Neoplasms in acquired immunodeficiency syndrome
Jeremy S. Abramson, MD, MMSc  David T. Scadden, MD
David T. Scadden, MD
Overview
Immunodeficiency of multiple etiologies is associated with an increased risk of malignancy, particularly lymphoma. The risk is variable, depending on the severity and extent of the immunologic abnormality. In the setting of the acquired immunodeficiency syndrome (AIDS) secondary to human immunodeficiency virus type 1 (HIV-1) infection, the range of tumor types is more extensive. Yet, the tumors are generally associated with oncogenic viruses and may be considered secondary, opportunistic neoplasms. Etiologic factors contributing to them include poor control of oncogenic viruses, altered cytokine regulation owing to HIV effects on immune cells and tissue stimulation from other AIDS-associated events. The interplay of immunity, infection, and oncogenesis is central to AIDS-related malignancies.
The spectrum of the tumor types seen in the context of immunodeficiency extends beyond that of lymphoma, but is quite limited. There appears to be little interaction between the conditions that predispose to the emergence of epithelial malignancies seen in the general population and immunodeficiency. Rather, immunodeficiency tumors represent a narrow subset of neoplasms, some of which are seen with only very low incidence in the general population. For example, primary central nervous system (PCNS) lymphoma and Kaposi sarcoma (KS) are extremely rare entities in all but the immunodeficient population, where they compose a large proportion of tumors. In addition, the incidence of specific tumor types varies according to the immunodeficient state. Non-Hodgkin lymphoma (NHL) is a common theme among all of the immunodeficiencies, yet in AIDS there is a broader spectrum of histologic subtypes than are seen in other immunodeficient states. KS is increased in subgroups of patients with HIV-related and pharmacologically induced immunodeficiency. Cutaneous tumors are common in many immunodeficient states, but the increase in squamous cell tumors of the skin is higher in the post solid-organ-transplantation population than in those with HIV-related immunodeficiency. In the latter, papillomavirus-related squamous cell neoplasia of the anogenital region predominates (Table 1).
Table 1 Tumor types with increased incidence in HIV disease
| Kaposi sarcoma |
| Non-Hodgkin lymphoma Diffuse large B-cell lymphoma Burkitt lymphoma Plasmablastic lymphoma Primary effusion lymphoma Primary CNS lymphoma |
| Squamous cell neoplasia |
| Hodgkin lymphoma |
| Leiomyosarcoma (in children) |
| Plasmacytoma |
| Seminoma |
| Skin cancers |
| Lung cancer |
| Osopharyngeal cancer |
| Liver cancer |
| Prostate cancer |
Shared among the tumors related to immunodeficient states is the frequent association with an infectious pathogen. The presence of Epstein–Barr virus (EBV) in immunodeficiency-related lymphomas is well known and likely a result of the direct stimulation that the virus provides to B-cell proliferation. In the absence of effective immunologic targeting of cells expressing EBV latency gene products, the overgrowth of cells may proceed unchecked, with the subsequent emergence of a transformed cell. This model for the direct ability of viruses to induce cell proliferation is a paradigm that may be applied to human papilloma virus (HPV)-related tumors as well. However, the model is less easily applied to the KS-associated herpesvirus/human herpesvirus-8 (KSHV/HHV8)-related tumors. The tumors associated with KSHV/HHV8 are more varied and are of less clear pathophysiologic relationship to viral gene products issues that are discussed in greater depth in sections that follow. In general, the tumors that do emerge in immunodeficiency are those in which a secondary pathogen can be implicated (Table 2). Immunodeficiency further leads to a failure of innate host tumor surveillance. In essence, the concept of inadequate immunologic control provides a unifying mechanism, and these tumors may be considered opportunistic malignancies, much the way in which specific infections are considered opportunistic infections. Indeed, the opportunistic malignancies of the immunocompromised patient represent the overlap between infectious diseases and oncology and provide unique insight into the intersection of immune function and tumor development.
Table 2 Secondary virus infections associated with AIDS-related malignancies
| Virus | Tumor |
| EBV | NHL (PCNSL; most systemic DLBCL; PBL) |
| HL | |
| Leiomyosarcoma (children) | |
| KSHV/HHV8 | KS |
| NHL (PEL) Multicentric Castleman disease (premalignant) | |
| HPV | Squamous cell neoplasia |
Abbreviations: ARL, AIDS-related lymphoma; EBV, Epstein–Barr virus HL, Hodgkin lymphoma; HPV, human papillomavirus; KS, Kaposi sarcoma; KSHV, Kaposi sarcoma herpesvirus; NHL, non-Hodgkin lymphoma; PCNS, primary central nervous system; PBL, plasmablastic lymphoma; PEL, primary effusion lymphoma; DLBCL, diffuse large B-cell lymphoma.
Epidemiology
The spectrum of tumors in the context of HIV-1 infection varies on the basis of risk group and is substantially affected by the use of modern combination antiretroviral therapy (cART).
Pre-cART
cART did not become available until 1996, with the introduction of the protease-inhibitor class of anti-HIV medications. Widespread use of protease inhibitors occurred rapidly in the United States, Western Europe, and Australia, altering the death rate and complication rate of HIV disease. The spectrum of opportunistic diseases also changed,1, 2 with an impact on malignant disease, discussed in the section “Post-cART.” Given the ongoing lack of access to cART in much of the developing world, and the number of patients unable to take or having failed cART, the profile of AIDS-related malignancies in the pre-cART era is still of considerable importance.
One of the first manifestations of the AIDS epidemic was the cluster of cases of a rare malignancy among men who have sex with men in the coastal cities of the United States. That tumor, KS, was identified as an AIDS-defining illness with the first attempt at classifying the immunodeficiency syndrome by the Centers for Disease Control and Prevention (CDC).3 The prevalence of KS among HIV-infected patients was approximately 20% early in the AIDS epidemic, but clearly was noted to be highest among patients whose risk factor for HIV transmission was men having sex with men.4 KS prevalence was substantially lower in the groups infected by blood products or through parenteral drug use.5 Subsequent behavioral studies indicated that specific types of sexual practice, including promiscuity and oral–fecal contact,6 had the highest risk and substantiated the impression that KS might be a manifestation of a secondary, transmissible pathogen. Indeed, it was strong epidemiologic data that galvanized efforts to identify a pathogen and that led to the molecular cloning of the KSHV, also known as HHV8.7
The second most common malignancy, which was recognized in 1984 to be increased among young men who have sex with men, was NHL. This disease was added to the list of AIDS-defining complications in the first revision of the CDC criteria for AIDS. It was noted that lymphomas that occurred in this population were generally of high-grade histology and followed extremely aggressive clinical courses. Unlike KS, this complication was much more broadly based in the risk groups for HIV infection. All groups had a high relative risk, estimated to be approximately 60-fold above that of the general population.6, 8–10 Subsets of infected individuals have been noted to have somewhat greater or lesser risk, such as the hemophiliac population, in which at least one study has noted an increased risk.6, 10 Similarly, it has been noted that risk among intravenous drug users or those from the Caribbean basin may be lower, but concern about confounding issues of care and surveillance complicate that analysis. However, the potential for important cofactors of lymphomagenesis within these subsets remains, and attention to this possibility may yield important information about the process of lymphocyte transformation.
The third most common malignancy occurring in the setting of HIV disease are anogenital squamous cell neoplasms, which are invariably associated with HPV infection of oncogenic serotypes. The majority, however, appear to be in situ carcinomas or high-grade intraepithelial neoplasia, while an increased incidence of invasive cancer remains controversial.11, 12 HPV-associated head and neck cancers may also be increasing in prevalence, related to the high seroprevalence of HPV, as well as additional concomitant risk factors for head and neck cancers including immunosuppression, tobacco, and alcohol use.
Post-cART
The introduction of cART has resulted in profound and dramatic changes in the nature of HIV disease. The inexorable decline in immune function and its attendant ravaging secondary infections and tumors in many cases is stopped, and indeed reversed, when combination therapy with protease inhibitors is introduced. The improvement in those patients with advanced disease has led to widespread use of the agents, including among those individuals who have recently acquired HIV-1 infection. The result has been a precipitous decline in the rate of death from AIDS in populations with access to the medications. Although death and debility from AIDS has decreased dramatically, there has not been a similar decline in new cases of HIV infection. Therefore, the total population with HIV infection in the West is rising, and those infected are living longer; globally, the epidemic goes unabated. Some changes in the epidemiology of malignancies in HIV infection have been immediately evident since cART was introduced, but the impact of longer periods of more modest immune dysfunction or even of the antiretroviral drugs themselves is only now becoming understood.
An observation immediately evident in the clinical care of patients with advanced HIV disease was the regression of KS following successful HIV suppression on cART. The impact on new cases of KS was also rapidly noticeable, and epidemiologic data have substantiated the magnitude of those clinically apparent effects. Multiple studies from sites in the United States, Europe, and Australia indicate the widespread decline in KS, with estimates of decline in incidence as high as 80-fold.13–16 The risk for development of KS, both pre- and post-cART, correlates directly with the depth of CD4 count suppression,17 though cases of KS in patients with increased CD4 counts are being increasingly reported in the post-cART era,18, 19 where the disease may be more localized to the skin of the lower extremities with less visceral dissemination, more akin to endemic KS seen in non-HIV-infected individuals in the Mediterranean basin.
As with KS, changes in the incidence of primary central nervous system lymphoma (PCNSL) are dramatic. Although this complication of advanced HIV disease was much less common than KS, and its decline was therefore less well documented, the impact in clinical terms has been comparable in magnitude. Cases are rarely seen except among those who have failed or have not been receiving antiretroviral therapy. This is a complication of severe immune suppression that, like the post-transplantation setting, is virtually uniformly associated with EBV detectable in tumor tissue. In general, the EBV latency genes expressed in these tumors are type III or those of lymphoproliferative disease (EBNA1-6, LMP1, and LMP2).20 These and other features distinguish PCNS lymphoma from other AIDS-related lymphomas (ARL) and may be the basis for clear differences in the impact of cART.
The incidence of systemic AIDS-related NHL has also decreased in the cART era, and the pattern of lymphoma subtypes in this population has also evolved.21–24 Early in the cART era, it became clear that there was significant variability in the impact of cART among lymphoma subtypes, with the greatest risk reduction seen in PCNSL and immunoblastic diffuse large B-cell lymphoma (DLBCL), while the incidence of Burkitt lymphoma (BL) and Hodgkin lymphoma (HL) did not decline.22, 25 One recent study of a cohort of 23,050 HIV-positive individuals in the United States found that 476 (2%) developed lymphoma between 1996 and 2010, among which 83% were NHLs and 17% were HL.26 The most common subtype in the cART era is DLBCL, accounting for 42% of cases, and it is also the most common type of NHL in non-HIV-infected individuals. BL accounted for 12% of cases, which is a significantly greater proportion compared to the general population. PCNSL has declined the most dramatically in incidence compared to the pre-cART era, accounting for only 11% of NHLs in this cohort. The risk for most HIV-related lymphomas correlates directly with decline in CD4 count, though less so for BL and HL than other histologies.17 Median CD4 counts in DLBCL and BL in this modern cohort were 68 and 118 cells/μL, respectively. Reflecting the HIV-infected patient population in the pre-cART era, the median CD4 count was lowest in PCNSL in this modern analysis with a median of 14 cells/μL.
The differential impact of cART within lymphoma subsets highlights biologic differences between these tumor types and suggests differential immune participation in tumor development.
Kaposi sarcoma (KS)
It was the announcement of clustered cases of KS in Los Angeles and New York that made headlines and first brought the AIDS epidemic to public awareness in 1981.27 Having originally been described by the Hungarian dermatologist Moritz Kaposi in 1872,28 KS was regarded as a tumor that generally had an indolent course in elderly men of Mediterranean extraction, but which could be problematic in the context of immunosuppressive medication for organ transplantation. It was this latter association that helped focus attention on an immune alteration spreading among subcommunities in urban centers. Recognized as a common entity among HIV-positive men who have sex with men, but not among groups with other risk factors for HIV infection (such as blood product exposure),5, 25 it was long suspected as being related to a second cofactor.29 A number of potential culprits were examined; none proved tenable until the identification of KSHV/HHV8. This virus was first recognized through the use of a genetic comparison of tissues from individuals with and without KS. A deoxyribonucleic acid (DNA) fragment that had partial homology with other members of the gamma-herpesvirus family was consistently noted.7 This subset of the herpesviruses includes several viruses with oncogenic potential, such as EBV, associated with a number of tumors and herpesvirus saimiri (HVS), associated with the ability to transform T cells. Because of the company KSHV/HHV8 kept and the high frequency of detectable signature DNA sequences in KS lesions, this virus rapidly and justifiably became the focus of investigation for a pathophysiologic basis of KS.
Viral epidemiology
KSHV/HHV8 is a 165-kilobase (kb) double-stranded DNA virus with features strongly supporting its causative role in clinical KS. There are data indicating that KSHV/HHV8 infection precedes tumor formation,29, 30 that populations with high seroprevalence for KSHV/HHV8 are also those with a high incidence of KS,25 and that the virus infects cell types within tumors.29
The definition of prior exposure to KSHV/HHV8 depends on documentation of antibodies specifically reactive against the virus. There have been a number of assays that have been tested with variable results. Data indicate seropositivity estimated at 3.5% in North America, up to 25% in the peoples of the Mediterranean basin, and up to 89% in sub-Saharan African populations.31–33
The much-suspected role of sexual transmission has been convincingly demonstrated in a longitudinal study of men in San Francisco over a 10-year period. Among exclusively heterosexual men, no KSHV/HHV8 seropositivity was detected; however, among men who have sex with men, the incidence of seroconversion was linearly related to the number of male sexual-intercourse contacts.34 Men who had more than 250 sexual partners in the preceding 2 years had a seropositivity rate of 65%. Yet, sexual transmission is not the exclusive basis of virus spread. KSHV/HHV8 can be identified in saliva, and it is thought that oral transmission can rarely occur.35 The higher incidence of KSHV/HHV8 seropositivity among family members in areas of endemic KS and given that children are often infected in sub-Saharan Africa indicate that nonsexual means of transmission do occur, but the specific basis is still to be fully defined.
Clinical manifestations
KS characteristically appears as pigmented macular-papular lesions on mucocutaneous surfaces (Figures 1 and 2). It is typically violaceous or erythematous in hue and may be associated with an ecchymotic halo. Typically, the lesions are multifocal and do not have a predictable order or pace of progression. Lesions may present as solitary nodules or plaques, but may also occur in clusters or simultaneously in multiple well-segregated sites. Although classic or endemic KS often favors the lower extremities, the pattern of involvement is much less predictable in the setting of HIV infection. Virtually any mucocutaneous site may be involved. On the face, the ears and nose are often affected, resulting in profound disfigurement. In addition to the disabling cosmetic effects of KS, lesions do occasionally become thick, uncomfortable plaques and can ulcerate with possible superinfection. Lesions are not generally destructive, however. The integument or mucous membrane overlying a lesion is most often intact, and deep invasion into muscle or bone generally does not occur.
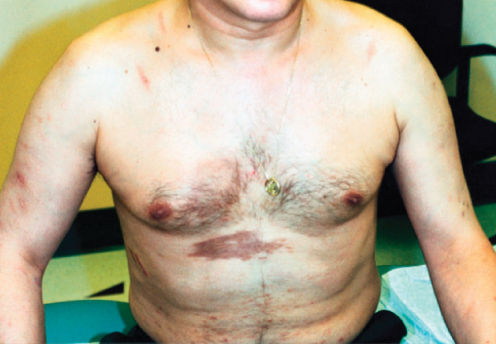
Figure 1 Cutaneous Kaposi sarcoma in a white patient with advanced HIV disease. The violaceous plaques on the chest are of characteristic appearance. These lesions entirely resolved on paclitaxel chemotherapy and antiretroviral medication.
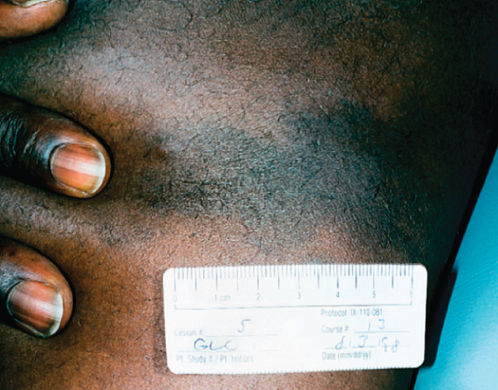
Figure 2 Kaposi sarcoma in an African American male with HIV-1 infection. Skin tone can make the lesions less readily distinguishable from other cutaneous processes and quite distinct from the appearance in lighter skinned individuals.
Edema often accompanies KS either locally or at a dependent site distal to KS lesions (Figure 3). The edema can be marked, with profound compromise of extremity mobility or occasionally with periorbital, peripubic, or genital edema. Two mechanisms are thought to contribute to the development of edema. One is the involvement of lymphatic vessels or lymph nodes with KS, thereby causing a mechanical obstruction to lymphatic flow. The other is the elaboration of permeability factors by KS lesions. The vessels that compose a KS lesion are themselves leaky, with extravasation of plasma proteins and cells into surrounding soft tissue. In addition, the vascular endothelial growth factor (VEGF) produced by KS lesions can alter the integrity of surrounding otherwise-normal vessels, increasing their permeability, and, thus, their contribution of fluid to interstitial fluid. The increased demand on lymphatic drainage and the compromised egress of lymph results in thickened skin locally and frank edema distally.
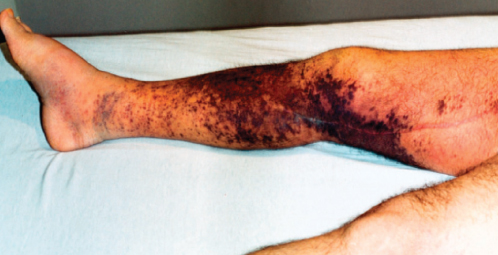
Figure 3 Lower extremity involvement by Kaposi sarcoma can result in marked edema and limited mobility. This patient had pedal edema that had limited response to chemotherapy despite marked improvement in the circumferential Kaposi sarcoma.
Involvement of organs other than lymph nodes and skin occurs frequently. The most common site is the gastrointestinal (GI) tract, where mucosal-based lesions are commonly observed in the course of endoscopic examination. The physiologic significance of these lesions is often minimal, however. Most patients will be unaware of GI involvement, and serendipitous observation of a mucosal KS lesion should not trigger a reflex to undertake aggressive therapy. However, there are some individuals for whom GI KS can be a symptomatic and even life-threatening complication. Massive bleeding has been observed, as has intussusception.
Pulmonary involvement may take several forms. Pleural-surface studding with KS lesions can result in pleural effusions, which are often bloody, but which do not have a characteristic set of diagnostic findings or cytologic abnormalities. Bronchial mucosa may be involved and, like GI mucosal surfaces, may be incidentally noted during bronchoscopic examination. The lesions are generally not destructive; but depending on location, they may be responsible for bronchial irritation, coughing, and hemoptysis. Involvement of the parenchyma of the lung occurs and is arguably the most serious complication of KS, because it is associated with life-threatening respiratory compromise and a high mortality rate if unsatisfactorily treated.36 Radiographically, involvement often takes the appearance of peribronchiolar cuffing on computed tomography (CT). Pathologically, this infiltration may extend into fine interstitial tissue and affect airspace function. This results in either a patchy or diffuse reticulonodular appearance on X-rays. The diagnosis of KS involvement of the lung is often difficult to firmly establish short of parenchymal thoracoscopic or open biopsy. Bronchoscopy is useful in assessing alternative infectious explanations for clinical findings and may identify mucosal lesions. However, mucosal lesions do not necessarily coincide with parenchymal infiltration, and transbronchial biopsy is often unrevealing. Notably, KS of the bronchial mucosa may coexist with opportunistic infections of the lung parenchyma, such as Pneumocystis jiroveci (P. jiroveci), cytomegalovirus (CMV) and others, so clinicians must maintain a broad differential diagnosis regarding lung findings in HIV patients, particularly with low CD4 counts. In some circumstances, the use of a therapeutic trial may also be helpful in establishing a presumptive diagnosis. If thorough microbiologic evaluation has been unrevealing, the chemotherapeutic agents discussed below have been well tolerated and associated with high rates of response, such that their use in select patients may be justified as a test for chemotherapy responsiveness of a parenchymal infiltrate. Such a strategy is generally reserved for those patients in whom (1) there is a diagnosis of KS already established from involvement of other sites, (2) there are no symptoms suggesting infection, or (3) aggressive assessment for infection is negative and there are no other contraindications to cytotoxic chemotherapy.
In addition to lung, GI tract, and lymph nodes, special sites of concern are areas of the upper airway. Involvement of the mucosa of the mouth, sinuses, pharynx, and larynx can result in distortion of soft tissues such that airway compromise or alteration of food ingestion can occur. These are lesions that generally respond rapidly to therapy and do not invade deeper structures. Therapeutic approaches are discussed in the section “Treatment.”
Given the common involvement beyond the readily apparent skin or oral mucous membranes, extensive staging evaluations are often considered. Although the bulk of tumor does influence prognosis, other characteristics of the patient’s immune and general health were incorporated into a KS staging system by the AIDS Clinical Trials Group (ACTG), based on pre-cART data on KS patients (Table 3).38 In the cART era, tumor extent and concomitant systemic illness appear to the most powerful predictors of prognosis in this model, with limited tumor involvement and lack of opportunistic infections predicting a 3-year overall survival of approximately 90%, compared to only 50% with extensive or visceral disease and presence of other HIV-related complications.39 An alternative prognostic model has been proposed in the cART era based on multivariate analysis in 326 AIDS-associated KS patients, which identified four favorable prognostic variables by multivariate analysis: (1) having KS as the AIDS-defining illness, (2) increasing CD4 count, (3) age <50 years, and (4) absence of another AIDS-associated illness.40 These variables generated risk scores predicting for 5-year overall survival ranging from 98% in the most favorable cohort, to only 8% in the least.
Table 3 Staging classification for AIDS-related Kaposi sarcoma
| Good riska | Poor risk (1)b | |
| Tumor (T) | Small tumor burden with limited involvement of one or more | Large tumor burden |
| Oral | ||
| Lymph nodes | Gastrointestinal | |
| Skin | Pulmonary | |
| Otherc | Other visceral with involvement of tumor associated edema or ulceration | |
| Immune system (I) | CD4+ cells >200 mm3 | CD4+ cells <200 mm3 |
| Systematic illness (S) | No opportunistic infection (including thrush), or B symptomsd | History of opportunistic infection or thrush B symptomsd |
| Other HIV-related illness (e.g., NHL or other malig-nancy, neurologic disease, wasting syndrome) |
a All the parameters listed.
b Any of the parameters listed.
c Limited oral disease is confined to the palate and is not nodular.
d B symptoms are unexplained fever, night sweats, weight loss of more than 10% of body weight, or diarrhea persisting for more than 2 weeks.
Abbreviations: HIV, human immunodeficiency virus; NHL, non-Hodgkin lymphoma.
Source: Krown 1989.37
The staging performed at diagnosis is often based on clinical presentation, with radiographic studies limited to a chest x-ray unless symptoms dictate otherwise. Testing of stool for fecal occult blood is a reasonable screening test for GI involvement, though specificity is poor. If localizing symptoms do suggest organ involvement, then more extensive radiographic and procedural interventions are appropriate, but they cannot be recommended routinely. Criteria for staging do include assessment of the underlying HIV infection, and all patients should have a careful history regarding medications, other complications of HIV infections, and documentation of the CD4 count in the blood.
Establishing the diagnosis of KS generally depends on simple punch biopsy of the skin or a snip biopsy of mucosal surfaces. The CDC criteria for an AIDS-defining diagnosis of KS do not require histologic confirmation if assessment is by an experienced clinician. However, biopsy is strongly recommended, given the broad differential diagnosis for nonblanching lesions resembling KS,38 including bacillary angiomatosis (usually caused by Bartonella infection, with increased frequency in advanced HIV-infection patients); hematoma; purpura; sarcoid plaques; lichen planus; pyogenic granuloma; mycosis fungoides; secondary syphilis; pityriasis rosea; drug-related erythema multiforme; prurigo nodularis; nevi; vascular lesions of the phakomatoses; epithelioid hemangioendothelioma; angiosarcoma; melanoma; and basal cell carcinoma.
Pathology and pathogenesis
The histologic appearance of KS belies its unclear association with the term “sarcoma.” No monomorphic array of mesenchymally derived cells is seen. Rather, the lesions are composed of endothelial cells lining ectatic vascular spaces surrounded by spindle cells of variable extent admixed with mononuclear immune cells and extravasated red blood cells. It is the red blood cells that provide the pigment to KS lesions and their breakdown that leads to the ecchymotic halo seen in actively growing lesions. The hemosiderin deposited locally yields a pigmented lesion that may remain even after effective therapy reverses the proliferative spindle and endothelial cell components. Cutaneous lesions are generally within the dermis and deep invasion to muscle is generally not seen.
The origins of the endothelial and spindle cell components remain controversial. The endothelial cells of KS lesions express the VEGF-C receptor characteristic of lymphatic endothelium and do not have detectable nitric oxide synthase, generally present in vascular endothelium.41, 42 The finding that the spindle cell expresses the mannose-binding receptor and CD68 suggests that the origin may be a macrophage-like cell type, perhaps emanating from the sinuses of secondary lymphoid organs and circulating in the blood before ultimately assuming its role in a KS lesion.43
The difficulty in defining the cell of origin emanates from its complex histology and the limitations of the available in vitro or in vivo models. In vitro culture has been established for some cell types, and outgrowth of cell lines has been documented, although the relationship to the primary disease process is unclear. In vivo transplantation of KS tissue into immunodeficient mice has resulted in tumors, but these are of murine origin. The potential for cytokine elaboration driving lesion development was hypothesized, and studies have extensively characterized cytokine production by KS lesions.44–46 The cytokines, particularly VEGF and basic fibroblast growth factor (bFGF), may play a role in the paracrine or autocrine sustenance of KS. Antibodies to bFGF block the proliferation of KS cells and prevent them from entering the S-phase of the cell cycle, even in the presence of exogenous growth factors like the interleukin (IL)-6–IL-6R complex, IL-10, tumor necrosis factor (TNF)-α, and oncostatin M.47 But exogenous growth factors do not completely explain the phenotype and growth potential of KS cells, which overexpress the antiapoptotic bcl-2 gene independently of any factors contained in conditioned medium from these cells.48
KSHV/HHV8 appears necessary for the induction of KS and is found in KS lesions regardless of whether the underlying context is HIV disease, organ transplantation, or endemic KS. However, the specific mechanism by which the virus participates in the oncogenic process is unclear and does not readily fit into paradigms established by other virus-related neoplastic disease. The latent genes implicated in EBV-induced transformation do not have homologs in KSHV/HHV8. The genes of HVS, which are known to transform cells, have homology only in the genome of a KSHV/HHV8 gene, K1, that is capable of transforming an immortalized cell line.49 However, this gene is not expressed in the latent phase of the KSHV/HHV8 life cycle. Similarly, a chemokine receptor-like KSHV/HHV8 gene product, open reading frame (ORF 74), is constitutively activated and is capable of transforming cells when transduced as a single gene; but it is also a lytic-phase gene.50 The encoded viral G-protein-coupled receptor activates multiple signaling pathways including the phosphatidylinositol-3 kinase (PI3K)/AKT, mitogen-activated protein kinase (MAPK), and Janus kinase/signal transducer and activator of transcription (JAK/STAT) pathways.51–53 These pathways exert protean antiapoptotic and proliferative effects that promote tumorigenesis. Two other gene products, K9 (a homolog of the interferon regulatory factor family) and K12 (with no clear gene family homology), are capable of transforming cell lines when transduced but are not expressed in latent phase.54–57 The lack of clear association with the latent phase would suggest that these gene products are not transforming in cis, but whether they may transform in trans cannot be excluded. A number of other mechanisms, including those that may be capable of acting at a distance, have been raised as possibilities for KSHV/HHV8. These include the chemokine-related gene products, vMIP-I (K6) and vMIP-II (K4), or the IL-6 homolog, K2. Each is capable of interacting with cognate receptors on target cells, either acting as agonists (K2 and K6) or antagonists (K4).58, 59 Finally, there are KSHV/HHV8 gene products that have antiapoptotic effects and that may enhance tumorigenicity. ORF 16 encodes a bcl-2-related gene product,60 and ORF 71 (K131) encodes a functional member of the Fas-associated death domain-like IL-10-converting enzyme-inhibitory protein (FLIP) family of antiapoptotic genes.61 The vFLIP of KSHV/HHV8 protects cells from Fas-mediated cell death and can enhance tumor progression of cell lines transplanted in vivo. KSHV/HHV8 further induces transcription of hypoxia-induced factor 1 alpha (HIF1alpha) and HIF2alpha resulting in upregulation of the proangiogenic factor, VEGF, as well as other proangiogenic and antiapoptotic factors62, 63 VEGF and VEGF receptors are richly expressed within KS lesions and appear to play a critical role in pathogenesis.64, 65 KSHV/HHV8 has further been shown to encode a microRNA that downregulates thrombospondin 1, a negative regulator of angiogenesis and tumor suppressor.66 Thus, KSHV/HHV8 encodes a range of gene products with potential for altering the growth, death, and immunologic characteristics of infected cells. It is not clear which of these mechanisms will be seen to play a dominant role in oncogenesis; further investigations into the process will certainly lead to new insights into viral-induced tumors and open new avenues for therapeutic attack.
The prevalence of KS in populations, (1) that are HIV infected, (2) that have undergone solid organ transplantation, (3) that are aged, and (4) that are of Mediterranean extraction, or live in economically disadvantaged parts of tropical Africa, suggests that expression of a KS disease phenotype requires a degree of immunosuppression. The advent of cART for HIV infection treatment offers dramatic support to a central role for immune suppression: Complete remissions of cutaneous and pulmonary KS are well recognized in the context of increases in CD4 count and declines in HIV load induced by cART.67, 68 Although HIV-induced immunodeficiency is only one type of immune abnormality that may predispose to KS, the relative risk among the population co-infected with HIV and KSHV/HHV8 is strikingly high, suggesting the potential for interaction between the two viruses in the pathogenesis of tumor. The HIV gene product, tat, affects KSHV/HHV8 replication itself,69 alters cytokine and cytokine receptor expression in target cells, and leads to proangiogenic effects.70, 71 Expression of tat may induce the lytic phase of KSHV/HHV8, resulting in increased viral transcripts, IL6 production, and stimulation of the JAK/STAT proliferation pathway, which offers a pathophysiologic rationale for the preferential occurrence of KS in HIV-infected patients versus solid-organ transplant recipients and other immunosuppressed populations.51 Retrospective analysis of the incidence of KS among HIV-infected patients receiving antiherpesvirus medications has suggested a direct role of replicating KSHV/HHV8 in the pathologic process. In several studies, there was a decreased incidence of KS in patients treated with ganciclovir or foscarnet.72–75 Therefore, suppression of replicating virus appears to lower the risk of KS. A randomized controlled clinical trial of valganciclovir in HHV8 seropositive individuals has also been shown to significantly decrease HHV8 replication,76 though benefits on clinically meaningful endpoints has yet to be established. Although transformation is generally associated with the latent phase of herpesvirus infection, control of the lytic phase (the only time at which the anti-herpesvirus medications have known activity) may limit the potential for transformation.
Control of virus by immunologic means also appears to be highly relevant to the risk of developing KS. It has long been known that there is an increased risk of KS in the context of multiple types of immunologic deficiency. The definition of epitopes of the virus that are recognized by cytotoxic T lymphocytes (CTLs) will allow for further definition of this important point and potentially lead to vaccine strategies.
Treatment
Treatment of KS should be guided by the impact of the tumor on the patient. The goals of treating this disease are to palliate symptoms, alleviate organ compromise, reduce edema, and improve quality of life and ultimately overall survival in affected patients. The variability in the course of HIV-infected patients with KS and the lack of clear association of tumor control with mortality suggests that aggressive therapy may not always be an appropriate response to the diagnosis of KS. This is particularly true among patients with advanced HIV disease or untreated HIV disease. In that setting, the toxicity of cytotoxic therapy may be daunting, and the potential therapeutic effect of anti-HIV medications is considerable. Suppressing HIV replication is associated with a rise in CD4 count, with attendant improvement in immunologic function, and also reduction in whatever the direct contribution of HIV to the pathophysiology of KS might be. The net result is a high frequency of clinical improvement among patients with established KS and, occasionally, complete eradication, simply by the introduction of cART. Therefore, for patients in whom the impact of KS is not organ threatening or profoundly symptomatic, a therapeutic trial of antiretroviral therapy alone is appropriate first-line therapy (Figure 4). This strategy was validated in a phase III placebo-controlled trial in sub-Saharan Africa, where 59 subjects were randomly assigned to cART alone or cART plus combination chemotherapy.77 Subjects treated with chemotherapy had a higher overall response rate at 1 year (66% vs. 39%), but no difference in overall survival. CD4 count and quality of life improved in both arms, without a statistically significant difference.
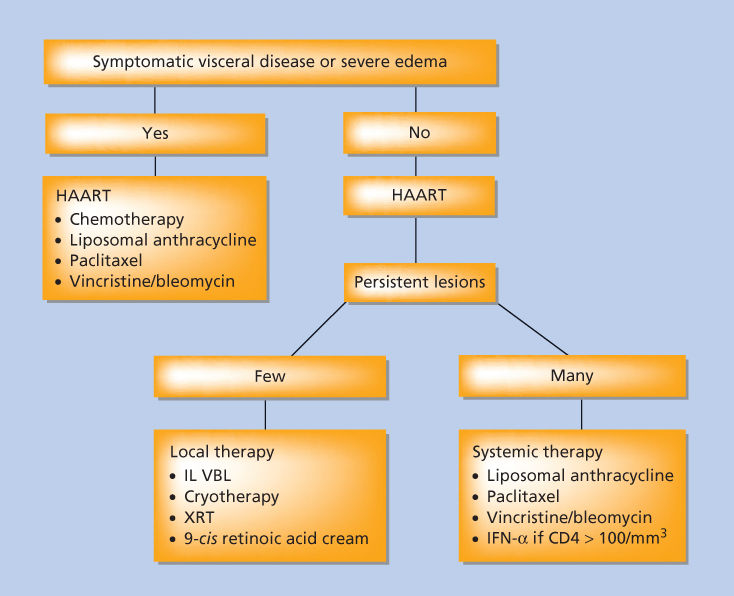
Figure 4 Treatment schema for patients with Kaposi sarcoma. Abbreviations: HAART, highly active antiretroviral therapy; IFN-α, interferon-α; IL, interleukin; VBL, vinblastine; XRT, radiation therapy.
In patients with highly symptomatic or organ-threatening disease, a more aggressive approach is warranted, concurrent with cART. Though anti-HIV therapy is an important component in the treatment of HIV-related KS, it should be recognized that not all patients will experience an improvement in their KS with antiretroviral therapy, and that improvement, when it occurs, may only be noted only after 4–8 weeks of therapy. If the patients do not show an improvement in KS by 12 weeks of initiation of anti-HIV therapy, it is unlikely that their disease will be controlled by that intervention alone; additional treatment options must then be considered. Another important phenomenon is that some patients will experience a flare of their KS shortly after beginning cART, evidenced by a rapid increase in size and number of KS lesions. Such flares may be self-limited and can regress with ongoing cART; however, this warrants close attention by treating physicians and consideration of additional KS-directed therapy if a flare becomes clinically significant. In contrast to antiretroviral drugs for HIV, anti-herpesvirus medications do not appear to have a therapeutic role in the management of active KS, where objective responses to ganciclovir and valganciclovir have not been observed. Anti-herpesvirus agents currently available, therefore, cannot be recommended as anti-KS therapy at this time.
Local antitumor chemotherapy
Therapies directed at KS tumors can be divided into either local or systemic therapies. Local therapies offer the benefit of deferring systemic chemotherapies and their attendant risks of increased immunosuppression in an already vulnerable patient population. The selection of a local therapy may be influenced by certain factors, such as the extent and location of the lesions (e.g., small, singular lesions on the trunk or on an extremity) and the rapidity of clinical change (e.g., indolent development of new lesions over months rather than weeks). Options for local therapy include intralesional injection of vinblastine, topical retinoid, radiotherapy and cryotherapy, among others.
For patients who have a small number of lesions that are unresponsive to anti-HIV medications, or who require more rapid improvement than the anti-HIV medications may offer, intralesional injection of vinblastine is a reasonable first-line approach. In particular, palatal or buccal mucosa lesions respond promptly to intralesional vinblastine.78 Practitioners most commonly use vinblastine at 0.1–0.4 mg/mL, injecting approximately 0.1–0.2 mL into a 1 cm2 lesion. Local discomfort may be considerable, and so the vinblastine may be admixed 1 : 1 with lidocaine to improve tolerability. Local reaction is generally modest, but skin breakdown can occur. Unfortunately, responses are usually short-lived, with most lesions progressing after a few months.
Topical 9-cis-retinoic acid (alitretinoin) gel has also been approved for treatment of cutaneous KS based on a randomized placebo-controlled trial in 139 patients, which demonstrated a sixfold higher response rate in the retinoic acid–treated group, as compared with the placebo-treated group.79 The difficulty with this medication is its potential for inducing an irritating local reaction when applied to normal skin. Consequently, patients must be counseled to be fastidious in their application of the compound exclusively to affected skin. Even with such care, some patients develop local reactions that may be troubling. Responses are not immediate, and may not be observed for 1–2 months after initiation of treatment, so patience is required. Of note, the same compound appears to have activity when given systemically. In two open-label multicenter trials, the tumor response rate was nearly 40%.80 Headache, dry skin, hyperlipidemia, and pancreatitis were notable toxicities.
Radiation therapy
Radiation therapy using either orthovoltage or electron beam may provide highly effective locoregional control of KS, even at relatively low doses. The great majority of treated patients will experience an objective response, many of which will be complete. A French study of 643 patients with AIDS-associated KS found a complete response rate of 92% when 20 Gy was delivered over 2 weeks, followed 2 weeks later with 10 Gy over 1 week.81 Modifications in dose schedule may reduce the complexity for patients. A 36-person study administered a total of 21 Gy in thrice weekly fractions for 2 weeks, achieving an overall response rate of 91% with complete response rate of 80%.82 Treatment with as little as one 8 Gy fraction has been reported to achieve a response in approximately three-quarters of patients.83, 84 Tolerance of therapy is generally good, though acute and late toxicities may occur including increased edema, ulceration, and chronic skin injury. Caution must be used when targeting mucous membranes, as the sensitivity of patients with AIDS to mucositis from radiation therapy appears to be heightened, and debilitating complications can occur.
Photodynamic therapy is an experimental modality employing light activation photosensitizing drugs to result in local tumor necrosis. A study of the photosensitizer photophrin in nearly 350 KS lesions yielded a 96% response rate, one-third of which were complete.85
Cryotherapy
Liquid nitrogen cryotherapy has been evaluated in a phase II clinical trial for local control of cutaneous KS.86 Twenty patients had 2–4 lesions and could be treated every 3 weeks until achievement of maximal response. Complete responses were observed in 80% of lesions with an average of three treatments per lesion (range 1–8). A subsequent retrospective analysis of 30 patients reported a complete response rate in 63% of patients with a mean of 3 sessions required per patient, and many of the remissions were sustained.87 Blistering occurs frequently with this technique, but bleeding, pain, and infection are uncommon. Liquid nitrogen cryotherapy can therefore be considered an effective and well-tolerated local therapy for discrete symptomatic KS lesions. Cosmetic effects of treating lesions in highly visible sites should be considered, however, as the blistering can be unsightly for days to weeks.
Systemic antitumor chemotherapy
Systemic therapy for KS is appropriate for advanced symptomatic disease, particularly for those patients with edema, extensive mucocutaneous disease, and pulmonary or symptomatic GI involvement. Type 1 interferons have demonstrated efficacy owing to their antiviral, antiproliferative, antiangiogenic, and pro-immunologic activities, though this treatment is rarely employed due to the slow onset of action and poor tolerability.88 Single-agent and combination cytotoxic chemotherapies are most commonly employed for those patients with symptomatic or organ threatening disease who are not candidates for local therapy or cART alone. Effective agents in this disease include doxorubicin, bleomycin, etoposide, taxanes, the vinca alkaloids, and gemcitabine. Combination strategies with either bleomycin and vincristine (BV) or doxorubicin (adriamycin), bleomycin, and vincristine (ABV) initially demonstrated tumor response rates of 57–88%.89, 90 The definition of response to chemotherapy in KS has historically been more ambiguous than with most other tumors because of the limitations of the bidimensional measurement in cutaneous, mucosal, and visceral lesions. KS can undergo complete regression of identifiable tumor on histology, yet the region of hyperpigmentation may not change in size. What does change is the nodularity of the lesion, the color characteristics (from a violaceous or salmon color to a gray-brown hemosiderin stain), and, when present initially, associated edema. Responses in the early literature are difficult to assess due to lack of uniform response criteria, while more recent studies have benefited by the introduction of standard response criteria initially defined within the ACTG and refined by a joint effort of the AIDS Malignancy Consortium, the National Cancer Institute, and the US Food and Drug Administration (FDA).
Combination cytotoxic therapy with ABV has served as a standard for comparison with newer treatment regimens. Although this regimen produces a substantial response rate, side effects are common, most prominently nausea, alopecia, fatigue, peripheral neuropathy, acral cyanosis, Raynaud phenomenon, cytopenias, and infection.91 These toxicities can be quite debilitating in a patient population on numerous other medications and often dealing with numerous other medical problems. The impetus for an active, but more easily tolerated, treatment program has therefore been particularly acute in KS. The liposomal encapsulated anthracyclines have provided that option and have emerged as a highly effective and tolerable treatment option. The leaky vasculature composing KS lesions predisposes to deposition of the drug, and lesion concentrations of drug have been shown to be almost an order of magnitude higher than in noninvolved tissue.92 Furthermore, the side effect profiles of these agents are more favorable. Two phase III studies with approximately 250 HIV-related KS patients in each found liposomal doxorubicin monotherapy superior to traditional cytotoxic combinations of either ABV or BV. Liposomal doxorubicin achieved responses in 46–58% of patients, compared to 25% with the traditional two- and three-drug combinations.93, 94 Time to response and tolerability were both improved in the liposomal doxorubicin–treated patients, as was health-related quality of life,95 though survival differences were observed. A large randomized study comparing the traditional ABV combination with liposomal daunorubicin found the liposomal drug to have less toxicity, but the tumor response rate was equivalent and not as high as that found for liposomal doxorubicin.96 A small trial compared the liposomal formulations of doxorubicin and daunorubicin, with the liposomal doxorubicin appearing superior, but the trial was too small to draw definitive conclusions.97 The overall body of evidence supports the use of liposomal doxorubicin as an initial chemotherapy of choice in advanced symptomatic KS; standard dosing is 20 mg/m2 every 2–3 weeks until maximal response or unacceptable toxicity.
Taxanes act upon neoplastic cells by stabilizing microtubules, and have emerged as highly active, well-tolerated agents for KS. After showing encouraging activity and safety in a phase I trial,98 phase II studies in previously treated patients with KS showed response rates of 56–71% with median durations of response of 9–10 months, longer than has been seen with any other therapy.99 Responses are seen in anthracycline-treated patients, and patients can tolerate low-dose paclitaxel (100 mg/m2 every 2 weeks) extremely well. Given the excellent efficacy and tolerability of paclitaxel, this was compared to liposomal doxorubicin in a randomized controlled trial as initial therapy.100 Seventy-three patients were enrolled, three-quarters of whom were on concurrent cART. The overall response rates for paclitaxel and liposomal doxorubicin were 56% and 46%, respectively, which was not a statistically significant difference. The majority of patients in both arms who presented with pain or KS-associated edema had clinical improvement with their respective therapy. The median progression-free survival was 18 months for paclitaxel and 12 months for liposomal doxorubicin (p = 0.66). Overall survival was identical in both arms, and both agents were well tolerated overall. Based on the aggregated data, either liposomal doxorubicin or paclitaxel can be considered an appropriate frontline systemic therapy for KS, with the alternate agent reserved for therapy at relapse if required. At our center, we typically employ liposomal doxorubicin as frontline therapy if there are not contraindications to anthracycline use, and reserve paclitaxel for relapsed disease. For patients who quickly progress off therapy, paclitaxel can be continued as ongoing maintenance therapy; and some patients have received this therapy for over 2 years at our center, with ongoing excellent tumor control. The newer taxane, docetaxel, also has demonstrated efficacy in advanced KS, including patients who have previously received anthracyclines or even paclitaxel, though neutropenia has been a problem.101, 102 We currently would not recommend docetaxel outside of a clinical trial.
Novel therapies
The highly vascular nature of KS lesions and their ready accessibility to study have made KS a candidate disease for assessment of anti-angiogenesis agents. A number of clinical trials involving a range of different agents have occurred or are under way. Bevacizumab is a monoclonal antibody against VEGF-A that was evaluated in a phase II clinical trial of HIV-associated KS patients who did not respond to cART alone.103 This was an overall high-risk population and the majority of patients had previously been treated with chemotherapy (most commonly liposomal doxorubicin), with a median time since last chemotherapy of 3 months. Bevacizumab was administered intravenously at 15 mg/kg on a 21-day cycle to a total of 17 subjects. The overall response rate was 31%, with 3 patients achieving a complete response. The median number of cycles administered was 10 (range 1–37), and the median time to progression was 8 months. Given the high-risk and pretreated nature of the study population, these results suggest encouraging activity of bevacizumab in HIV-associated KS. Studies combining bevacizumab with other agents are ongoing. The agent thalidomide has a number of properties, including inhibiting angiogenesis, and has been assessed in early-phase studies in patients with KS. A phase II study with 20 patients demonstrated partial responses in 8 patients and a median time to progression of 7 months.104 Neutropenia, depression, somnolence, and neuropathy were notable toxicities. The next-generation agent lenalidomide has shown responses in case reports, and a prospective trial for lenalidomide in KS is ongoing.105, 106
Membrane metalloproteinases (MMPs) are overexpressed in KS cells and may facilitate tumor invasion and metastasis.107 The MMP inhibitor COL-3 demonstrated efficacy and safety in a 75-patient randomized phase II trial of low-dose and high-dose oral COL-3.108 The overall response rate was 41% in the low-dose and 29% in the high-dose group, with responses correlating with decline in plasma MMP levels. Rashes and photosensitivity were the most frequent adverse events.
KSHV/HHV8 induces the PI3K/AKT/mTOR pathway, resulting in pro-proliferative and anti-apoptotic effects which contribute to KS pathogenesis.53 Targeting of the mTOR pathway has therefore garnered attention as a rational therapeutic target in this disease. Enthusiasm for this strategy was generated by a report of 15 patients with KS post renal transplantation who had a striking 100% complete response rate when immunosuppression was changed from cyclosporine to sirolimus.109 A small study by the AIDS Malignancy Consortium of seven subjects with HIV-associated KS on cART showed responses in three subjects, all of whom were on protease-inhibitor-containing therapy which resulted in higher sirolimus exposure.110 The drug was well tolerated and did not result in significant changes in HIV viral load or a significant decrease in CD4 counts. Additional data will hopefully further inform the role of this agent in HIV-associated KS.
Tyrosine kinase inhibitors have attracted attention as a possible treatment strategy in KS due to the role of platelet-derived growth factor (PDGF) and c-KIT activation in KS biology.111, 112 The AIDS Malignancy Consortium conducted a 30-person phase II trial of imatinib mesylate in HIV-associated KS.113 Seventy-seven percent of patients had prior cART, and 57% had prior chemotherapy for KS. The overall response rate was 33%, all partial responses, with a median time to response of 21 weeks. Among responders, the median duration of response was 9 months.
The viral process underlying KS and the relationship of the disease to immune function also suggests that immunologic approaches may ultimately have therapeutic value. KSHV/HHV8 can alter both macrophage and CTL function. Macrophage inhibitory chemokines are encoded by the virus and produced in KSHV/HHV8-infected cells. The cells also produce an OX-2 homolog termed K-14. OX-2 receptors are present on monocyte/macrophages and stimulation dampens the ability of macrophages to be activated by other stimuli. K14 is capable of reducing activated macrophage production of inflammatory cytokines, possibly restricting host response to KSHV/HHV8.114 In addition, KSHV/HHV8 encodes genes that alter the ability of infected cells to be targeted by CTLs. The K3 and K5 viral gene products limit CTL engagement of MHC class I molecules, and K5 also inhibits interaction with the coreceptor (B7) complex necessary for CTL activation.115 However, a number of reports have defined epitopes within KSHV/HHV8 gene products that are recognized by CTLs.116, 117 Definition of whether reactivity to certain epitopes associates with protection from disease and whether immune inhibitory pathways may be further considered if a vaccine or adoptive cell therapies become of interest in this disease.
Non-Hodgkin lymphoma (NHL)
Lymphoproliferation occurs in the context of immunodeficiency of many different types. It is a common complication in individuals born with congenital abnormalities of T-cell function, individuals receiving immunosuppressive medications for organ transplantation, and in individuals with HIV infection. The common theme among these is the role of EBV going unchecked in its ability to induce B-cell proliferation. However, only a minority of ARL resembles the lymphoproliferative disease of the congenital or post-transplantation setting. ARL comprises a complex set of tumors with challenging clinical scenarios. It has been, and remains, the most lethal complication of HIV infection, demanding new approaches and new understanding of the pathophysiology underlying it. The most common ARL is DLBCL, followed by BL, PCNSL, primary effusion lymphoma (PEL) plasmablastic lymphoma (PBL), and rarely peripheral T-cell lymphomas (PTCL). These entities show significant variation in incidence based on the depth of HIV-related immunosuppression.
Epidemiology
The association of NHL with HIV infection was evident within the first half-decade of the AIDS epidemic, when an unusual number of lymphomas among young men became evident in cancer registries in California. The first revision of the definition of AIDS by the CDC, in 1987, included NHL, and it has remained an important and devastating manifestation of HIV infection. Unlike KS, in which select subsets of HIV-infected individuals have a unique risk, NHL is more uniformly distributed among risk groups for HIV, with little variation. What variation is present may be attributable partly to issues of care.
The risk of NHL among HIV-infected individuals is in part determined by the level of immunosuppression, with higher risk noted among those with low CD4 cell counts; however, specific subsets of lymphoma have a stronger association with severe immunosuppression than others. The occurrence of PCNSL is restricted to those with very advanced immunodeficiency, as are the PEL and PBL subsets of systemic ARLs. These manifestations of profound immune dysfunction have significantly decreased in incidence since the advent of cART. Among the other ARLs, DLBCL has declined in incidence due to cART, but continues to be the most common ARL. Unlike other subtypes of ARL, BL occurs most commonly in the setting of preserved immune function, and constitutes an increasing proportion of ARLs over time as individuals enjoy longer lives with well-controlled HIV.26, 118 The median CD4 counts in most treatment reports of ARLs are in the range of 50–100 cells/mm3, with the BL group often having a CD4 count >200 cells/mm3. In contrast, reports of patients with PCNSL demonstrate profound CD4 count depression).26, 119 Thus, NHL may occur across a broad range of contexts in HIV disease, including those with virtually no other manifestations of immunosuppression, and may be the presenting illness of HIV infection. Possible unrecognized HIV infection should therefore be considered in any person presenting with an NHL histology that may occur in the context of HIV infection. The duration and severity of persistent immunosuppression is important in determining risk for developing an ARL, with a relative hazard of approximately 1.4 for each 50% decline in CD4 count. Reassuringly, as the CD4 count responds to cART, the risk of lymphoma reduces, but there is a lag time of approximately 1 year, somewhat longer than for other opportunistic diseases.21 Interestingly, the reduction in risk appears to proceed with longer time on cART in parallel and likely reflects the gradual repair in immune function seen over long periods of time on cART. Immune function is accompanied by HIV ribonucleic acid (RNA) level as a predictor of ARL risk. In the EuroSIDA study, each log of HIV RNA was associated with a relative hazard of 1.51.21 Therefore, controlling HIV is a critical determinant of ARL, presumably because of the immunologic alterations it engenders, though other pathophysiologic mechanisms may also participate.
Pathology and pathogenesis
The development of lymphoma among HIV-infected individuals is virtually always associated with transformation of a B cell, a cell type that HIV itself is unable to infect. Thus, HIV does not play a direct role in the development of most ARL; rather, it provides the background immunosuppression either for transforming viruses or for proliferative triggers for B cells to go unabated. The rare exception to this is a small subset of tumors of T-cell origin (PTCL).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree








