(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Risk factors of EsCa
Screening for esophageal cancer (EsCa)
Molecular pathology of EsCa
Circulating EsCa biomarkers
Circulating EsCa cells
Key Points
EsCa remains a disease characterized by dismal outcomes. Geographically, esophageal adenocarcinomas (EACs) are more common in the resource-rich parts of the world, while those of squamous cell histology (ESCC) are more prevalent in middle- to low-income countries. The multistep EsCa progression model offers opportunity for noninvasive early detection; however, Barrett’s esophagus is monitored by invasive endoscopic evaluations.
Noninvasive biomarkers such as epigenetic alterations (e.g., promoter methylation of CDKN2A), miRNA deregulation (e.g., miR-18a), changes in protein levels (e.g., CYFRA 21-1), metabolite profiles (e.g., alterations in ketone bodies and glycolysis), and circulating EsCa cells should augment current efforts at disease detection and management.
Circulating biomarkers are being pursued. However, while the molecular genetics of EAC progression is much better characterized, circulating biomarkers have been more extensively studied in ESCC.
5.1 Introduction
Cancer of the esophagus has a major global presence. As the eighth and sixth cause of global cancer diagnosis and deaths, respectively, 456,000 new people were afflicted with 400,000 deaths in 2012. The 2016 estimated incidence and mortality are 16,910 and 15,690, respectively. Notice how the incidence closely mirrors the mortality, which is indicative of a cancer that has a high case fatality rate. Indeed, the 5-year survival is woefully 5–15 %. It is the third cause of gastrointestinal cancer mortalities. The global distribution of cases, which differs by 20-fold in order of magnitude between countries, reflects on the various etiologic risk factors. Seventy-five percent of all cases are diagnosed in Asia, with ~80 % of the global cases being clustered in the less developed world, where most of the mortalities also occur. Thus, attention to prevention, early diagnosis, and improved interventional measures need to be focused on this sector of the global population and not the currently otherwise observed.
There are two major pathologic subtypes of esophageal cancer (EsCa), esophageal squamous cell carcinoma (ESCC) and esophageal adenocarcinoma (EAC). These two subtypes differ in their global distribution patterns, clinical and molecular features, as well as predisposing risk factors. The highest rate of EsCa, primarily ESCC, occurs in the less developed world, mainly in Eastern Asia, Southern and Eastern Africa, and what is described as the “esophageal cancer belt,” which extends from Northern Iran through Central Asia into Northern and Central China. The lowest rates are observed in Central America and Western and Middle Africa. On the contrary, EAC is more common in the developed than the developing world. Obesity, which may be associated with gastrointestinal reflux disease (GERD) and Barrett’s esophagus (BE), may help explain this prevalence. Lifestyle is a major contributor to EsCa, because in many Western countries, EAC is on the rise (mirroring the increase incidence of obesity), while ESCC is declining (due to declining tobacco use and moderate alcohol consumption). The reverse appears true for the developing world where smoking and alcohol use are on the rise, and hence the increasing incidence rates of EsCa.
As alluded to above, lifestyle plays a major role in the etiology of EsCa. Thus, to decrease the current rising trend, health educational and motivational efforts should be implemented to help people change the known risky habits. Complementing these efforts will be biomarkers that can play a key role in primary and secondary prevention. Moreover, while BE is a known risk factor for EAC, the majority of people with BE do not progress to develop invasive cancer and yet are subjected to the associated anxieties and importantly invasive endoscopic surveillance procedures. The clinical availability of validated biomarkers that can be used to stratify people with BE into “progressors” and “nonprogressors” will be invaluable in improving the current dismal outcomes of EsCa. Given that the majority (~80 %) of EsCas occur in the less industrialized world where endoscopy is not readily available, biomarkers that can be assayed noninvasively, especially in body fluids such as plasma, serum, and saliva, or otherwise, will make an even much impact on the global burden of this affliction. This chapter examines the molecular pathology of EsCa and the current efforts at identifying and developing circulating biomarkers for this disease.
5.2 Risk Factors of EsCa
Although some overlap exists, such as tobacco and alcohol use, which may reflect on some of the identical molecular alterations, the risk factors for ESCC and EAC are quite different. Risk factors for both types of cancer include age (50+ years), thoracic radiation, and male sex (male:female ratio of 3:1 for ESCC and 8:1 for EAC). Familial predisposition is rare for ESCC but could be as high as 7 % for BE and EAC in European population.
Heavy alcohol consumption and smoking are established risk factors for ESCC. For alcohol use, the risk is further elevated especially in people with loss of activity of aldehyde dehydrogenase 2 (ALDH2). The glutamine polymorphic change to lysine at position 487 in ALDH2 identified in Eastern Asians inactivates the protein. Drinking hot beverages or liquids is an identified risk factor for ESCC. This is one theoretical explanation for the increased ESCC in the “esophageal cancer belt.” Achalasia (the incomplete relaxation of the lower esophageal sphincter on swallowing) is strongly associated with ESCC, while accidental caustic injury, especially during childhood, is associated with either ESCC or EAC. Other implicated risk factors for ESCC are fungal contamination of food, consumption of food poor in vegetables and fruits but high in nitrosamines, celiac disease, esophageal diverticula, and HPV 16 and 18 infections, as well as tylosis.
Chronic GERD that may lead to BE, increased body mass index (BMI), and smoking are proven risk factors for EAC. The frequency of GERD symptoms is associated with the risk of progressing to BE. In comparison to those who experience infrequent symptoms, weekly symptoms are associated with a fivefold risk, but this increases to sevenfold in if GERD symptoms occur daily. Smoking is associated with a relative risk of ~2.32 compared with nonsmokers, and BMI ≥ 35 confers a hazard ratio of 3.67 compared with those with normal BMI. Drugs such as β-blockers and anticholinergics that relax the gastroesophageal sphincter elevate the risk of EAC due to increased reflux.
5.3 Screening Recommendations for EsCa
The best approach to reducing the incident rates of EsCa is obviously prevention (avoidance of the lifestyle risk factors). Because the risk factors are different for ESCC and EAC, different interventional approaches are required. In the developed world, >90 % of ESCCs are caused by excessive alcohol and tobacco use. Conclusively, smoking cessation has reduced the incidence of ESCC in the developed world. GERD, especially in chronic situations, is associated with BE, both of which are risk factors for development of EAC. However, the eradication of GERD and the associated reductions in the development of EAC are uncertain. But, as established risk factors, both GERD and BE warrant close monitoring for possible early detection of cancer.
In view of the grounded knowledge of chronic GERD and BE being risk factors for EAC, several organizations have recommended screening and surveillance procedures for people with these conditions. These organizations all agree on the recommendation that patients with chronic GERD and associated EsCa risk factors such as age (≥50 years), male sex, white race, obesity, and the presence of hiatal hernia should have endoscopic evaluation of the upper gastrointestinal tract. The American Society for Gastrointestinal Endoscopy (ASGE) further refines these guidelines to include the following:
Surveillance should stop in the event that there is no BE or EAC.
In patients with non-dysplastic BE, surveillance intervals should be 3–5 years, and lesions should be biopsied for evaluation.
Repeat biopsy in 6 months in patients with low-grade dysplasia (LGD) to confirm diagnosis and annually thereafter with biopsy of lesions. Consider lesion ablation at this time.
In patients with high-grade dysplasia (HGD), endoscopic resection or radiofrequency ablation (RFA) of flat lesions is indicated. Surveillance should only be offered for those patients unfit or unwilling for surgery or RFA therapy.
To these surveillance and treatment guidelines by the ASGE, the American Gastroenterological Association (AGA) adds the following surveillance guidelines intervals:
In the absence of dysplasia, repeat endoscopy in 3–5 years.
In the presence of LGD, repeat endoscopy in 6–12 months.
In the presence of HGD, for patients without eradication therapy, repeat endoscopy every 3 months.
The American College of Physicians issued in 2012 the following recommendations for endoscopic evaluation of patients with GERD:
Endoscopic screening should not be offered to women of any age or men <50 regardless of risk factors, due to the low cancer incidence in these populations.
Screening should be offered to both men and women with GERD, especially those associated with dysphagia, bleeding, anemia, weight loss, or recurrent vomiting.
Surveillance should be stopped in those who have negative findings for BE on endoscopy.
In patients with BE without dysplasia, surveillance should occur at 3–5-year intervals. For those with dysplastic lesions, surveillance intervals should be shorter.
Although there is evidence that some people can develop EAC without the classic metaplastic sequence through BE, there is no recommendation for population-wide screening or at least for all men over the age of 50. Moreover, although >80 % of all EsCas occur in the resource-poor parts of world, where the subtype is primarily ESCC, there are no established recommendations for screening to detect early lesions of ESCC. This lack of generalized screening for the entire population is due partly to the disease prevalence and importantly the cost, invasive, and discomfort nature of endoscopy. Noninvasive body fluid biomarkers could make a difference, if developed and made available for screening of everyone above the risk age of 50.
5.4 A Place for Early Detection Biomarkers for EsCa
In patients with chronic GERD and/or BE, periodic endoscopic evaluation with detection and biopsy for grading of dysplasia is currently used to monitor risk for progression to EAC. Even with current improved endoscopic imaging techniques, including confocal laser endomicroscopy, narrow band imaging, and chromoendoscopy, there are still limitations to surveillance such as sampling errors or bias to this approach. The Seattle protocol attempts to provide gastroenterologists with a guide to comprehensive sampling, by recommending biopsy of all suspicious lesions, and four quadrant biopsy every 1–2 cm of the entire BE segment. While laudable, it has not been easily embraced, as complains surrounding this protocol stems from being time-consuming, tedious, and yet is still associated with sampling bias. Even if lesions are accurately sampled, histopathologic evaluation of biopsies are subject to the well-known inter-pathologist variable observations and opinions. Another issue with histopathologic evaluation and risk prediction is the fact that BE is not a boni fide index for developing EAC. While this invasive procedure is the recommended surveillance protocol for BE, the absolute risk for individuals to develop EAC following BE diagnosis is 1:200 per year (0.5 % or less per year). Indeed 90–95 % of patients with BE will not progress to EAC. The need to limit this invasive and costly procedure to BE patients at disproportionately high risk for progression is obviously laudable. However, endoscopy is not ubiquitous in the developing world where the majority of EsCas are diagnosed. Thus, an objectively measurable biomolecules (biomarkers) that can accurately predict the risk of lesion progression to either ESCC or EAC should complement histopathologic evaluations. Of even much better value and cost-effectiveness will be those biomarkers that can be evaluated in body fluids such as blood.
5.5 Molecular Pathology of EsCa
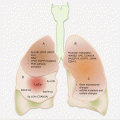
Cancers of the esophagus are anatomically mostly located in the distal two thirds. While ESCC is mostly found in the middle and lower thirds of the esophagus, EAC is mostly restricted distally close to the gastroesophageal junction (Fig. 5.1). In fact, it is often difficult to distinguish between cancers of the gastric cardia and EACs.
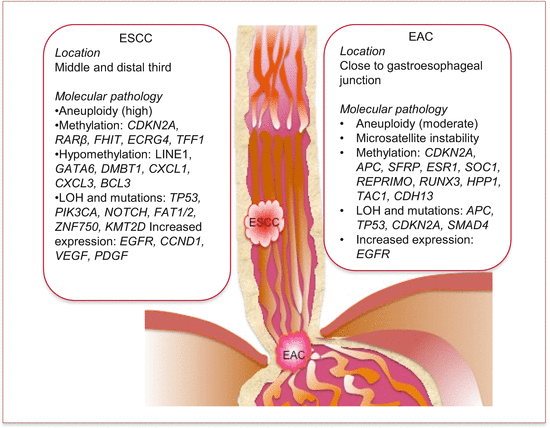

Fig. 5.1
Common anatomic locations and molecular pathology of esophageal adenocarcinoma (EAC) and squamous cell carcinoma (ESCC)
5.5.1 Barrett’s Esophagus
Barrett’s esophagus (BE), by definition, is the replacement of the stratified protective squamous epithelium of the lower esophagus with simple columnar epithelium. The metaplastic columnar epithelial cells are of three types, gastric-fundic type, cardia type, and intestinal type, that may contain goblet cells. Of the three, only the intestinal type of metaplasia carries a risk for progression and hence is the recommendation by the AGA and the American College of Gastroenterology for the diagnosis of BE. It is also established that noxious chemical substances including acid and bile from chronic GERD mediate the development of BE. The noxious chemical damage is mostly repaired by replacement with normal stratified squamous epithelium. But in some individuals (probably those with increased frequency of GERD episodes or with different types of noxious substances that are yet to be determined), the epithelium trans-differentiates into the columnar type. The columnar epithelium is intended to be protective against reflux-mediated damage but may progress to invasive cancer. The metaplasia has been thought of as being a consequence of recommitment of the esophageal cells. However, other findings suggest different pathologic mechanisms. The cells that eventually differentiate into the columnar epithelia are suggested to be of three different “stem cell” origins: (i) the proximal migration of stem cells from the gastric cardia into damaged esophageal epithelium [1], (ii) the docking of circulating bone marrow stem cells at the damaged epithelial site, and (iii) the presence of embryonic stem cells located at the gastroesophageal junction [2].
The intestinal-type metaplastic change in the epithelium is an established predisposing risk factor for the development of EAC. In reference to only BE patients, the development of dysplasia, especially high-grade form, carries a risk of 10 % annually for progression to EAC. This figure contrasts sharply with the 0.2–0.5 % annual risk in patients without dysplasia. Globally, the incidence of EAC is on the rise, which suggests the ineffective detection of many people with BE and BE with dysplasia. To help curtail this trend, people with GERD, especially those with chronic and frequent symptoms, should be screened for early detection of BE that will inform actionable surveillance plan.
The need for objective measures to help stratify BE patients into risk categories is urgent. Biomarkers are available for assessing various lesions for predicting the risk of progression. Immunohistochemical stains for cyclin D1, p53, β-catenin, and α-methylacyl-CoA racemase (AMACR) are in clinical use. The established epigenetic and chromosomal changes in BE are also used as single or panel biomarkers to complement surveillance protocols. These biomarkers include aneuploidy and increasing tetraploidy, LOH at 9p and 17p, and methylation assays for targeting tumor suppressor genes such as CDKN2A.
5.5.2 Esophageal Cancer
ESCC is the most common pathologic type worldwide. It is more prevalent in males than females, and the median diagnostic age is 65. The numerous risk factors culminate in creating inflammation of the esophagus, the so-called chronic esophagitis. Squamous esophageal tumors are often located in the middle or lower third of the esophagus. Similar to other EsCas, presentation is often late, leading to poor survival outcomes.
Globally, EAC is the second most common cancer of the esophagus. These tumors are located at the lower esophagus (close to the gastroesophageal junction), because they arise on the background of BE, especially when it progresses to high-grade dysplasia. Similar to ESCC, men are more frequently affected than women. Many patients present with late disease, whereby BE had not previously been diagnosed, and hence are often associated with poor prognosis. This is one of the rationales for the efforts to detect BE early, so that surveillance can be instituted for early detection of curable cancers.
5.5.3 Epigenetic Changes in EsCa
The epigenome is exploited early in EsCa progression. While these epigenetic alterations occur in both ESCC and EAC, they are better characterized in EAC. Evidence from BE, BE with dysplasia, and EAC conclusively demonstrates that these early epigenetic events in association with other genetic alterations drive EsCa progression.
5.5.3.1 Epigenetic Changes in EAC
Many genes, mainly tumor suppressor genes, are silenced through promoter hypermethylation in BE, BE with dysplasia, and EAC. While variable, many of the hypermethylation of these genes occur at a frequency of >50 % in EAC and at various reasonable frequencies in early lesions.
The tumor suppressor gene, CDKN2A, that encodes p16/INK4A is inactivated early in EAC development. This loss of function is due partly to promoter hypermethylation and LOH at 9p21, the CDKN2A locus. The early loss of this gene function in BE is associated with aneuploidy and LOH at 17p, the TP53 locus. Together, these genetic changes drive clonal expansion of BE cells to invasive cancer. Other epigenetic alterations that may be associated with clonal expansion are promoter hypermethylation in APC and ESR1 in BE and BE with dysplasia. Similarly, LOH and/or mutations in APC, CDKN2A, and TP53 in early lesions may drive clonal progression. The methylation frequencies of REPRIMO, SOC1, and SOC3 also suggest increased clonal expansion with disease progression. Promoter hypermethylation of REPRIMO is present in 36 % of BE cases but increases to 64 % and 63 % in BE with dysplasia and EAC, respectively [3]. Similarly, SOC3 is methylated at a frequency of 13 % in BE, 22 % in BE with low-grade dysplasia, 69 % of BE with high-grade dysplasia, and 74 % of EAC. SOC1 had similar progressive patterns of methylation, though at lower frequencies than SOC3 [4].
Of major importance will be biomarkers that inform disease progression status. To address this question, Schulmann et al. identified inactivation via methylation of a number of genes associated with predictive increased risk of disease progression from BE to HGD or EAC [5]. These genes are CDNK2A, HPP1, and RUNX3 with odd ratios of 1.74, 1.77, and 1.80, respectively. To improve disease stratification, the three methylated genes were combined with three other parameters, sex, BE segment length, and pathologic features, to generate ROC curves for prediction. For progression of BE to HGD or EAC, these ROC curves could accurately stratify patients into low-, intermediate-, and high-risk categories [6]. To further augment the predictive accuracy of these biomarkers for disease progression, a multicenter retrospective study included five more relevant genes that are methylated in early disease, to create an eight-marker risk-of-progression panel. Using these eight gene panel (CDKN2A, RUNX3, HPP1, TAC1, SST, NELL1, CDH13, and AKAP12), when the specificity was set at 90 % using ROC curves, the predictive sensitivity was ~50 %. While not the best, this demonstrates an improvement and indicates that the selection of an appropriate panel could improve detection of disease progression.
5.5.3.2 Epigenetic Changes in ESCC
Several genes demonstrate promoter hypermethylation in ESCC. The frequencies though tend to be lower in low-, intermediate-, and high-grade dysplastic lesions than ESCC. Unlike EAC, the frequencies of gene promoter hypermethylation in ESCC, except for a few such as CDKN2A, RARβ2, ECRG4, and FHIT, are generally low.
Global methylation studies using genome-wide approaches have been applied to EsCa. Agarwal et al. demonstrated that BE patients who progressed to EAC were more likely than those who did not, to harbor hypomethylation in growth-promoting genes, including genes involved in insulin-like growth factor signaling [7]. Hypomethylation of long interspersed elements 1 (LINE1) is a frequent feature in ESCC, which correlates with poor prognosis [8]. In general, outside CpG islands, methylation is low in BE and EAC. Another study of genome-wide methylation, coupled with CGH, found that loss of methylation was an early event in disease progression and that global hypomethylation in association with gene amplification and increased expression of GATA6, DMBT1, CXCL1, and CXCL3 may underlie disease progression [9].
5.5.4 Genetic Changes in EsCa
5.5.4.1 Chromosomal Alterations in EsCa
Chromosomal numerical (aneuploidy) and structural (LOH, chromosomal instability, amplification) changes are associated with EsCa. The frequency of aneuploidy is very high in ESCC, being present in up to 90 % of poorly differentiated tumors. Aneuploidy is also an early occurrence in EAC, and the frequency increases with lesion progression to invasive cancer. About 86 % of EACs harbors aneuploidy and may be associated with lymph node metastasis. The presence of these chromosomal numerical changes in BE was strongly associated with the risk of progression to HGD (69 % of “progressors” vs. 0 % of “nonprogressors”) [10].
LOH in several tumor suppressor genes and genes involved in DNA repair and cell cycle control occur in EsCa. Among these are losses at 2p21 (MSH2 locus), 3p21 (MLH1 locus), 5q21 (APC locus), 9q21 (CNDN2A and CDKN2B loci), 13q14 (RB locus), 17p13 (TP53 locus), and 18q21 (DCC and DOC4 loci). Similarly, growth factor genes, and genes involved in cell cycle progression, are amplified in EsCa. These include the following loci with involved genes; 7p12–13 (EGFR locus), 8q24 (MYC locus), 11q13 (CCND1 locus), and 17q21 (HER2 locus).
5.5.4.2 MSA in EsCa
Although not the predominant genetic alteration, MSI is associated with some EsCas. MSI often occur as a consequence of replicative error due to ineffective DNA mismatch repair, stemming from loss of function in repair genes including MLH1 and MSH2, which are altered in EsCa. The frequency of MSI is higher in EAC than ESCC. In EAC, 10–20 % of cases demonstrate MSI. While variable, a good proportion of high-grade dysplastic lesions of EAC (~33 %) and ESCC (~23 %) samples harbors MLH1 promoter hypermethylation, implicating them in possible MSI.
5.5.4.3 Alterations in Cell Cycle Regulators in EsCa
RB loss of function is common in both ESCC (30–50 %) and EAC (35–50 %). The primary mechanism of RB inactivation is LOH. In ESCC, this appears to be a late event that occurs on a background of increased expression of CCND1, loss of TP53, and decreased expression of CDKN2A. However, in EAC, RB loss of function is an early occurrence, with increasing rates of loss in dysplastic lesions and carcinoma.
TP53 LOH and mutations are common in both major types of EsCa. In ESCC, TP53 mutations occur in up to 93 % of cases (range 40–75 %) and have been observed in early mucosal lesions. The frequency of these mutations increases in a tumor-progressive fashion, from hyperplasia to dysplasia to carcinoma. As an early event, TP53 mutations or LOH precede RB loss of function. The types of TP53 mutations appear as a fingerprint of the causative agent. Of interest, most TP53 mutations commonly occur in the DNA-binding domain encoded by exons 5–8. In about 40 % of ESCC, TP53 mutations are A/T base pairs, which are changes that can be induced by a metabolite of ethanol, acetaldehyde. Additionally, 15 % of cancers harbors G to T transversion mutations, which are reminiscent of tobacco-related exposure. In 18 % of cases, C to T transition mutations are observed.
TP53 loss occurs in 45–75 % of EACs. Similar to ESCC, these mutations mostly involve exons 5–8, and are mainly base transitions. TP53 mutations also demonstrate clonal expansion in the epithelium and mirror the progressive metaplasia-to-dysplasia-to-carcinoma sequence. The mutation rate ranges from ~5 % in BE to 65–75 % in dysplastic lesions and to 90 % in EAC. In the progressive sequence, TP53 mutations precede and may contribute to the development of aneuploidy, because diploid dysplastic cells with TP53 mutations arrest at G2 phase and undergo aberrant replication that can give rise to aneuploidy. While TP53 lesions coexist with those of CDKN2A, it is evident that 9p LOH (CDKN2A locus) occurs prior to 17p (TP53 locus), and these early events precede LOH at 5q, 13q, and 18q.
The gene products from the 9p21 locus, p16 and p15, inhibit cyclin D1:CDK4/6 complexes to promote cell cycle arrest at G1. The 9p21 locus is frequently lost in dysplastic lesions (90 % frequency) and EAC (80 %). Although inactivation of CDKN2A by mutation, primarily in exons 1 and 2, occurs in ~25 % of cases, promoter hypermethylation is more common and is very frequent in dysplasia and adenocarcinoma. Similarly, loss of CDKN2A via promoter hypermethylation is found in ~50 % of ESCC. Genetic defects of CDKN2B in EsCa are less well documented. LOH at 9p also precedes losses at 5q and 13q.
Overexpression of CCND1 (11q13) due to gene amplification occurs in 30 % of BE or dysplastic lesions but in as many as 70 % of ESCC. About 92 % of EACs overexpress CCND1, but gene amplification is rare, suggesting alternative mechanisms that are less well understood. The overexpression of CCND1 is of prognostic significance, being associated with metastasis, advanced tumor grade and stage, poor chemotherapy response, and decreased overall survival. Cyclin E is also overexpressed in BE with dysplasia, EAC, and ESCC.
5.5.4.4 Growth Factor Alterations in EsCa
Exogenous and endogenous growth ligands can propel cell cycle uncontrollably by inducing cyclin expression. Tyrosine kinase receptors of importance in EsCa are the EGFR and FGFR-α. Also elevated in EsCa are VEGF and PDGF, which are associated with angiogenesis.
While growth factors may not be overexpressed in ESCC, the amplification and overexpression of their receptors are well documented in ESCC. EGFR (c-erb B1) is overexpressed in 40–70 % ESCCs and may predict poor chemotherapy response and adverse outcome. Both EGFR and TGFα are overexpressed in BE and EAC, and EGFR overexpression correlates with lymph node metastasis.
EGFR2/HER2 (c-erb B2) is overexpressed in both EAC (~20 %) and ESCC (~10 %). The mechanism of elevated expression is gene amplification. Whereas its clinicopathologic relevance in ESCC is less understood, HER2 overexpression in EAC predicts disease progression and correlates with aneuploidy. The elevated levels of HER2 are strong independent prognostic factors of EAC.
Angiogenesis is a hallmark of cancer, necessary for sustained growth, invasion, and metastasis. VEGF and VEGFR overexpression are detected in BE. VEGF overexpression can be observed in ~58 % of ESCC and is an early event in EAC, being present in metaplastic lesions. PDGF overexpression in ESCC is associated with increased VEGF levels, increased microvasculature, venous invasion, and poor survival.
5.5.4.5 Apoptosis and Immune Escape in EsCa
The ability to avoid death by any mechanism is another hallmark of all cancer cells. For EsCas, this is achieved through multiple mechanisms, as summarized below:
EsCa cells alter the expression of death receptors. The interaction of the surface receptor FAS, with its ligand FAS-L, leads to cellular death via apoptosis. Normal cells express FAS; however, both ESCC and EAC cells lose surface expression of FAS, so as to avoid apoptotic cell death. Both ESCC and EAC and their precursor lesions overexpress FAS-L. Thus, these cells avoid apoptotic cell death by decreasing expression of FAS but are able to kill FAS-expressing cells such as immune surveillance lymphocytes through their interactions with FAS-L on cancer cells. Expression of FAS-L is thus associated with decreased tumor-infiltrating lymphocytes. Thus, tumors that express FAS receptor confer independent prognosis of prolonged disease-free survival.
The loss of p53 functions, as is common in EsCa, prevents activation of pro-apoptotic genes in situations of irreparable DNA damage.
EsCa cells increase expression of Cox2 enzyme, which is an inhibitor of apoptosis. Cox2 is overexpressed in BE and shows progressive increases in levels in dysplastic lesions and carcinoma cells.
5.5.4.6 Adhesion Molecules and Invasion of EsCa Cells
The two adhesion molecules of importance in EsCa are E-cadherin and β-catenin. E-cadherin is important in cell–cell adhesion and is anchored to the cell cytoskeleton via α-, β-, and γ-catenins. Loss of E-cadherin thus causes cellular detachment and invasion. β-catenin also serves adhesive functions, but free unantagonized β-catenin can translocate into the nucleus to trigger signal transduction that enhances cellular proliferation, growth, and antiapoptosis. Expectedly, therefore, E-cadherin expression is lost or decreased in 45–80 % of ESCC, with decreasing levels being associated with degree of metastasis. The prognostic value of E-cadherin has been proven. Low levels are associated with worse outcome variables such as increased hematogenous spread and mortality. Loss of E-cadherin is associated with poor 5-year survival outcome. The progression of BE to EAC is also associated with decreased E-cadherin expression. In 65 % of EAC, E-cadherin expression is reduced via LOH at 16q22, the CDH1 locus. In both ESCC and EAC, β-catenin changes from membrane to become cytoplasmic with disease progression.
5.5.4.7 Other Alterations in EsCa
APC tumor suppressor gene inactivation via LOH occurs in about 55–80 % of ESCC and in 20–55 % of EAC. Mutations are infrequent in EAC, occurring in < 10 % of cases. However, loss of APC is a late event in EsCa. Cancer cells avoid telomere shortening that halts the cell cycle, by increasing the expression of telomerase. Telomerase helps stabilize the lengths of telomeres in these cancer cells. Almost all (100 %) EsCas (ESCC, EAC, BE with dysplasia) overexpress telomerase.
5.5.5 Multistep Esophageal Carcinogenesis
The multistep carcinogenic pathway is well charted for EsCa. Histopathologic, molecular, and genetic characterization of tumors has enabled two different pathways for EAC and ESCC progression. EAC progression employs the metaplasia–dysplasia–adenocarcinoma sequence, while the hyperplasia–dysplasia–squamous cell carcinoma sequence characterizes ESCC progression.
The progression of BE through LGD, HGD, and eventually EAC requires epigenetic and genetic changes with clonal selection and development of expansive preconditioned cancer fields. Subsequent additional genetic alterations usher in the invasive pathology. Myriads of molecular genetic lesions occur to drive EAC progression, but a number of genetic changes have been well placed in the progressive sequence. Hypermethylation of CDKN2A and loss of cell cycle control, followed by loss of TP53 through mutations that may contribute to G2 arrest, abnormal replication, and hence, subsequent aneuploidy, underlie development of BE and eventual dysplastic lesions. Loss of RB tumor suppressor functions adds to other cell cycle deregulation. Overexpression of HER2 further confers in these cells the ability of growth independent of exogenous factors. Additional factors are mutations in SMAD4 in HGD and EAC.
TP53 mutations (distinct from those in EAC) occur early in ESCC and may be involved in cell cycle deregulation and genomic instability. Augmenting this, and to offer the cell with uncontrollable proliferation, is CCND1 overexpression. Self-sufficiency in growth signals is partly acquired via EGFR overexpression. However, acquisition of invasive and metastatic phenotype is through loss of CDH1 expression and alterations in β-catenin levels.
5.6 Circulating EsCa Biomarkers
Circulating cell-free DNA has diagnostic potential in EsCa; however, targeting tumor-specific genomes through detection of epigenetic and genetic alterations may be more clinically useful. Changes in coding and noncoding transcripts, serum proteins, and metabolites in circulation of EsCa patients have been demonstrated. The potential utility of circulating EsCa cells in disease management is worthy of validation.
5.6.1 Circulating Cell-Free Nucleic Acid Content as EsCa Biomarkers
The clinical relevance of ccfDNA has been addressed in EsCa patients. Esophageal and gastric cancer patient plasma samples were collected preoperatively for diagnostic assessment. Short (102 bp) and long (253 bp) DNA amplicons and DNA concentration were measured. DNA concentrations of both short and long fragments were much significantly elevated in cancer patients compared to controls. This difference was more pronounced in EsCa patient samples. The AUROCC was 0.83 and 0.91 for short and long fragments, respectively, for EsCa, and 0.75 and 0.67 for short and long fragments for the gastric cancer cohorts. DNA integrity index defined as the ratio of short to long fragments, significantly differentiated EsCa patients from normal healthy controls [11]. Other studies support the elevated levels of ccfDNA in patients with EsCa. Banki et al. found that plasma DNA was more reliable than CEA for the detection of recurrences [12]. They concluded that elevated ccfDNA was significantly higher in cancer patients than controls but that these high levels returned to normal following complete surgical resection. Similar to other cancers, EsCa is associated with increased ccfDNA.
5.6.2 Circulating EsCa Epigenetic Biomarkers
Because of the importance of epigenetic alterations in EsCa progression, attempts have been made to uncover their clinical relevance in circulation. APC, CDKN2A, TAC1, MSH2, and global methylation changes in circulation are associated with EsCa.
Circulating methylated APC has been assayed as a prognostic biomarker of EsCa. APC (5p21–22) is frequently silenced via methylation in EsCa, and this has been investigated in plasma samples as a prognostic biomarker. Tumor and matched plasma samples were tested, and hypermethylation was in 92 % of EAC, 50 % of ESCC, and 39.5 % BE. The detection rates in plasma samples were 25 % and 6.3 % for EAC and ESCC, respectively. However, high circulating levels were significantly associated with reduced survival [13]. Hoffmann and colleagues also targeted methylation of APC and DAPK in plasma samples from patients with EsCa [14]. Methylation of either gene was found in 61 % of cancer patients. Preoperative levels of methylated APC and DAPK predicted poor survival outcomes. Both biomarkers significantly increased the accuracy of discriminating between short-term (<2.5 years) and long-term survival, and postoperative detection of methylated APC promoter predicted residual tumor presence.
Given the early promoter methylation of CDKN2A in EsCa progression, it has been explored as diagnostic biomarker. Promoter hypermethylation of CDKN2A, CDH1, and RARβ assayed in blood was compared to the expression of CEA (marker of CTCs) for the detection of EsCa. At least one gene was methylated in 37 % of the samples. CTCs (CEA expression) were detected in 37 % of samples as well. Methylation had no correlation with CEA expression, which suggests complementary utility for EsCa detection [15]. In a follow-up study, CTCs were detected at a rate of 27 %, and methylation of at least one of the three genes was in 37 % of the samples. The combined application of the four biomarkers enabled the detection of 53 % of EsCa patients. This assay was specific, because all control samples were negative [16]. In an earlier study, Hibi et al. detected CDKN2A promoter methylation in as many as 82 % of serum samples from EsCa patients [17].
Other genes assayed in circulation of EsCa patients are TAC1 and MSH2. TAC1 promoter methylation was analyzed in 258 EsCa samples at various stages of disease progression and 126 plasma samples. Gene methylation status could significantly distinguish cancer from normal tissues (p < 0.0001), and the frequency of TAC1 methylation increased with disease progression from BE (55.6 %), BE with dysplasia (57.5 %), to EAC (61.2 %). Moreover, TAC1 methylation was associated with BE segment, which is a clinical measure of risk for progression. Mean normalized methylation values and frequencies in plasma were significantly higher in EsCa patients than controls. TAC1 was methylated in 50 % of ESCC tissue samples as well, and this was associated with poor OS [18]. The promoter methylation of MSH2 in plasma samples was used to monitor disease-free survival after esophagectomy in ESCC patients. In this cohort, methylation was found in 48.3 % of matched tissue and plasma samples, of which 76.2 % harbored same methylation in both sample types. Postoperative DFS was lower in patients with high MSH2 methylation compared to those without [19].
A methylome approach was explored by Zhai et al., who used the Infinium HumanMethylation 27 BeadChip that covers 27,578 CpG loci in 14,495 genes to interrogate tissue and matched sera from EAC, BE patients, and healthy controls [20]. In cancer patients who provided both tissue and serum samples, there was a strong correlation (r = 0.92) in methylation patterns between the two. Using the most differentially methylated loci for hierarchical clustering, 911 loci could perfectly separate EAC patients from controls, 554 loci distinguished between BE and EAC, and finally 46 loci discriminated between BE and controls.
5.6.3 Circulating EsCa Genetic Biomarkers
Genetic alterations as circulating biomarkers of EsCa have been assayed. In both tumor and matched serum samples from patients with ESCC, 92.9 % had at least one MSA in primary tumor tissue samples, when 12 markers located at 5q (APC), 9p (CDKN2A), 17p (TP53), and 18q (SMAD4) were used. Interestingly, 96.4 % of the positive cases also had at least one alteration in matched serum samples. Controls were all negative [21]. Using the same markers in a follow-up study, the detection frequencies were 84.4 % and 81.3 %, respectively, for tissue and serum samples. As an early detection biomarker, all early stage disease patients (no lymph node involvement, pT1pN0) harbored serum MSA with none in control subjects [22]. Similar findings were further uncovered in serum and tissue samples. In this cohort of patients with EsCa of distal esophagus and gastric cardia, LOH in tissue and serum samples ranged from 77 to 96 %, and similarly with none in control serum samples [23].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree