(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Molecular pathology of endometriosis.
Circulating endometriosis miRNA biomarkers.
Circulating endometriosis proteomic biomarkers.
Circulating endometriosis immunologic biomarkers.
Endometriosis biomarkers in other body fluids.
Key Points
Endometriosis is a major cause of morbidity in women in their reproductive age. It is also a major global economic burden on health care. Thus, noninvasive early detection biomarkers of endometriosis are needed to mitigate these situations.
Although mostly benign, ~1 % of endometriosis will progress to malignancy mostly involving the ovaries. The lesions with malignant potential often demonstrate LOH, MSI, and mutations in members of key signaling pathways including PTEN, TP53, and CTNNB1.
Emerging noninvasive biomarkers of endometriosis include miRNAs, immunologic mediators, angiogenic factors, and novel proteomic discoveries in serum and urine (also in peritoneal fluids).
14.1 Introduction
Endometriosis is a condition of implantation and growth of endometrial tissue outside the uterus. It is a debilitating disease that can in some instances (a slight elevated risk) progress to ovarian cancer. It is a morbid condition of women in their reproductive ages and is associated with subfertility, nulliparity, and their associated psychological effects. The ectopic endometrial tissue is associated with inflammation, which alters ovarian functions and endometrial quality necessary for good oocyte production and nidation. Between 5 and 10 % of women in their reproductive ages are living with this condition, and for those reporting with subfertility and/or pelvic pain, as many as 35–40 % are diagnosed with endometriosis. Endometriosis is a major cause of disability in women and a great global economic burden on health care. It is the third leading gynecologic-related hospitalization in the USA and accounts for 25 % of gynecologic surgeries in China.
While not well grounded, the putative risk factors for endometriosis include genetics, nulliparity, pelvic infections, inflammation, and any gynecologic pathology that precludes normal outflow of menstrual tissue. Population and monozygotic twin studies suggest a familial component to endometriosis. The risk is six times higher in women who have first-degree relatives of severe endometriosis. Linkage analysis implicates chromosomes 7 and 10, but the specific genes implicated in endometriosis are still a challenge to gynecologic oncologists for the fact that it remains an enigma.
There currently are no validated circulating biomarkers for the noninvasive detection of endometriosis. Thus, the diagnosis still relies on the gold standard of invasive laparoscopic visualization and acquisition of endometrial samples for histopathologic evaluation. Transvaginal ultrasound, CT, and MRI scans are useful adjuncts to the diagnostic work-up. It will therefore be of considerable economic importance to have noninvasive molecular signatures for the diagnosis and management of endometriosis, and these are being pursued. Ideally, such noninvasive tests should include validated accurate biomarkers in the peripheral circulation, urine, saliva, or menstrual fluid. This chapter considers all such possible biomarkers for endometriosis management.
14.2 Screening Recommendations for Endometriosis
There are unfortunately no recommended screening tests or guidelines for endometriosis despite its high incidence and prevalence among women of reproductive age and the economic and psychologic burden on society. A cost-effective noninvasive test should reverse this situation. Currently, only a diagnostic workflow is offered for symptomatic women. These include thorough clinical history, physical examination, possible therapeutic trial (especially in women presenting with pelvic pain), imaging and eventual laparoscopic pelvic visualization, and biopsy of lesions for histopathology.
14.3 Molecular Pathology of Endometriosis
Endometriosis is a complex multifaceted disease with yet unclear etiopathogenesis, possibly due to the myriads of etiologies and molecular pathologies. Thus, a network of communications between environmental, epigenetic, genetic, hormonal, and immunologic factors underly endometriosis pathogenesis. A hereditary component is also suggested because there is an increased risk of 5–7 % in women who have affected first-degree relatives.
Endometriosis is a disease of women in their reproductive age, afflicting 5–10 % of women in this age group. However, there is a disproportionate clustering among infertile women (30–40 %) and/or those who report with pelvic pain. Several treatment modalities are available, but surgery is often offered to remove the endometriotic tissues. Recurrences after surgery are common, being 30 % at 3 years and up to 50 % in 5 years after surgery.
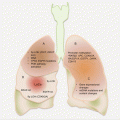
Anatomically, endometriotic tissues are found on the ovaries, fallopian tubes, cervix, vagina, rectovaginal septum, pelvic peritoneum, uterosacral ligaments, bladder, rectum, and the intestines (Fig. 14.1). They could, however, be found anywhere in the body as in very rare occasions ectopic endometrial tissues have been detected in the pleura and brain.
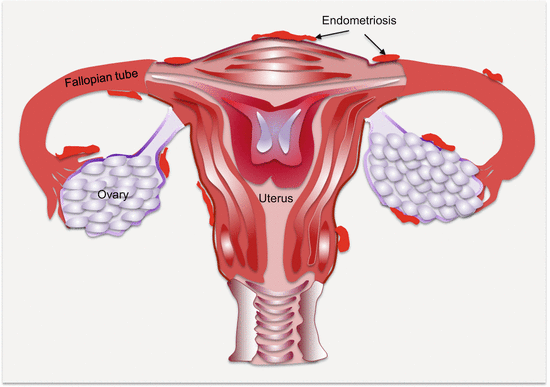

Fig. 14.1
Common anatomic locations of pelvic endometriosis (other common sites not shown are the “cul de sac,” bladder, and broad ligament)
The American Society for Reproductive Medicine stages endometriosis based on the number, location, and depth of invasion of lesions, as well as the presence of endometriomas and filmy or dense adhesions. Using these criteria, there are four stages of endometriosis:
Stage I or minimal disease is characterized by a few superficial implants.
Stage II (mild) or disease is defined by more lesions that are slightly deeper implanted than lesions in stage I.
Stage III or moderate disease has many lesions deeply implanted, in association with small endometriomas, and some filmy lesions.
Finally, the severest stage IV lesions consist of many deeply implanted lesions in association with large endometriomas and many dense adhesions.
This classification helps guide clinical decision-making on patient management.
Several mechanistic theories are provided for the emergence of endometriosis. As a complex disease, endometriosis may be mediated by multiple mechanisms. For instance, the pathogenic process of pelvic endometriosis may differ from the rare findings of extra-pelvic endometrial tissues. Thus, the diverse proposed mechanisms include the classic retrograde menstruation model due to uterine hyper-peristalsis or dysperistalsis, coelomic metaplasia, cellular induction, and possible circulatory spread via either lymphatic or blood vessels.
14.3.1 Molecular Alterations in Endometriosis
Endometriosis is a hormonal disease due to its specific addiction to estrogen. However, the cell of origin demonstrates some aspects of the hallmarks of the cancer cell. Thus, alterations in the epigenome, genome, and transcriptome that reflect on cellular processes such as altered hormonal signaling, immunologic responses, angiogenesis, and oxidative stress are all features of this enigmatic disease.
14.3.1.1 Hormonal Alterations in Endometriosis
Part of the ability of endometrial cells and tissues to survive in ectopic locations depends on hormonal control, analogous to the regulatory roles in the normal endometrium. Unlike the normal phases of the menstrual cycle whereby hormonal control is physiologically regulated, in endometriotic tissues, cyclical hormonal control is disrupted. The endometriotic cells are estrogen loving and progesterone resistant, with altered prostaglandin levels and signaling. The dependence of endometriosis on the estrogen axis is revealed by the deregulation of several mediators of estrogen signaling in endometriotic tissues. ERβ is upregulated in endometriotic tissues, and this is associated with cell cycle deregulation and aberrant proliferation of endometrial tissues. Additionally, polymorphisms in the ERβ, specifically +1730G:A, increase the risk of endometriosis and associated infertility. Estradiol levels are elevated in menstrual blood of women with endometriosis. A target gene of estradiol, CYR61, is also upregulated in endometrial tissues from women with endometriosis. Altered PGE2 levels also impart the hormonal derangement in endometriosis. In normal endometrium, PGE2 and estradiol levels are low. Additionally, in normal endometrium, increased progesterone production in the luteal phase is associated with increased HSD17B2 levels involved in estradiol metabolism to estrone. On the contrary, these physiologic processes are deranged such that in ectopic endometrial tissues, PGE2 and estradiol levels are high, HSD17B2 levels are low, and this is associated with resistance to progesterone-mediated metabolism of estrogen. These hormonal alterations are explored for medical treatment of endometriosis using estrogen suppression.
14.3.1.2 Epigenetic Alterations in Endometriosis
The hormonal derangement and other pathophysiology of endometriosis are partly due to epigenetic alterations in specific genes. Similar to the cancer cell, the progenitor endometriotic cell has to undergo similar hardships to establish a successful niche. This phenotypic change is mediated in part by deregulated gene expression, possibly as a result of epigenetic gene modification.
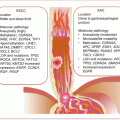
The epigenome in endometriosis is modified at the DNA, histone, and miRNA levels. In 2005, Wu et al. first demonstrated promoter hypermethylation of the homeobox gene, HOXA10, in eutopic endometrial tissues from women with endometriosis [1]. HOXA10 has functions in uterine physiology, being elevated in endometrial tissues at the mid-secretory phase of the menstrual cycle, coincident with the possible implantation of a fertilized ovum. Functionally, HOXA10 regulates progesterone receptor cofactors (e.g., KLP9) to mediate endometrial responsiveness to progesterone. Thus, decreased expression of HOXA10 in endometrial tissues from women with endometriosis could partly account for the associated infertility (lack of implantation). In support of endometriosis being a disease of epigenetic alteration is the overexpression of DNA methyltransferases (DNMT1, DNMT3A, and DNMT3B), in endometriotic tissues, which could mediate promoter hypermethylation of multiple genes. Thus, hypermethylation of progesterone receptor isoform B (PR-B) in endometriotic tissues is associated with progesterone resistance; reduced E-cadherin expression due to CDH1 promoter hypermethylation favors detachment and invasiveness. As a disease addicted to hormonal influence, altered expression of steroidogenic genes underlies the pathology of endometriosis. The expressions of steriodogenic genes are repressed in normal endometrial tissues partly due to promoter hypermethylation of steriodogenic factor-1 (SF-1), a transcription factor responsible for their expression. In contrast, endometriotic stromal cells have promoter hypomethylation of SF-1 leading to overexpression of these steriodogenic genes involved in estrogen biosynthesis. Finally, the overexpression of ERβ in endometriotic tissues is due to unregulated promoter hypomethylation.
14.3.1.3 Immunologic Alterations in Endometriosis
Endometriosis may also be a disease of awry immunology at the molecular level. First, IL-6 and some other cytokines are elevated in endometriotic tissues. Second, epidemiologic evidence indicates the association of endometriosis with other autoimmune disorders. There are increased rates of fibromyalgia, autoimmune thyroiditis, hypothyroidism, multiple sclerosis, alopecia universalis, allergies, asthma, and chronic fatigue syndrome among women with endometriosis. Similarly, SNPs associated with rheumatoid arthritis (CCL21 rs2812378 and HLA-DRB1 rs660895) are associated with endometriosis.
Autoantibodies are also observed in tissues and circulation of women with endometriosis. Autoantibodies against laminin-1 are found in a vast majority (~90 %) of lesions from infertile-associated endometriosis. Consequently, elevated levels of anti-laminin-1 antibodies are in body fluids (serum and peritoneal fluid) of women with endometriosis and this could serve as a diagnostic biomarker. Autoantibodies against carbonic anhydrase are common in autoimmune diseases such as rheumatoid arthritis and systemic lupus erythematosus. In infertile women with endometriosis, circulating anti-carbonic anhydrase antibodies are equally present. Other autoantibodies implicated in endometriosis are those against endometrial transferrin and α-2-Heremans Schmidt glycoprotein.
14.3.1.4 Mediators of Angiogenesis and Oxidative Stress in Endometriosis
A feature of endometriotic tissues that mimics some solid tumors is the high levels of vascularization. This is a necessary requirement for the endometriotic cell to sustain its existence as it proliferates and grows into a solid mass in its new environment. While the molecular mechanisms involved in new blood vessel formation in endometriotic lesions are poorly understood, hormonal signaling and immune response have been implicated. Estrogen signaling is involved in neovascularization, while inflammatory and other immune cells such as macrophages and dendritic cells contribute to the pro-angiogenic endometrial tissue environment. Additionally, angiogenic factors including VEGF, tissue factor, and its receptors are upregulated in endometriotic lesions.
Another feature of endometriosis is oxidative stress, as is common in solid tumors. Elevated free radicals in association with decreased antioxidant mechanisms play a role in endometriosis. Consistently, biomarkers of oxidative stress are elevated in sera from women with endometriosis compared to those without. Similarly, there are lower levels of the antioxidant, vitamin E, and its extracellular binding protein, afamin, in peritoneal fluid from women with endometriosis.
The Warburg phenomenon of aerobic glycolysis is also a feature of endometriosis. TGFβ1 signaling induces transcription of glycolytic gene (HIF-1α, PDK1, LDHA, and SLC2A1) expression accompanied by elevated lactate in peritoneal fluid and endometriotic tissues, and these levels are significantly higher than in eutopic endometrium from women without endometriosis.
14.3.2 Malignant Potential of Endometriosis
Although endometriosis is quite prevalent, only ~1 % of cases will undergo malignant transformation, which mostly involves the ovaries. Women with endometriosis have elevated risk (OR, 1.46) for developing ovarian cancer, and the prevalence of endometriosis is 39 % and 21 % in clear cell and endometrioid ovarian cancer, respectively. Thus, ovarian clear cell and endometrioid cancers account for ~76 % of all endometriosis-mediated ovarian cancer.
The molecular mechanisms that control the transformation of benign endometrial tissues into malignant tumors are not fully elucidated. However, epigenetic aberrations have been implicated. Hypomethylation of LINE1 and RASSF2 inactivation through promoter hypermethylation are early events in malignant progression of endometriotic lesions. Other relevant genes involved in malignant transformation of ovarian endometriosis include RUNX3 inactivation and loss of MMR gene hMLH1 via promoter hypermethylation. Additionally, LOH at 10q23.3 and PTEN mutations occur in up to 56 % and 20 % of endometriotic cysts, respectively. Moreover, mutations in PTEN (~20 %) and CTNNB1 (16–54 %), as well as TP53 overexpression and mutations (42–63 %) and MSI (12–18 %), occur in ovarian endometrioid adenocarcinomas.
While it is not recommended to refer women with endometriosis for oophorectomy (as practiced in women with BRCA mutations), women with endometriosis require close surveillance for early cancer detection. Women at elevated risk of progression are those who are diagnosed at an early age, and/or with long-standing histories of endometriosis, as well as women with associated endometriomas.
14.4 Endometriosis Biomarkers in Circulation
Noninvasive biomarkers are needed to curtail the morbidity associated with endometriosis. Towards achieving this has been the efforts at uncovering circulating endometriosis biomarkers. Of interest, changes in miRNA, immunologic and angiogenic factors, novel serum, and urine proteomics are all potential noninvasive biomarkers of endometriosis. The levels of ccfDNA are significantly higher in women with endometriosis than healthy controls (p = 0.046), suggesting the possible targeting of endometriosis-specific genetic alterations in circulation [2].
14.4.1 Circulating Endometriosis miRNA Biomarkers
MiRNA deregulation in the peripheral circulation as a diagnostic biomarker of endometriosis has been evaluated. Elevated in circulation of patients are miR-16, miR-122, miR191, miR-195, and miR-199a, while decreased are miR-9*, miR-17-5p, miR-20a, miR-22, miR-141*, miR-145*, and miR-542-3p [3, 4]. Wang et al. profiled and validated a select set of diagnostic serum miRNAs in women with endometriosis [3]. Levels of serum miR-199a and miR-122 were elevated, while miR-9*, miR-141*, miR-145*, and miR-542-3p were reduced in women with endometriosis. A panel composed of miR-199a, miR-122, miR-145*, and miR-542-3p achieved a diagnostic sensitivity, specificity, and AUROCC of 93.22 %, 96.00 %, and 0.994, respectively, for endometriosis. Additionally, the elevated levels of miR-199a and miR-122 could differentiate between women with mild and severe endometriosis, while miR-199a levels correlated with possible disease severity in terms of pelvic adhesion and lesion distribution. Focusing on let-7 family members, Cho et al. demonstrated that let-7b, let-7d, and let-7f levels were reduced in serum samples from women with endometriosis [5]. During the proliferative phase of the endometrial cycle, levels of Let-7 b, let-7c, let-7d, and let-7e are significantly reduced in circulation of these patients. A panel of let-7b, let-7d, and let-7f (at proliferative phase of the cycle) achieved an AUROCC of 0.929 in detection of endometriosis. The circulating levels of let-7b correlate with CA125, and as a single biomarker achieved an AUROCC of 0.692.
14.4.2 Circulating Endometriosis Protein Biomarkers
Proteomic approaches have enabled the use of serum spectral peaks to discriminate between women with and without endometriosis. Additionally, several circulating proteins, including glycoproteins, cytokines and other immune mediators, growth and angiogenic factors, adhesion molecules, and mediators of redox status have all been evaluated as potential diagnostic biomarkers of endometriosis. While there are currently no validated and hence commercially available circulating biomarkers for endometriosis, some of the findings show great promise.
14.4.2.1 Circulating Endometriosis Proteomic Spectral Peak Biomarkers
Proteomic profiling of samples to uncover diagnostic signatures of endometriosis has demonstrated potential. Jing et al. used SELDI-TOF MS to identify two serum proteins peaks that were associated with endometriosis [6]. As diagnostic biomarkers of endometriosis, these achieved a sensitivity of 86.67 % and specificity of 96.7 %. Importantly, these peaks declined to levels in control women following surgery. Zheng and colleagues uncovered three peptide peaks that were used in a diagnostic model to detect endometriosis [7]. This peptide-peak model achieved a sensitivity of 91.4 % and specificity of 95 % when applied to women with (n = 126) and without (n = 120) endometriosis. In an independent validation set, this model performed at a similarly high sensitivity and specificity of 89.3 % and 90 %, respectively, in detecting endometriosis. Another study using SELDI-TOF MS uncovered five peptide peaks that achieved a sensitivity of 88% and specificity of 84 % in stratifying women with and without endometriosis [8]. Thirteen and twelve peptide peaks were found deregulated in women with endometriosis and adenomyosis, respectively, when compared to healthy controls. Five common peaks were decreased in both patient groups, and the two conditions could not be separated based on these serum peptide peak profiles [9].
14.4.2.2 Circulating Endometriosis Glycoprotein Biomarkers
Currently, the only circulating biomarker used by some centers to help detect endometriosis is CA125. Circulating CA125 is well studied as a diagnostic biomarker of endometriosis. It has demonstrated clinical utility in endometriosis management, but the sensitivity limits its general recommendation as a screening biomarker. A meta-analytical study revealed a sensitivity of 50 % at a specificity of 72 % for all stages of endometriosis, with a slight increased performance in women with late stage III/IV disease (sensitivity of 60 % and specificity of 80 %) [10]. Other members of the CA family, especially CA19-9 and CA15-3, are significantly elevated in circulation of women with endometriosis. In the analysis by Tuten et al., CA125 achieved a better AUROCC of 0.928 for the detection of endometriosis; however, levels of CA19-9 and copeptin were significantly much higher in stage III/IV than stage I/II disease patients [11]. Copeptin levels correlated with disease burden in terms of stage and size of endometriosis.
Aside from CA, many other glycoproteins are evaluated singly as potential diagnostic biomarkers. These include glycodelin (sICAM-1), follistatin, Zn-α2-glycoprotein, and several others. Serum levels of glycodelin failed to delineate adolescent women with endometriosis from those without [12]. However, in women with endometrioma, glycodelin was elevated in patients compared to controls and achieved a diagnostic sensitivity and specificity of 82.1 % and 78.4 %, respectively [13]. The levels of circulating follistatin, an inhibitor of activin, were elevated in women with endometriosis [14], but this data could not be reproduced [15]. In a pilot study using mass spectrometry, Zn- α2-glycoprotein was identified as differentially expressed between cases and controls. An ELISA assay was developed for this biomarker and confirmed the significant differential expression in women with and without endometriosis (p = 0.019). When applied to samples from 120 cases and controls (n = 20), this assay could detect endometriosis at a sensitivity of 69.4 % and specificity of 100 % [16]. A panel of biomarkers including glycodelin, annexin V, VEGF, and CA125 achieved a sensitivity of 74–94 % and specificity of 55–75 % in a training set [17].
14.4.2.3 Circulating Endometriosis Immunologic Biomarkers
As a disease of inflammation, circulating levels of immunologic mediators have been explored as biomarkers of endometriosis. Cytokines, chemokines, and cells of the immune system have all been explored as diagnostic biomarkers with mixed outcomes. With the exception of RANTES, IL-4, IL-8, MCP-1, TNF-α, YKL-40, and copeptin, many immunologic circulating biomarkers have demonstrated no discrimination between women with and without endometriosis. In adolescent women with endometriosis, IL-4 is significantly elevated in their sera [18]. Tuten et al. performed a pilot study of the circulating levels of the inflammatory mediator, copeptin, between cases and controls [11]. Levels were significantly increased in cases compared to controls. As a potential circulating biomarker of endometriosis, copeptin performed at a sensitivity and specificity of 65 % and 58.3 %, respectively, when using a cutoff value of 251.18 pg/ml. Another inflammatory biomarker identified by Tuten et al. is YKL-40, which is significantly elevated in women with and without endometriosis [19]. In a comprehensive review by May et al., significant increases in circulating levels of RANTES (concordant in 75 % of the studies), MCP-1 (concordant in 50 % of the studies), and IL-8 (concordant in 46.1 % of the studies) were uncovered in women with endometriosis, and may have potential as early detection biomarkers, especially if used in a panel [20]. A panel of three plasma biomarkers including IL-8, TNF-α, and CA125 impressively achieved a sensitivity and specificity of 89.7% and 71.1%, respectively, in differentiating between women with (n = 201) and without (n = 93) endometriosis [21].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree