subtypes of bladder cancer have long been recognized to have different biologic behavior, these subtypes became the focus of molecular analysis.5 The earliest cytogenetic studies in bladder cancer demonstrated alterations in chromosomes 9, 11, and 17, reflecting the possible presence of tumor suppressor genes in these areas.5, 6 On the basis of consistent and frequent genetic defects in bladder tumors, it has become clear that there are at least two distinct molecular pathways involved in bladder cancer tumorigenesis and progression (Figure 1), as reviewed elsewhere.5, 6 Papillary tumors frequently show alterations in chromosome 9, particularly at the INK4a/p16 locus. Further, these tumors frequently show constitutive activation of the receptor tyrosine kinase–Ras pathway, exhibiting activating mutations in the HRAS and fibroblast growth factor receptor 3 (FGFR 3) genes. In contrast, flat CIS and invasive tumors frequently show alterations in the p53 gene and protein (TP53) and the retinoblastoma (RB) gene. Similar molecular changes are found in upper tract tumors, and added abnormalities in chromosomes 5q, 1p, 14q, and 8p have been identified.
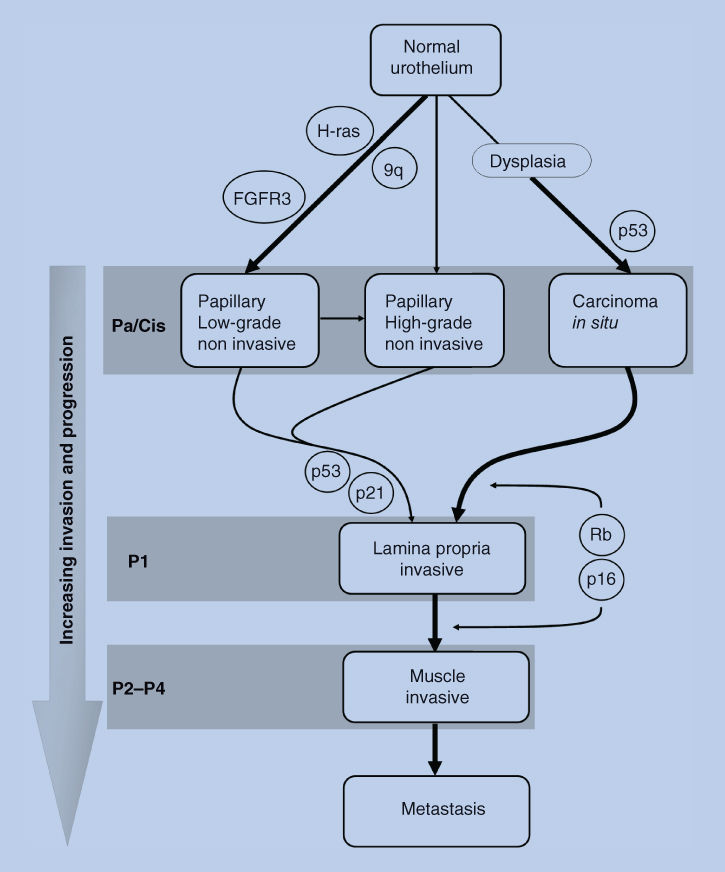
Figure 1 Proposed model for urothelial tumorigenesis and progression. Superficial and invasive tumors have unique molecular profiles and arise from distinct pathways. The locations of molecules indicate events that pose a risk for progression of a particular phenotype. The rare papillary carcinomas that invade are more likely to have genetic alterations at crucial loci. The thickness of arrows represents the relative frequency of occurrence.
Source: Mitra 2006. Reproduced with permission of the American Society of Clinical Oncology.
The RAS–MAPK signal transduction pathway is also important in noninvasive papillary tumors. Most noninvasive papillary UC’s show activation of this pathway, generally through the activation of FGFR3, and potentially presenting a target for novel therapies. Other receptor tyrosine kinases are also involved, such as epidermal growth factor receptor (EGFR) and Her2-neu, as reviewed previously.5
Cell cycle regulation has clearly been shown to be a critical pathway in flat CIS and invasive UC. A central molecule in this pathway is the p53 tumor suppressor protein, encoded by the p53 gene. Patients showing alterations in two or three out of the three critical genes (p53, p21, RB) in this pathway have much poorer outcomes than patients with tumors showing alterations in none or only one of these determinants (Figure 1).
Tumor angiogenesis and epigenetic alterations are also important in the genesis and control of UC. Comprehensive whole genome sequencing efforts have now confirmed the prevalence of mutations in chromatin remodeling genes, underscoring the potential importance of epigenetic dysregulation in UC carcinogenesis. Genes known to be affected by methylation in UC include RASSF1A, DAPK, and INK4A.5
Multiplex genome-wide expression analysis has become increasingly important in the analysis of UC and other cancers, leading to more detailed molecular profiles with histogenetic, prognostic, therapeutic targeting, and predictive implications (Figure 2).5, 6
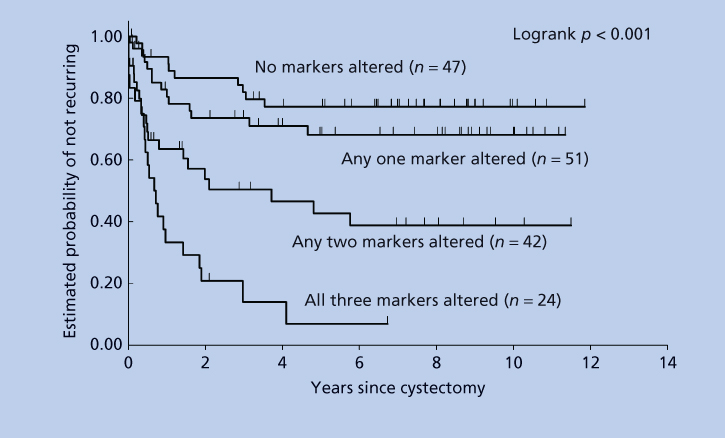
Figure 2 Probability of recurrence-free survival in 164 bladder cancer patients, who underwent radical cystectomy, based on alterations in p53, p21, and/or RB expression. The combined analysis shows an increased risk of recurrence with increasing number of deregulated molecules (logrank p < 0.001).
Source: Chatterjee 2004. Reproduced with permission of the American Society of Clinical Oncology.
Clinical presentation
The presentation of bladder cancer usually reflects the extent of disease, with somewhat different patterns associated with non-muscle-invasive tumor, invasive disease, metastases, and the nonmetastatic manifestations of malignancy.1, 8 Patients with noninvasive tumors may present with asymptomatic hematuria (diagnosed on urinalysis), visible hematuria, and irritative patterns such as frequency, dysuria, burning, or nocturia. In patients with a prior history of non-muscle-invasive bladder cancer and prior transurethral resections (TURs), irritative bladder symptoms may be more prominent. Invasive tumors have a similar pattern of presentation, although more advanced tumors may be associated with pelvic pain, slowing of urinary stream, dyspareunia, and occasionally pneumaturia or fecal incontinence. Occasionally, tumors involving the trigone will cause obstruction of ureters, with concomitant flank pain. Flank pain or more generalized abdominal pain occasionally reflects the presence of an upper tract tumor, although usually such features are associated with more advanced local disease.
The presenting features of metastatic disease usually reflect the site(s) of involvement. Common sites include distant lymph nodes, lung, liver and bone, and less commonly brain, skin, other viscera. In the present era of aggressive imaging, many metastases will first be detected upon routine follow-up scans. Pulmonary involvement will classically be associated with cough and dyspnea and occasionally with hemoptysis or chest pain. Liver involvement may present with right upper quadrant pain or shoulder tip pain, and occasionally disruption of function, most commonly manifested by jaundice. Osseous metastases are often associated with bone pain, and less commonly with pathologic fracture, with common sites of involvement including spine, ribs, pelvis, and skull. Brain metastases may be suggested by the development of headache, confusion, or other motor features. A computed tomography (CT) or magnetic resonance imaging (MRI) brain scan will usually reveal the problem, although rarely a spinal tap will be required to diagnose carcinomatous meningitis in a patient with a normal scan (especially in a patient with suspicious symptoms who has received extensive prior chemotherapy for metastatic disease). Skin metastases are uncommon but usually are manifest by an infiltrative pattern or isolated cutaneous or subcutaneous nodules.
The nonmetastatic manifestations of malignancy consist predominantly of serological syndromes, although occasional patients will present with the thromboembolic syndromes classically associated with advanced adenocarcinomas of the gastrointestinal (GI) tract. Bladder cancer is occasionally associated with the production of granulocyte-macrophage colony-stimulating factors or other cytokines, and a greatly elevated white blood cell count may reflect this phenomenon, rather than underlying infection. Tumors with squamous differentiation may sometimes be associated with hypercalcemia, owing to excess production of immunoreactive parathyroid hormone (PTH)-like substance. In general, these syndromes should be taken in context and generally do not require specific management unless causing clinical problems—for example, severe hypercalcemia and significant thrombotic episodes.
Investigation and staging
The specifics of the presentation will usually govern the nature of the investigations. Presentation with hematuria or other urinary symptoms will usually lead to urinalysis and assessment of possible infection or urinary calculi. The absence of these conditions or the presence of sterile pyuria is usually grounds for assessing urinary cytology and/or progressing to cystoscopic examination. In some clinical practices, to improve upon the sensitivity of urine cytology and to reduce the need for periodic cystoscopy in the follow-up of patients with non-muscle-invasive bladder cancer, novel biomarker dipstick assays have been developed, based on soluble bladder tumor antigens or cell-based markers (NMP22, BTA-TRAK, BLCA-4, and Immunocyt).9 Molecular analysis (Urovysion) allows detection of aneuploidy reflecting changes in chromosomes 3, 7, and 17, which are associated with high-grade tumors, and loss of the 9p21 site that is characteristic of low-grade disease. Case–control and cohort studies have suggested that several of these markers may have higher sensitivity than urine cytology, and some have been approved by the FDA (Food and Drug Administration) for use in screening (UroVysion and NMP-22) and in combination with cystoscopy for the diagnosis of recurrence.9 False positives can occur in cystitis, urolithiasis, bowel interposition, or in the presence of foreign bodies. Urinary cytology is said to be more than 95% specific, and a positive reading mandates further investigation; however, negative findings are less helpful. A more recently introduced approach that will require further validation is the use of microfiltration devices for capture and characterization of bladder cancer cells in the urine.10 The technology of endoscopic examination has improved in recent years with the introduction of more sophisticated endoscopic cameras, high-resolution videography, fluorescence cystoscopy, and narrow banding imaging cystoscopy, leading to improved specificity and sensitivity.11, 12 This has also facilitated instrumentation of the upper tracts.
There is no specific serological workup for bladder cancer. Routine hematological and biochemical testing may reveal chronic anemia of chronic disease or from blood loss, renal dysfunction (from obstruction or the underlying cause of the cancer), and occasionally evidence of metastases, such as raised alkaline phosphatase or liver function tests. No tumor markers have been shown to be specific to bladder cancer, although occasional elevation of HCG, CEA, CA 19-9, or CA125 will be seen, the latter particularly in the presence of elements of adenocarcinoma.
Imaging of the urinary tract may be carried out before or after cystoscopy. A relatively standard approach is to obtain an excretory urogram to delineate the anatomy of the urinary tract, including the presence of tumors of the bladder and upper tracts or hydronephrosis.8 CT urography is more commonly used in the current era, based on its ability to evaluate the renal parenchyma in addition to the urothelium, and it is performed more rapidly than excretory urography. MRI may also be helpful to define the local anatomy and the extent of an invasive tumor, while also providing staging information about involvement of lymph nodes and distant sites. However, the sensitivity and specificity of non-muscle-invasive pelvic imaging are somewhat limited. Also of importance, CT and MRI scans performed soon after transurethral resection of bladder tumor (TURBT) will often suffer from the artifact of apparently increased depth and invasion owing to the impact of postresection inflammatory infiltrate. The role of the positron emission tomography (PET) scan is limited, although we occasionally use this modality to assist in defining potential metastatic sites for further investigation, with a “positive” result being investigated further and a negative result having limited implications.
Definitive investigation involves TUR with the usual goals of complete tumor eradication and accurate staging. Bimanual examination at the time of TUR allows assessment of tumor stage and the presence of extravesical disease. In the setting of high-grade cancer, the existence of detrusor muscle invasion is important to determine. Unless cystectomy is planned, repeat TUR (in patients with non-muscle-invasive disease) within 4–6 weeks shows upstaging in 30% of patients with muscle identified in the original specimen and 60% of patients in whom no muscle was present initially.
Prognosis
The prognosis of bladder cancer largely reflects several of the factors already discussed, including stage and grade of the tumor, multifocality, the presence of lymphovascular invasion, association with CIS, morphology, pattern of gene mutation, and the presence of anemia or hydronephrosis. Similar factors govern the prognosis of upper tract tumors, including grade, stage, the presence of lymphovascular invasion, aneuploidy, EGFR expression, location of tumor (ureters with worse prognosis than renal pelvis), and the presence of residual disease after surgery.1
The AJCC (American Joint Committee on Cancer) Staging Classification13 generally correlates well with outcome. Although the bladder is heavily invested by fat and muscle, this is not the case in the upper tracts, and thus the barriers to spread and patterns of metastasis are somewhat different from tumors in the bladder.
In addition, Bajorin et al.14 have developed an algorithm for estimating risk and prognosis for patients with advanced disease, focused on the presence of visceral metastases, performance status, and anemia; this has recently been focused and updated to increase the precision of prediction.15 Several modifications have been proposed, including prognostic criteria for second line and salvage chemotherapy, but they have not led to improved survival figures, although they may have contributed to avoidance of futile chemotherapy (Table 1).
Table 1 Risk factors in metastatic bladder cancer
| Variable | Statistical significance (p) | Risk ratio |
| Three variables | ||
| Visceral metastases (yes/no) | 0.0001 | 1.99 |
| Karnofsky PS (</≥80%) | 0.0001 | 2.05 |
| Hemoglobin (normal/abnormal) | 0.0103 | 1.41 |
| Two variables | ||
| Visceral metastases (yes/no) | 0.0001 | 2.10 |
| Karnofsky PS (</≥80%) | 0.0001 | 2.20 |
Source: Adapted from Bajorin 1999.
Management of non-muscle-invasive bladder cancer
The key to effective management of non-muscle-invasive bladder cancer involves cystoscopy and resection of visible bladder tumor(s),16, 17 sometimes followed by postoperative use of intravesical therapy (immunological or cytotoxic reagents) to reduce the risk of recurrence.8, 16, 17 As bladder cancer is associated with a field defect, multiple random biopsies of apparently normal urothelium should be performed to identify occult CIS if urine cytology is positive or in the presence of high-grade disease when bladder conservation is contemplated. Usually, endoscopic resection is repeated within 4 weeks of the initial resection in patients with high-grade disease and/or T1 tumors, as up to 50% will have evidence of invasive bladder cancer into muscularis propria on rebiopsy.
The grade and stage of the tumor will dictate subsequent management. Patients with non-muscle-invasive, low-grade papillary bladder cancer are at low risk of progression to invasive disease, although the risk of recurrence may be as high as 60–80%. Patients at increased risk for recurrence on the basis of tumor size, multifocal tumors, or prior recurrent tumors are often given adjuvant intravesical therapy (usually weekly instillations for 6–8 weeks) following resection, mostly with bacillus Calmette Guerin (BCG), which reduces the risk of recurrence by up to around 40%.14 The mechanism of action of BCG is based on local immunological stimulation, perhaps with alteration of suppressor-helper T cell ratios. Effectively, such treatment allows the bladder to “reject” implantation and recurrence of bladder cancer. This may be a harbinger of the apparent utility of PD-1 targeting, which releases the brake on T cell function, for invasive and metastatic disease.
Randomized trials suggest that BCG is superior to other intravesical agents at preventing tumor progression,17 and an initial bladder preservation strategy involving intravesical BCG is associated with long-term outcomes similar to early cystectomy for low-grade tumors.17 Maintenance BCG is associated with a reduction in tumor recurrence and reduced requirement for cystectomy, compared to a single 6-week induction regimen. The optimal schedule of BCG administration has not been defined, and, similarly, the optimal commercial preparations and the ideal duration of administration remain controversial.
The side effects of all the intravesical agents in common use include irritative symptoms and hematuria. BCG may also cause a flu-like syndrome and, because it is an attenuated mycobacterium, it can produce local, regional and systemic TB (tuberculosis)-like infections. Granulomatous infections can occur at extravesical sites, including the prostate, epididymis, testes, kidney, liver, and lungs. BCG sepsis is the most serious complication, and can be life threatening, and should usually be treated with triple-antituberculous therapy.
In some centers, cytotoxic agents such as mitomycin C or gemcitabine18 are preferred because of purportedly reduced toxicity, although the certainty of this is not substantiated. For patients who refuse cystectomy for relapsed non-muscle-invasive disease, several lines of immunological or cytotoxic intravesical therapy may be feasible and may delay recurrence and progression.
After completion of treatment, patients should be monitored closely with periodic cystoscopy and selective urine cytology and/or tumor marker evaluation at 3–6 months intervals to detect recurrence early. Patients with high-risk non-muscle-invasive bladder cancer (high-grade Ta, T1, or CIS) have at least a 50% risk of developing invasive bladder cancer and a 35% risk of dying from bladder cancer. Moreover, those with persistent or recurrent high-grade disease after one or two courses of intravesical therapy will develop muscle invasion and progression in 80% of cases. Thus, we advocate timely radical cystectomy with urinary diversion for relapsed high-risk disease, particularly for patients with long life expectancy.19, 20 Cure rates approach 90% in this setting, but when cystectomy is delayed, deeply invasive disease may develop and is associated with diminished survival.19
Management of invasive bladder cancer
Definitive surgery
Over the past 20 years, radical cystectomy with bilateral pelvic lymphadenectomy has been viewed as the standard treatment for clinically localized invasive bladder cancer.1, 8, 20, 21 Traditionally, this requires the en bloc removal of the anterior pelvic organs, which include the bladder, prostate, and seminal vesicles in men and the bladder, urethra, uterus, ovaries, and vaginal cuff plus anterior vaginal wall in women.20 A urinary diversion is formed by the connection of the ureters to detubularized intestinal reservoir. Continent reservoirs, such as the Indiana pouch and orthotopic neobladder, are now standard approaches because they offer improved continence without the need for an external collecting bag. The orthotopic neobladder involves creation of an intestinal reservoir that is attached to the urethra and enables the patient to void normally without self-catheterization.
Radical cystectomy, without adjuvant therapy, is curative in up to 60% of patients with invasive bladder cancer,20, 21 depending on stage and other prognostic factors. The 5-year overall survival rates in large series of patients with T2–T3 disease range from 40% to 65%. Relapse rates reflect stage, grade, the presence of lymphovascular invasion, and expression of adverse molecular prognosticators. Radical cystectomy alone has been reported to be curative in 20–40% of patients with regional metastasis to pelvic lymph nodes, and the outcome is influenced by the primary tumor stage, number of involved lymph nodes, and the presence of extranodal extension.1, 8, 20, 21 Extended template node dissection may improve cure rates.20, 21 However, this may reflect the case selection bias, surgical skill, or support and salvage techniques available in centers of excellence.
Advances in instrumentation
Laparoscopic radical cystectomy, with or without robotic assistance, has been reported in modest series from centers experienced in laparoscopic surgery.22, 23 The cystectomy and lymph node dissection are commonly performed laparoscopically and the urinary diversion is carried out through a midline incision smaller than is usual for conventional surgery. The potential advantages include reduced blood loss, less postoperative pain, and shorter convalescence, although most of the data have been derived from nonrandomized series, carried out by technically superb surgeons, with careful case selection and relatively short follow-up. More recently, Bochner et al.24 have reported preliminary outcomes of a randomized trial, which suggested much higher cost for laparoscopic cystectomy, with equivalent toxicities. As this was an early report focused on acute morbidity, long-term outcomes are not yet available.
Another innovation has been the use of prostate-sparing cystectomy, with the intent of ameliorating the extent of mutilation and late effects, although this has not yet been validated by randomized trials. This approach is not useful for patients with extension of tumor into the prostate or with incidental prostate adenocarcinoma.
Role of radiotherapy
For patients with invasive, clinically nonmetastatic bladder cancer who are not surgical candidates, by their choice, technical considerations, or physical fitness, radiation is the treatment of choice.25–27 There have been no well-designed, randomized studies comparing radiation with surgery in patients with similar characteristics. The optimal technique of dose delivery, either conformal or IMRT (intensity-modulated radiotherapy), remains controversial.25 Favorable prognostic features for use of radiotherapy include small, localized, T2 tumors, absence of hydronephrosis, normal renal function, maximum debulking by TUR, and absence of anemia.1, 26, 27
A relatively standard radiotherapy approach is to deliver more than 65–70 Gy over 6–7 weeks, with 40 Gy delivered to the bladder, and the highest doses confined to the tumor plus a reasonable margin, as defined by diagnostic scans. Mapping is done at the time of TURBT with CT simulation films in the prone position.26, 27 It is less common today for radiotherapy to be delivered in isolation than for it to be administered in combination with systemic chemotherapy, based largely on the studies of the RTOG (Radiation Therapy Oncology Group) and a randomized trial conducted by the National Cancer Institute of Canada.28 These studies have shown significantly improved local control from chemoradiation, although a statistically significant survival benefit remains unproved. In the United Kingdom, a randomized trial of chemoradiation with 5-fluorouracil and mitomycin C versus radiation alone showed a significant increase in local control and a strong trend toward a survival benefit from the combination.29
Toxicities of radiation include cutaneous inflammation, proctitis occasionally complicated by bleeding and/or obstruction, cystitis or bladder fibrosis, impotence, incontinence, and development of secondary malignancies in the region surrounding the radiation field. Importantly, if radiotherapy fails, salvage surgery is much more complex because of the formation of fibrosis in the irradiated field.
Several innovations in radiation planning and treatment have been introduced in recent years, including devices for tracking physiological movement of the tumor tissue and adjusting the radiation beam, and particle therapy, such as proton beam, with more focused beams and potentially less normal tissue toxicity. No level 1 evidence supports the use of proton beam therapy for bladder cancer, which remains investigational.
Combined modality strategies
Neoadjuvant (preemptive) chemotherapy
We first studied pre-emptive or neoadjuvant systemic chemotherapy plus local treatment more than 30 years ago,30 based on the rationale that chemotherapy might reduce the extent of local tumor while controlling occult metastases. Our preliminary studies showed that this can shrink primary bladder cancers and result in downstaging, sometimes achieving a complete clinical and pathological remission.30 However, initial randomized trials did not confirm a survival benefit for single agent regimens. The introduction of multidrug chemotherapy regimens, such as methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC), and cisplatin, methotrexate, and vinblastine (CMV), adapted from use in metastatic disease into neoadjuvant protocols yielded survival benefit, confirmed by randomized clinical trials (Table 2).31–33
Table 2 Results of clinical randomized trials of neoadjuvant chemotherapy for invasive bladder cancer, stages T1–T4
| Series | Neoadjuvant regimen | Definitive therapy | Median survival with/without neoadjuvant therapy (months) | Actuarial long-term survival with/without neoadjuvant therapy |
| Neoadjuvant | ||||
| MRC-EORTC | CMV | RT/cystectomy | 44/37.5 | 35%/30% at 10 years |
| Intergroup | MVDC | Cystectomy | 77/46 | 42%/35% at 10 years |
| Nordic 1 trial | DC | Cystectomy | Not reached/72 | 59%/51% at 5 years |
| Adjuvant | ||||
| EORTC | MVDC | Cystectomy | 81/55 | 44%/39% at 5 years |
| Stanford | CMV | Cystectomy | 63/36 | 42%/38% at 5 years |
| USC | CDCy | Cystectomy | 52/30 | 44%/39% at 5 years |
| Cognetti | GC | Cystectomy | 38/58 | 44%/44% at 6.5 years |
Abbreviations: C, cisplatin; D, doxorubicin; M, methotrexate; Cy, cyclophosphamide; V, vinblastine; G, gemcitabine; MRC-EORTC, Medical Research Council/European Organization for Research and Treatment of Cancer; RT, radiotherapy.
Thus, the consensus is now that neoadjuvant MVAC or equivalent chemotherapy affords an absolute survival benefit of 7–8%, with an increase in median survival of up to 3 years, when added to radical cystectomy. A statistically significant survival benefit has not been proved when the primary treatment is radiotherapy. Recent national surveys of patterns of practice have indicated that most patients do not receive such treatment, suggesting that change has come slowly in this area of clinical work.34, 35
To date, no multidrug cytotoxic regimen has been shown to be superior or even equivalent to the MVAC or CMV regimens for neoadjuvant chemotherapy. However, the newer gentle regimens, such as gemcitabine-cisplatin or gemcitabine-carboplatin, are being used increasingly for neoadjuvant therapy. This may be reasonable for the older or frail patients, but may lead to a greater risk of death from cancer for the more robust patient without intercurrent medical disorders. Ideally, a well-powered, randomized clinical trial would be needed to resolve this issue.
The importance of dose-dense MVAC, as initially developed and tested by the European Organization for Research and Treatment of Cancer (EORTC) in the metastatic setting, remains unclear and controversial. Toxicity may be reduced by this approach, but whether long-term results are equivalent is unclear, despite the imprimatur of the NCCN (National Comprehensive Cancer Network) guidelines.
Adjuvant chemotherapy
Chemotherapy administered after radical cystectomy for patients with T3–T4 tumors and/or lymph node involvement improves disease-free survival, as one would expect for any effective chemotherapy.36–41 However, in the randomized trials reported to date, most of which have been flawed by poor design, a disease-free statistical target, or inadequate sample size, a statistically significant improvement in total survival has never been demonstrated, as previously discussed.42 An Italian group tested the use of adjuvant gemcitabine-cisplatin and demonstrated a statistically nonsignificant inferior survival in the chemotherapy arm.41 An attempt was made to address these problems in the EORTC international randomized trial that had been in progress for several years, and which suffered from poor accrual, leading to premature closure. This study confirmed a disease-free survival benefit, the largest benefit counterintuitively in patients without node metastases, but no overall survival benefit.43 This suggests that the adjuvant chemotherapy may have compensated for inadequate definitive surgery.
Although meta-analysis can sometimes help to resolve the failure of small trials to resolve an issue, the study published by the Cochrane group was flawed.44 It grouped a heterogeneous set of small trials that were poorly designed, poorly executed, or which did not actually compare adjuvant chemotherapy with chemotherapy at relapse and thus confused the issue. However, understanding the significant limitations of historical controls and poorly executed randomized trials, our group has concluded that a survival benefit from adjuvant chemotherapy is still possible, and a survival deficit is unlikely; thus, we sometimes offer this approach to carefully selected otherwise healthy, postcystectomy patients with high-risk disease. A well-powered randomized chemotherapy trial is unlikely to ever answer this question.
Metastatic bladder cancer
Chemotherapy is the first-line treatment of choice for patients with metastatic bladder cancer. The single agent activity of 5-fluoruracil, methotrexate, the vinca alkaloids, doxorubicin, and cisplatin was demonstrated between the 1960s and early 1980s.45 The combination of methotrexate, vinblastine, and cisplatin, with46 or without47 doxorubicin, first produced objective responses in more than 60% of cases, with a median survival of 1 year. Investigators in the United States, Canada, and Australia proved the utility of the MVAC regimen in a randomized trial against single agent cisplatin and confirmed that the benefit persisted with a median follow-up beyond 6 years.48 The major limitation of the MVAC regimen was substantial toxicity, including grade 3–4 GI effects, stomatitis, and myelosuppression, as well as occasional cases of renal dysfunction and cardiotoxicity.46, 48 Attempts were made to improve the regimen, and Sternberg et al.49 demonstrated, in a randomized trial, that a dose-intense variant of MVAC yielded higher response rates and reduced toxicity compared to the original regimen, but without achieving a major increment in median survival. However, at 5 years, the number of surviving patients was greater than for standard-dose MVAC but did not reach statistical significance.
Single agent response rates of around 20–30% have been reported for paclitaxel, gemcitabine, docetaxel, ifosfamide, and pemetrexed.45 The combination of these agents with other standard or investigational drugs has resulted in response rates of 50–80%, sometimes with less toxicity than the conventional-combination MVAC regimen, but median survival figures have remained in the range of 12–20 months, and cure rates for patients with visceral metastases have not exceeded 10–15%.45 After initial studies with the combination of gemcitabine and cisplatin revealed apparently equivalent response rates and substantially less toxicity than the MVAC regimen,50 a randomized trial comparing gemcitabine-cisplatin versus MVAC confirmed these observations and showed that survival was similar.51 Consequently, an international consortium added paclitaxel to this doublet for patients with previously untreated metastatic UCs in an effort to improve cure rates, but without major survival impact in a randomized trial.52 Several other doublets and triplets have been assessed in phase I–II trials, but none has emerged as a major advance.
An important caveat in interpreting modern clinical trial data is that stage migration has occurred in the management of advanced bladder cancer, largely due to the increased use of aggressive postsurgical imaging via CT, MRI, and PET scans, and there has been increased use of systemic chemotherapy to treat patients with small volume, asymptomatic metastases. This should be borne in mind when considering the utility of novel combinations, such as the ITP regimen (ifosfamide, paclitaxel, and cisplatin), which yields a median survival of about 18 months, which is similar to outcomes in the current use of the MVAC regimen. Before novel regimens can be accepted into routine clinical practice, their safety and efficacy should be defined in randomized trials against accepted current standards. Despite modest progress in the past two decades, the majority of patients with metastases still die of progressive tumor. It is particularly important, in an era of increasing cost awareness and focus on the value proposition, that the aims of chemotherapy are detailed clearly, and that patients unlikely to secure life prolongation or improved quality of life are referred either into Hospice programs or, if still sufficiently fit, into clinical trials of novel approaches. Focal radiotherapy for symptomatic local or metastatic disease may also confer a palliative benefit for such patients.
Because cytotoxic chemotherapy regimens have not improved the cure rate dramatically, alternative approaches are being investigated. In the past decade, we have focused on novel compounds that target the genes and proteins that control cellular growth, differentiation, and apoptosis. Agents that modulate the function of EGFR and other tyrosine kinase inhibitors have been studied as monotherapy and in combination with chemotherapy. The ability to identify expression of the HER-2/neu oncogene, EGFR, and other molecular predictors of response allows some tailoring of treatment. Hussain et al.53 have assessed herceptin, in combination with a regimen of chemotherapy, against bladder cancers expressing the HER 2/neu gene, and showed a response rate of 70%; however, the median survival of 14 months did not suggest that this was a major advance; in this study, a higher response rate was seen in tumors expressing EGFR.
The tyrosine kinase inhibitor, sunitinib (see Chapter 97), can cause partial remissions in heavily pretreated bladder cancer but of only relatively short duration.54 Although this suggests some utility of the targeting of anti-angiogenesis pathways in bladder cancer, other studies have shown lack of activity of this group of agents against bladder cancer, and this approach is not gaining traction.
However, two novel approaches appear more promising. Recent data indicate that the MET gene is heavily expressed in urothelial cancer and prostatic adenocarcinoma. Early trials showed significant activity against prostate cancer, and a more recent study has suggested similar anti-cancer efficacy against advanced urothelial cancer.55
Preliminary studies have shown substantial expression of programmed death-ligand 1 (PD-L1) in urothelial malignancy, and phase I trials have suggested substantial anti-tumor effect from PD-L1 inhibitors, such as MPDL3280A.56 Phase II trials are in progress. Despite early promise, such agents, because of their uncertain long-term impact and very high cost, should be validated against established standards before introduction into routine clinical practice.
Another approach that is being investigated anew is the use of surgery to consolidate remissions achieved from chemotherapy.57 This approach was pioneered in bladder cancer by Alan Yagoda more than 20 years ago. The rationale is based on the high relapse rate observed at responding sites of disease and is supported by the 33% incidence of viable cancer found within resected specimens after complete clinical response. Five-year survival rates, as high as 30–40%, have been reported in patients following complete resection of metastatic sites after cisplatin-based chemotherapy, these represent very heavily selected cases, dominated by single metastases.
Uncommon histologic variants
A detailed discussion of the management of adenocarcinoma, squamous carcinoma, small cell carcinoma, and sarcoma of the bladder is beyond the scope of this brief review and has been detailed elsewhere.58, 59 However, certain principles of management can be noted.60 All of the uncommon variants tend to be more resistant to chemotherapy than are the pure UCs, and thus a greater emphasis is placed on surgical resection or definitive radiotherapy when possible. Where unusual histologic patterns are noted, it is also important to ensure that the diagnosis is confirmed by an expert tumor pathologist and also to exclude the diagnosis of a metastatic second primary cancer. In general, we recommend referral to a center of excellence at least for confirmation of the diagnosis and a second opinion regarding management.60
The prognosis of metastatic tumors of non-transitional type reflects the sites of involvement, growth characteristics, and bulk of disease. As the yield from chemotherapy is less impressive than for urothelial cancer,48, 58, 59 consideration of context (age, anticipated active life expectancy, intercurrent disease, sites of metastases) is important when planning the approach to chemotherapy.60
As a general rule, squamous carcinomas are sensitive to combinations that include a platinum complex, paclitaxel, and gemcitabine, and occasional responses have been reported after treatment with methotrexate, bleomycin, and ifosfamide. We have shown that the MVAC regimen is not especially useful for squamous carcinoma of the bladder.48 Patterns of practice vary with the combinations of gemcitabine, ifosfamide, and cisplatin or paclitaxel, gemcitabine, and cisplatin, occasionally producing long-term survival for metastatic disease. Adenocarcinomas tend to respond transiently to regimens used for cancers of the GI tract, such as combinations involving oxaliplatin, irinotecan, and fluoropyrimidines, such as 5-fluorouracil or capecitabine, although there is a paucity of data from well-structured phase II or phase III clinical trial data.
Investigators at the MD Anderson Cancer Center have reported a substantial experience with metastatic small cell anaplastic cancer of the bladder, revealing anticancer efficacy but few cures.59 The regimens with most utility resemble those used for small cell cancers of the lung and generally involve combinations that include a platinum complex, etoposide, doxorubicin, a taxane, and an oxazophorine. However, there is a general consensus that these tumors are more resistant to chemotherapy than are bronchogenic small cell tumors, and there is thus a greater emphasis on the role of surgical extirpation in the control of the primary tumor. In the metastatic setting, this is less relevant. In addition, there is good level-2 evidence that chemotherapy adds to the survival impact of surgical resection for clinically nonmetastatic disease.
Upper tract tumors
The approach to upper tract urothelial cancers is very similar to that employed for cancers of the bladder, with the caveat that the extent of surrounding fat and muscle is less, thus constituting less obstruction to metastasis. In addition, the phenomenon of “drop metastasis” may occur, in which tumor deposits from the upper tract(s) may seed to the urothelium of the bladder; whether this is the only mechanism of metachronous tumors, or whether this reflects the presence of field defect remains unclear. Details regarding etiology, epidemiology, clinical presentation, and investigation have been addressed in the relevant sections earlier.61–63
Surgical treatment
The surgical approach to upper tract tumors is quite different from that employed for the bladder.64–67 The standard treatment for localized upper tract UC is radical nephroureterectomy, with complete removal of the kidney, surrounding fat and Gerota’s fascia, removal of the affected ureter, and the en bloc resection of a bladder cuff. We believe that ipsilateral node dissection or extensive sampling should be performed for prognostication purposes, although there is no level 1 evidence to prove a therapeutic impact from the procedure.
Increasingly, laparoscopic radical nephroureterectomy is being considered as a viable alternative to open surgery, although long-term outcome equivalence has yet to be proven. Nonrandomized series appear to indicate that the results are comparable with respect to tumor control and possibly with less operative morbidity.65, 66
Nephron-sparing surgery is considered for settings such as bilateral disease, solitary kidney, impaired renal function, or significant comorbid medical conditions. This can be achieved by partial nephrectomy, partial ureterectomy, partial resection of renal pelvis, and percutaneous resection of a renal pelvic tumor. The decision to take this approach is essentially a cost-effectiveness choice, and one which must take into consideration the likely outcome of tumor management versus the morbidity of treatment and its impact on the comorbid state. Low grade tumors, in particular, may be treated safely and effectively by endoscopic means.67 Case selection is of critical importance, and recurrence rates reflect surgical experience and technique, instrumentation employed, and the prognostic determinants of the tumors being treated.
Radiotherapy and chemotherapy
There is remarkably scanty levels 1–2 information to support the use of radiotherapy for upper tract tumors, beyond palliation for inoperable cases. Dosing is limited by the sensitivity of the normal tissues to the impact of radiotherapy. Furthermore, those tumors with sufficiently poor prognosis to require consideration of radiotherapy for local control actually have a high chance of synchronous or metachronous distant nodal or metastatic involvement, thus vitiating the true role of radiotherapy. In structured trials, adjuvant radiotherapy has not been shown to have a major survival impact for upper tract tumors.68, 69
The efficacy of intravesical therapy for bladder cancer led investigators to use these agents in upper urinary tract tumors. The most common approach has been to place bilateral ureteral stents followed by instillation of cytotoxic agents (usually BCG) via a urinary catheter, as for bladder cancer; however, uncertainty remains as to how well the agents are being delivered upstream. Alternatively, there have been reports of transcutaneous insertion of flexible catheters into the ureters, followed by infusion of agents. Anecdotal data suggest that tumor regression occurs in response to topical delivery of chemotherapy or immunotherapy.
The use of adjuvant intravesical instillation of BCG, doxorubicin, and mitomycin C after endoscopic treatment has been reported, with evidence of anti-cancer effect on tumors in the upper tracts.70 These agents have also been delivered via nephrostomy tube after percutaneous treatment. The quality of the data, including length of follow-up, has been variable, but the overall consensus is that relapse and progression can be reduced by this type of treatment. Approximately 30% of patients with upper tract urothelial cancer will develop a recurrence in the bladder, thus requiring long-term cystoscopic surveillance of the bladder for patients with upper tract UC.71
The considerations for systemic chemotherapy for upper tract urothelial cancers are essentially identical to those pertaining to urothelial bladder cancer.45–52 In the past, upper tract tumors were considered to be less responsive than those arising in the bladder. However, there is little evidence to support this, and the international randomized study of MVAC versus cisplatin confirmed similar response rates and survival.48 Thus, systemic chemotherapy is covered in detail in the section on chemotherapy for bladder cancer.
Summary
Significant progress has been made in the management of bladder cancer over the past 30 years, with refinement of our understanding of the underlying biology, relevance of gene expression and stem cell function, molecular prognostication, improvement in the nature of surgery, reduction in morbidity of surgery, and rationalization of the role of chemotherapy for advanced disease. There is also a place for bladder conservation via chemoradiation. Despite progress, many patients with metastatic disease die of their disease, and this has led to the search for new systemic therapies, including novel cytotoxics and the assessment of targeted therapies, with agents targeting the MET gene and PD-L1 currently appearing most promising. New agents should be compared against standard regimens in well-structured trials before introduction into routine clinical practice.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








