(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Molecular pathology of bladder cancer (BlCa)
Circulating cell-free nucleic acids as BlCa biomarkers
Circulating BlCa miRNA biomarkers
Circulating BlCa serum protein biomarkers
Circulating BlCa cells
Key Points
Being the most commonly diagnosed urinary tract cancer, BlCa is among the top ten cancers in the world. Early stage low-grade tumors are associated with high 5-year survival rate of >90 %. However, this survival rate is only ~6 % for advanced stage tumors, emphasizing the need for early detection.
Non-muscle invasive bladder cancer (NMIBC) and muscle invasive bladder cancer (MIBC) may originate from different basal stem cells with distinct genetic lesions. NMIBCs are genetically stable and harbor FGFR3 mutations, while MIBCs are genetically unstable with TP53 mutations.
Potential circulating biomarkers of BlCa are ctDNA, miRNA, serum proteins, and circulating BlCa cells.
11.1 Introduction
Being the most commonly diagnosed cancer of the urinary tract, the 2012 global estimated cases of bladder cancer (BlCa) were 330,380 for men and 99,413 for women. For both sexes, however, BlCa is the ninth in incidence (429,793 for 2012) and thirteenth cause of cancer-related mortality (165,084 for 2012). But for men, it is the ninth course of cancer-related deaths (123,051 for 2012). In the United States, the projected 2016 estimates for BlCa are 58,950 and 18,010 for men and women, respectively, while mortality figures stand at 11,820 for men and 4,570 for women. Although the incidence of BlCa has remained quite stable over the past few years, it is among the top ten most commonly diagnosed cancers in the United States.
BlCa is a disease mostly caused by environmental carcinogenic effects on the genome. Tobacco smoke carcinogens cause a substantial (~50 %) number of BlCas. Additionally, occupational chemical exposures such as working in the rubber industry are established risk factors. Because of these risk associations, the prevalence of BlCa is five times higher in men than women. Additionally, this is a disease of the aged, having a median diagnostic age of 65.
Over 90 % of BlCas are urothelial carcinomas. Many (~75 %) of these are diagnosed early when the lesion is confined to the mucosa and submucosa. These tumors are referred to as non-muscle invasive BlCas (NMIBC). The remaining 25 % of BlCas are MIBC, whereby cancer cells will have already infiltrated the muscle wall of the bladder. But ~70 % of these NMIBC patients will develop recurrences within 2 years after treatment as a consequence of field cancerization (and tumor evolution), whereby almost the entire urothelium is exposed to the same carcinogen and hence at risk of developing cancers in the future. BlCas are also dichotomized into low-grade and high-grade tumors based on tumor biology. Low-grade tumors are superficial papillary multifocal tumors that may progress to advanced stage disease. These tend to be associated with good prognosis. High-grade tumors are nodular, and tend to invade early, and hence are associated with adverse outcomes. In general, early stage in situ BlCas or low-grade tumors have a favorable outcome with a 5-year survival rate of about 94–96 %. But this survival rate is markedly reduced to ~6 % in advanced stage disease.
Early stage BlCa is usually asymptomatic. Clinical suspicion is made in patients presenting with blood in the urine (hematuria), increased frequency (polyuria) and urgency of urination, or discomfort/pain on urination. But these symptoms are nonspecific to BlCa and could occur in other bladder conditions such as bladder infection. Thus, a diagnostic work-up including urine cytology, cystoscopy, and possible biopsy of suspicious lesions for histopathologic evaluation is needed. But early BlCa may not shed cancer cells into the urine, thus making urine cytology insensitive (apart from issues with pathologist experience). Cystoscopy is invasive and may not pick up early lesions (without overt histopathologic appearance). Thus, the need for authenticated early detection noninvasive biomarkers should improve the outlook, at least for patients with elevated risk factors. This has primarily been explored in urinary samples for BlCa screening and management, but circulating biomarkers have also been examined and could be useful in disease prognosis and treatment decision-making.
11.2 Molecular Pathology of BlCa
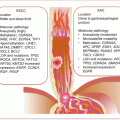
Urothelial BlCas are dichotomized based on histopathologic and molecular characteristics into NMIBC and MIBC. NMIBCs develop from epithelial hyperplastic lesions that progress to low-grade superficial tumors. On the other hand, flat dysplastic lesions that progress to carcinoma in situ give rise to MIBCs. These two pathologic subtypes also have distinct and overlapping molecular pathology. Evidence suggests BlCa develops from two possible BlCa stem (or tumor-initiating) cell lineages. A population of SHH-expressing basal cells with tumor-initiating cell features has been identified in mouse models [1]. Additionally, non-basal tumor-initiating cells are present in the urothelium. Putatively, the duality of this population of cells belies the two pathways, with NMIBC and MIBC emanating, respectively, from the non-basal and basal stem cells niches [2].
The permissive state for progression into either tumor subtype appears to be 9p/9q loss. Further molecular progression of the two pathways can be described simplistically as (Fig. 11.1):
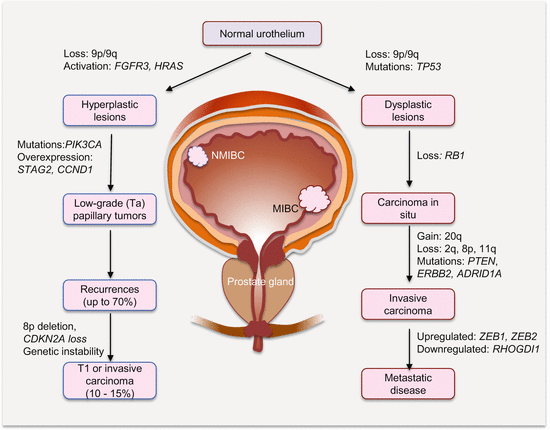
Superficial NMIBCs are mostly characterized by FGFR3, PIK3CA, and HRAS alterations leading to RAS-MAPK and PI3K pathway activation. These signaling pathways then mediate increased cell proliferation and survival with subsequent development of hyperplasia and low-grade tumors. A significant number (~70 %) will recur, of which a small percentage (10–15 %) may acquire further genetic lesions (e.g., 8p loss, TP53, and RB mutations) and instabilities to become high-grade MIBC.
LOH at 9p/9q in association with deregulated cell cycle genes (e.g., TP53 mutations) characterizes flat dysplastic lesions that often progress to CIS with loss of RB1 functions. Subsequent gain at 20q, losses at 2q, 8p, and 11q in association with mutations in PTEN, ERBB2/HER2, and ARID1A causes progression to invasive disease. Metastasis is mediated by alterations in EMT (e.g., CDH1, ZEB1, ZEB2, and MMP9), as well as angiogenic and inflammatory (e.g., Cox2, RHOGDI2, and VEGF) gene expression.

Fig. 11.1
Molecular progression pathways of NMIBC and MIBC. Not shown is the existence of a mixed population of hyperplastic and dysplastic clones that can progress to papillary Ta high-grade tumors
However, this simplistic dichotomized progression model cannot explain the heterogeneity of BlCas. Tumors with features of both types exist. Indeed, within each pathway are different BlCa subtypes, which suggest there are alternative progression pathways. Also low-grade papillary tumors (NMIBC) and their recurrences can display features of high-grade T1 lesions and invasive carcinomas (MIBC). The progression from NMIBC to aspects of MIBC usually involves acquisition of genetic instability (e.g., 8p loss) and deregulated cell cycle control genes (e.g., CDKN2A, TP53, and RB loss of function). A mixed dysplastic/metaplastic lesion progression to papillary high-grade lesion is also possible.
11.2.1 Epigenetic Alterations in BlCa
The epigenome is deregulated in BlCa and has been explored for discovery of noninvasive biomarkers. In addition to miRNA deregulation, aberrant methylation and histone modifications characterize both NMIBC and MIBC. DNA hypomethylation in non-CpG islands is common in NMIBC, while CpG island hypermethylation and gene silencing are a feature of MIBC. Sequencing data has revealed mutations in genes involved in chromatin regulation in ~90 % of MIBC. Commonly mutated and inactivated are MLL3 involved in histone H3K4 methylation and KDM6A that demethylates H3K27 (both create a euchromatin state). Also commonly mutated is a member of the SWI/SNF chromatin-remodeling complex, ARID1A.
11.2.2 Chromosomal Alterations in BlCa
While NMIBC tends to display diploid chromosomes indicative of limited genomic instability, MIBC is characterized by aneuploidy, with possible chromothripsis (massive chromosomal damage from a single mutational event) in some cases. Several technical analyses, including FISH, LOH, SNP, CGH, and genome sequence copy number, have uncovered several chromosomal variations in BlCa. Chromosomal deletions, in particular at 9p and 9q and also at 11p, 13q, 14q, and 17p, underlie BlCa development. Chromosome 9 deletions occur in 30–60 % of BlCas and are a major alteration frequently observed in superficial papillary and invasive flat tumors. Deletions at 2q, 5q, and 8p are common in aggressive disease, and gains at 3q, 7p, and 17q, as well as deletions at 9p21, have been identified as clinically relevant diagnostic and prognostic biomarkers. Because of the pathogenic role of 9p/9q loss in BlCa, a number of relevant tumor suppressor genes at these loci have been identified. The following chromosomal locations contain some important genes involved in BlCa: 9p21 (CDKN2A and CDKN2B), 9q22 (PTCH), 9q32–33 (DBC1/BRINP1), and 9q34 (TSC1).
11.2.3 FGFR Alterations in BlCa
FGFR3, and to a lesser extent FGFR1, demonstrates alterations in BlCa. FGFR aberrations induce MAPK and PLCγ signaling leading to cellular proliferation, growth, and survival. Hence FGFR activation is mostly associated with NMIBC. The majority (~80 %) of superficial NMIBC harbors activating FGFR3 point mutations. However, 10–20 % of T1 and invasive BlCas also demonstrate these mutations. While the mechanisms mediating the development of this subset of BlCas (FGFR3 mutant invasive BlCa) are not well established, these tumors frequently harbor CDKN2A homozygous deletions.
The increased FGFR signaling in BlCa can be explained by several mechanisms:
As noted, activating FGFR3 mutations occur in a vast majority of these cancers.
Expression of FGFR3 and/or its ligand is increased in BlCa. Increased expression of FGFR3 could be augmented by a SNP in TACC3, which is separated from FGFR3 by 70 kb DNA sequence. This conclusion is because this SNP is associated with BlCa risk and recurrence of Ta disease with FGFR3 mutations.
Chromosomal translocation and formation of fusion genes occur in a subset (~5 %) of BlCas. These fusions often involve FGFR3IIIb isoform kinase domain in frame to oncoproteins. These oncoproteins include TACC3 and possibly BAI1-associated protein 2-like 1 (BAIAP2L1), leading to increased FGFR3 activation.
Isoform switches can augment FGFR3 signaling. FGFR3 exists in different isoforms. Isoform FGFR3IIIb, which is expressed by normal urothelial cells, interacts with FGF1 ligand to control the levels in the urothelium. Another mechanism of reducing the levels of ligand is the expression and secretion of a spliced variant, FGFR3Δ8–10, which binds and sequesters FGFs. In BlCa, a different isoform, FGFR3IIIc, is overexpressed, in association with downregulation of isoforms FGFR3IIIb and FGFR3Δ8–10. Because isoform FGFR3IIIc interacts with several FGF ligands, increased expression can mediate ubiquitous autocrine and paracrine signaling in urothelial cells.
Unlike BlCas with epithelial features, some BlCas overexpress FGFR1 and FGF2 ligands, and these tumors demonstrate EMT phenotypes. Of interest, levels of FGFR1β, which has increased sensitivity to FGF1 ligand, and also activates the MAPK and PLCγ pathways through interaction with FGF2, are much higher than FGFR1α spliced variant in these tumors.
11.2.4 MAPK Pathway Alterations in BlCa
The MAPK pathway is activated in BlCa, especially NMIBC. Multiple mechanisms may explain pathway activation. Mutations in RTKs (e.g., FGFR3, RAS, and PIK3CA) are very frequent (>80 %) in early stage (Ta–T1) tumors. While RAS and FGFR3 mutations are mutually exclusive in BlCa, suggestive of functional redundancy, the disproportionately more FGFR3-mutant than RAS-mutant BlCas also indicates a possible selective need or oncogenic mechanisms not yet understood. Another poorly understood observation is the distribution of these mutations between NMIBC and MIBC. More NMIBCs harbor FGFR3 than RAS mutations. But RAS mutations occur at similar frequencies in both cancer types. However, FGFR3 and PIK3CA mutations tend to occur together in NMIBC and may function to induce both the MAPK and PI3K pathways.
11.2.5 PI3K Pathway Alterations in BlCa
BlCa is also characterized by activation of the PI3K pathway. In ~25 % of NMIBC, the PIK3CA is mutated and activate this pathway. Mutations in PIK3CA may cooperate with RAS in orchestrating signaling networks in BlCa. Also PTEN function is commonly lost in MIBC (LOH is more common than biallelic loss). PTEN loss is associated with TP53 loss, and both may cooperate at some level in the development of invasive disease. Apart from these mutations, several other mechanisms are involved in PI3K pathway activation. EGFR/ERBB1 activates the pathway through RAS. ERBB2/ERBB3 heterodimers may activate the pathway through the interaction of ERBB3 with p110α. Functionally, ERBB activation of the pathway mediates metastasis. Additionally, mutations in TSC1 and activation of MET and RON/MST1R can activate the PI3K pathway in BlCa. While a role for FGFR3 is not established in this pathway, increased levels of pAKT are associated with FGFR3-mutant tumors [3].
11.2.6 Cell Cycle Alterations in BlCa
The cell cycle is deregulated in BlCa, especially in MIBC, and this is due to myriads of controlling factors including altered signaling pathways and cell cycle regulators. Members of the cell cycle, especially those involved in G1/S phase transition, are abnormally expressed in nearly all MIBCs and are mostly associated with poor prognosis. Established tumor suppressors, including RB, TP53, and CDKN2A, are inactivated in MIBC. On the contrary, genes involved in propelling the cell cycle, including E2F3, MDM2, CCND1, and CCND3, are amplified or overexpressed in many MIBC. For example, amplification of CCND1 occurs in ~20 % of BlCas [4], while The Cancer Genome Atlas data demonstrate inactivation of TP53 in ~76 % of MIBC. Thus, abnormal cell cycling underlies BlCa proliferation and invasiveness.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree