(1)
Medical Sciences Division Northern Ontario School of Medicine West Campus, Lakehead University, Thunder Bay, Ontario, Canada
Key Topics
Molecular pathology of cervical cancer (CvCa).
Circulating CvCa and high-risk HPV DNA as CvCa biomarkers.
Circulating CvCa miRNA biomarkers.
Serum proteins as CvCa biomarkers.
Circulating CvCa proteomic biomarkers.
Circulating CvCa cells.
Key Points
Globally, CvCa is the fourth most commonly diagnosed cancer in women, but in general is associated with good prognosis. Unfortunately, though, the vast majority is diagnosed in the resource-poor parts of the world and is associated with most of the fatalities.
The major etiologic factor of CvCa is high-risk HPV infection of cervical epithelia cells. The pathogenesis is thus deregulation of signaling pathways by E6 and E7 viral oncoproteins.
Established noninvasive biomarkers of CvCa include CA125, SCCA, and CYFRA 21-1. Circulating miRNAs are potential CvCa biomarkers; however, the role of serum proteomics and circulating CvCa cells awaits further discoveries and evaluation.
16.1 Introduction
Cervical cancer (CvCa) is one of the three most commonly diagnosed tumors of the female reproductive tract. It is the fourth most common cancer in women with global estimates of 578,000 new cases and 260,000 deaths in 2012. Unlike other cancers, only 12,990 new cases and 4,120 deaths are estimated for the US in 2016. Due in part to active screening programs that enable early CvCa detection, the 5-year prevalence is as high as 1.55 million. Unfortunately, though, the global burden of CvCa is high in the less developed world, where ~ 85 % of all cases are diagnosed. The lack of early detection also partly account for the high mortalities associated with CvCa in these countries. Thus, 87 % of all CvCa deaths occur in the less developed world. Age-standardized rates of >30 per 100,000 are found in parts of Sub-Saharan Africa and Melanesia, with the lowest in Australia and New Zealand.
Although not sufficient as a causative factor of CvCa, high-risk HPV infection of the uterine cervix is an established risk agent of cervical epithelial cellular transformation. Heightened screening programs by Papanicolau (Pap) smear cytology beginning at age 21, coupled with HPV co-testing at 30, have translated into lowered incidence and mortality rates. Unfortunately, the achievements made by these screening efforts are realized only in the developed world where the annual incidence rates of CvCa are as low as 83,000 compared to the alarming rates of 445,000 in the less developed world. Expectedly, the ineffective screening in the developing world is associated with diagnosis of advanced stage disease, culminating in the high annual mortality of 230,000 cases, compared to the 35,000 deaths in the developed world.
The poor performance of the CvCa screening program in the developing world is due to myriads of factors including cultural, inaccessible, and inadequate healthcare services. Thus, accurate biomarkers in noninvasive clinical samples such as body fluids that can be deployed at the point-of-care through lab-on-a-chip technology should help curtail the disease burden in women in the resource-poor countries and communities.
16.2 Screening Recommendations for CvCa
Cancer of the uterine cervix is one of the few cancers where intense screening programs are in place. It is recommended to begin at age 21 for all women and continue till age 65. Pap smear cytology is the recommended screening method, and this should be performed at three yearly intervals beginning at age 21. Because cervical HPV infection is an etiologic factor, it is also recommended to include HPV testing beginning at age 30, and this Pap smear and HPV co-testing should be performed every 5 years. For women with normal screening results, it is not recommended to continue screening past age 65. However, for those with histories of CIN2 or higher in the last 20 years, continued surveillance is recommended.
16.3 Molecular Pathology of CvCa
Acquired knowledge on the molecular pathology of CvCa has led to the identification of viral and cellular biomarkers currently in clinical practice or being evaluated for translation. This session examines the molecular events in CvCa development and progression.
16.3.1 Types of CvCa
Any cellular composition of the uterine cervix can undergo transformation, hence the elaborate WHO classification of CvCas. However, the commonest cancers are cervical carcinomas, which either originate from precursor lesions in the squamous (squamous cell carcinoma—SCC) or glandular (adenocarcinomas) epithelial cells. The precursor lesions of SCC of the cervix are cervical intraepithelial neoplasia (CIN), types 1–3, and those of adenocarcinomas are adenocarcinoma-in-situ (ACIS). The majority of CvCas are squamous cell carcinomas (85 %), followed by adenocarcinomas (10 %) and the remaining 5 % constitute rare cancer types such as melanocytic and mesenchymal tumors.
16.3.2 Etiologic Agents of CvCa
HPV infection of the uterine cervix is the established etiologic agent of CvCa based on epidemiologic, clinical, pathologic, and molecular genetic evidences. Indeed, almost all SCCs (99.7 %) and adenocarcinomas (94–100 %) harbor HPV genomes. The risk of developing CvCa first depends on the type of HPV infection. Established data dichotomizes all HPVs (~120) into low-risk (LR-) and high-risk (HR-) HPVs. Low-risk or non-oncogenic HPVs (e.g., HPV6 and HPV11) tend to predominate in CIN1 lesions, whereas the oncogenic HR-HPVs are encountered more often in CIN3 lesions and CvCas, offering further support for their oncogenic functions. The majority (~53 %) of CvCas are caused by HR-HPV16, with the remainder being attributed to other HR-HPVs including HPV18 (15 %), HPV45 (9 %), HPV31 (6 %), and HPV33 (3 %). HPV infections are ubiquitous, with the cumulative lifetime risk for cervical infection of a woman being ~80 %. But the immune mechanisms of many infected women efficiently ward off the infections without any sequelae. However, ~20 % of HR-HPV-infected cases proceed to develop CIN1. Similarly, many of these lesions will not progress further. It is the few women who fail to fight off the infections and, hence, live with persistent HR-HPV infections that will progress to CIN3 and eventually develop CvCa.
16.3.2.1 HR-HPV and CvCa
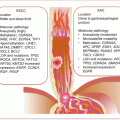
Having succeeded in overcoming the host immune system, the molecular pathology of disease progression is mediated primarily by viral oncoproteins E6 and E7. Following entrance into the cervical squamous epithelium (due to failed barrier functions), HR-HPV infects the basal stem cells, and viral genomes or episomes can be visualized in the cytoplasm. As the stem cells differentiate and move towards the epithelial surface, viral encoded early genes, including E1, E2, E4, E5, E6, and E7) mediate viral genome transcription and replication to increase viral copy number. When close to the outermost layer, late viral genes, L1 and L2, are encoded to provide viral capsid proteins needed for the packaging and release of viruses in exfoliated epithelial cells. This cyclical process or productive infection is harmless to the infected host. However, in persistent infections, and under certain circumstances, loss of genetic control of viral genome transcription can lead to overproduction of early viral oncoproteins including E6 and E7, which can be pathogenic. In a nutshell, E6 and E7 mediate cellular escape from apoptosis and uncontrolled proliferation, immortalization, and eventual genomic instability with development of aneuploidy. These deregulated control mechanisms drive the development of dysplastic lesions and CvCa.
The success or failure to form dysplastic lesions that will progress to CvCa is the consequence of the interplay of three main factors. First, upon viral infection, the host immune system gets activated and attempts to mount an efficient immunity against the infection. In many cases, this is successful and the infection is cleared. Second, the infected viruses adopt several mechanisms to escape the immune system, rendering it inefficient in viral clearance. These mechanisms include downregulation of cytokine production and inhibition of type I interferon pathways to reduce interferon-mediated gene expression that can establish the antiviral state. Finally, host factors play a role in efficient viral clearance. For example, the HLA allele DQB1*03 is associated with increased risk, while allele DRB1*13 confers reduced risk for developing CIN lesions. Thus, the dynamic interplay between HR-HPV infection, immune mechanisms, and host factors determine disease behavior.
Another mechanism of interaction between the virus and infected host is integration of viral DNA into the host genome. While this may cause genomic instability, viral integration is rather thought of as the “chicken and not the egg”—thus integration of viral genomes is a consequence and not the cause of genomic instability. Upon entry into the cervical epithelial cell, the circular HPV genome can be linearized in the E1–E2 genes, which disrupts the E2 open reading frame. In the situation whereby only episomal DNA is present in the cell, E2 protein controls viral replication by suppressing expressing of early genes. Putatively, loss of E2 should increase E6 and E7 expression. But this is not a likely mechanism of viral transformation because the negative feedback loop is abrogated in the simultaneous presence of both episomal and integrated genomes in a cell. Moreover, in high-grade lesions, aneuploidy and polyploidy can be detected before or in the absence of any viral DNA integration.
16.3.3 Molecular Pathogenesis of CvCa
The uncontrolled proliferation, escape from apoptosis, cellular immortalization, genomic instability, and eventual transformation of HR-HPV-infected cervical epithelial cell are mediated primarily by viral oncoproteins E6 and E7.
Host chromatin structure can be modulated by HPV infection leading to epigenetic alterations. One mechanism of increased CDKN2A/INK4A expression by E7 is through modification of histone architecture at the CDKN2A locus. Moreover, promoter methylation of cell adhesion molecule 1 (CADM1) and T-lymphocyte maturation-associated protein (MAL) is demonstrable in CIN3 lesions and have been explored as potential biomarkers.
The master regulator of cell cycle progression into S-phase, RB, sequesters E2F in a complex and hence prevents its entry into the nucleus to induce expression of genes required for S-phase entry. HR-HPV oncoprotein E7 plays major regulatory roles at this stage of the cell cycle. First, E7 forms complexes with RB, thus releasing E2F to induce genes such as cyclin A and E, which interact with cyclin-dependent kinases to drive S-phase entry. Second, E7 increases the levels of cyclin A and E through binding to their inhibitors, p27 (cyclin A) and p21 (cyclin E). Probably because of the importance of early cell cycle deregulation in malignant transformation, low-grade HR-HPV lesions express high levels of cyclin E.
Viral oncoprotein E7 also increases the expression of CDKN2A/INK4A and TP53. This effect of E7 on CDKN2A/INK4A should result in cell cycle arrest, because p16 inhibits cyclinD-CDK4/6 complexes, thus preventing hyperphosphorylation of RB to enhance sequestration of E2F. But the effect of or binding of E7 to RB continues to result in the release of E2F independent of RB phosphorylation. Thus, while elevated p16 serves as a biomarker of CvCa, it fails to perform its functions of halting cell cycle progression.
The increased expression of p53 by E7 should maintain genomic stability by its induction of genome repair or apoptosis in cells with irreparable genomic damage. However, the effects of E6 also abrogate this function. Viral oncoprotein E6 forms complexes with p53 and E3 ubiquitin ligase E6-associated protein (E6AP) to ubiquitinate p53 for proteasomal degradation. Apart from loss of p53 apoptotic functions, E6 also prevents cellular apoptosis via other mechanisms. First, E6 can bind to TNFR1, thus interfering with extrinsic apoptosis induction by TNF-α. Second, E6 can bind to proapoptotic BAX and BAK, as well as cause increased levels of apoptotic inhibitors including inhibitors of apoptosis 2 (IAP2) and survivin, thus abrogating the mitochondrial apoptotic pathway as well.
Another mechanism by which HR-HPV infection causes cellular transformation and dysplastic lesion formation is through induction of genomic instability through various mechanisms. First, E6 and E7 oncoproteins can induce aberrant number of centrosomes during cell division in infected cells. The abnormal number of centrosomes may lead to multipolar mitotic figures with subsequent formation of aneuploidy and polyploidy. Second, the sequestration of p53 by E6 prevents its normal functions of maintaining genomic stability during cell division. Finally, viral genome integration into host genome requires host genome damage by E6 and E7 that could potentially lead to genomic damage.
Maintenance of immortality also relies on reversal or prevention of telomere attrition. In HR-HPV infection, this is partly achieved by increased expression of telomerase reverse transcriptase (TERT) to repair shortening telomeres. Additionally, cervical cancers demonstrate gains of chromosome 5p, the TERT locus, and 3q that contain the RNA component of telomerase (TERC).
16.4 Circulating CvCa Biomarkers
Consistent with many gynecologic cancers, serum proteins including SCCA, CA125, and CYFRA 21-1 are clinically useful CvCa biomarkers. To augment disease management, circulating miRNA, serum proteomics, and circulating CvCa cells are being sought as CvCa biomarkers.
16.4.1 Circulating Cell-Free DNA Content as CvCa Biomarkers
Stage-wise elevation of ccfDNA was demonstrated in CvCa patients [1]. Plasma DNA levels were significantly much lower in CIN3 (8.1 ng/ml) than in stage I CvCa (12.78 ng/ml) patients (p = 0.001), and stage II and III levels were much higher (17.99 ng/ml) than stage I (p = 0.02). With a diagnostic reference cutoff level at 15.70 ng/ml, a sensitivity, specificity, PPV and NPV were 38.1 %, 92.5 %, 84,21 % and 58.73 % respectively for CvCa detection. Circulating DNA levels in CvCa patients are much higher than in healthy controls [2]. Median plasma DNA was 61.59 mg/l in patients compared to 16.35 mg/l in controls (p < 0.01). Similarly, median plasma DNA concentration was lower in stage I (46.02 mg/l) than stage II and III (71.35 mg/l) patients.
A prognostic relevance of DNA methylation in serum samples from CvCa patients has been demonstrated. Hypermethylation of the promoters of APC and RASSF1A were present in 48 % of plasma samples from patients with high-grade cervical lesions and cancer, compared to 3 % of those with low-grade lesions. The frequency of methylation correlated with FIGO stage [3]. Methylation in any of CALCA, hTERT, MYOD1, PGR, and TIMP3 in serum was positive in 87 % of women with CvCa, and these were present in matched cancer tissue samples [4]. MYOD1 methylation was associated with advanced stage disease and poor disease-free survival and OS. Similarly, methylation of CDH1/CDH13 in serum samples was associated with poor DFS [5].
16.4.2 Circulating HPV DNA as CvCa Biomarkers
To study CvCa circulating viral DNA, an assay targeting HPV-insertion sequence was developed. This concept proved useful in detecting specific ctDNA in many CvCa patients, and the levels or amounts reflected tumor burden. In principle, the assay can be used to monitor for residual disease or for detection of subclinical relapse in patients with HPV-positive tumors [6].
HPV DNA has been detected at various frequencies in CvCa patients, and some are associated with clinical parameters. Moreover, viral load appears to increase with disease progression or following relapse. Plasma HPV DNA was positive in 12 % of patients, but those with metastatic disease had higher viral load and were more likely to develop recurrent disease [7]. HPV16, HPV18, and HPV52 DNA were present in blood from 27 % of patients with invasive CvCa, but not in those with CIN or microinvasive disease. Disease recurrence with distant metastasis was associated with positive circulating levels. Similarly, circulating HPV16 DNA was detectable in 30 % of patients with SCC of the cervix, and the copy number was higher in those with invasive cancer compared to patients with CIN3 [8].
Plasma HPV DNA was evaluated for assessing disease progression and response to therapy. HPV16 and HPV18 DNA were detectable in 50 % of cases, and the median level was much higher in patients with cancer and high-grade lesions than those with low-grade pathology. In 79 % of patients, plasma HPV DNA was undetectable following complete response to treatment, but returned to detectable levels in those with recurrences [9]. Widschwendter et al. detected HPV DNA in 45 % of CvCa patients at diagnosis, but these converted to negative following treatment and without recurrences [10]. This serum HPV positivity was present for up to 423 days (median 72 days) prior to clinical diagnosis of relapse
16.4.3 Circulating CvCa miRNA Biomarkers
16.4.3.1 Circulating CvCa Diagnostic miRNA Biomarkers
A number of potential diagnostic circulating miRNA biomarkers for CvCa have been detected. Solexa deep sequencing of pooled serum samples from CvCa patients and healthy controls, followed by library construction and analysis using MIREAP, enabled the identification of two novel miRNAs for CvCa detection [11]. As a single biomarker, one of these achieved a sensitivity, specificity, and AUROCC of 85.7 %, 88.2 %, and 0.921, respectively. Of 12 miRNAs markedly elevated in serum samples from CvCa patients, 5 were validated as CvCa biomarkers [12]. The 5 miRNAs, miR-21, miR-29a, miR-25, miR-200a, and miR-486-5p, as a panel was more superior at CvCa detection than each marker alone, and outperformed serum SCCA and CA125. A potential association between miR-29a and miR-200a with tumor stage and grade was also suggested. In screening of serum samples from patients with CvCa, CIN, and healthy subjects, miR-16-2*, miR-195, miR-2861, and miR-494 were uncovered as discriminatory diagnostic biomarkers for CvCa [13]. As a signature panel, the four miRNAs yielded an AUROCC of 0.849 and 0.734 in discriminating CvCa from CIN and healthy controls, respectively. While miR-16-2* shares similar circulating profiles as in breast and ovarian cancer patients, the remaining three miRNAs may be unique to CvCa.
16.4.3.2 Circulating CvCa Prognostic miRNA Biomarkers
Circulating miRNAs are associated with clinicopathologic variables and prognosis of CvCa patients as well. Cervical cancer metastasis to lymph nodes is associated with alterations in the levels of miR-20a and miR-203 in lymph node tissues [14]. To evaluate the possible noninvasive utility of these in predicting lymph node metastasis in early-stage disease, pretreatment samples from stage I–IIA patients were obtained for analyses. MiR-20a levels were significantly higher in CvCa patients than controls and were even much significantly higher in those with node metastasis than patients without (p = 000, OR, 1.552). Paradoxically, miR-203 levels were significantly higher in CvCa patients than controls, but node metastasis was associated with decreased levels (p = 0.001, OR, 0.849). But the levels of miR-20a were more accurate than miR-203 in predicting nodal involvement at a sensitivity, specificity, and AUROCC of 75 %, 72.5 %, and 0.734, respectively. Analyses of both serum and tissue samples from cervical SCC patients and controls uncovered six miRNAs (miR-20a, miR-1246, miR-2392, miR-3147, miR-3162-5p, and miR-4484) that could accurately predict lymph node metastasis with AUROCC of 0.932 for serum samples (sensitivity of 85.6 % and specificity of 85 %) and 0.992 for tissue samples (sensitivity of 96.7 and specificity of 95.0 %) [15]. Although inferior to tissue levels, the serum levels of this signature were superior to SCCA (AUROCC of 0.713) for predicting lymph node metastasis in early-stage SCC of the cervix. Differential circulating levels of miR-141*, miR-646, and miR-542-3p were uncovered between cervical SCC patients and controls [16]. The abnormal postoperative levels of the three miRNAs in patients could serve as biomarkers for treatment monitoring, but were also suitable as diagnostic biomarkers.
A significant decrease in the tumor suppressormir, miR-218, in both tissue and serum samples of patients compared with controls (p < 0.001) was uncovered [17]. The decreased levels in cancer patients were associated with aggressive phenotypes as determined by advanced FIGO stage, invasiveness, and lymph node metastasis. MiR-218 has also been investigated for its possible clinical utility in serum samples for CvCa management [18]. There were significant reductions in circulating levels in CvCa patients compared with controls (p < 0.001), and the decrease in the levels was associated with adenocarcinoma histology, advanced tumor stage, and lymph node metastasis. A noted elevated level of miR-205 in CvCa patients is significantly associated with poor tumor differentiation, lymph node metastasis, and advanced tumor stage [19]. The elevated levels could differentiate early from advanced stage disease, metastatic from nonmetastatic disease, and differentiated from poorly differentiated tumors with AUROCC of 0.740, 0,694, and 0.717, respectively. Importantly, Kaplan–Meier analysis associated high levels of miR-205 with shorter OS, and in multivariate Cox regression analysis, miR-205 was an independent prognostic predictor.
16.4.4 Circulating CvCa Protein Biomarkers
Several proteins are overexpressed as a consequence of CvCa progression. These proteins are released into interstitial fluids, such that their levels are subsequently elevated in serum samples from cancer patients. The circulating levels of serum proteins have been investigated for their potential clinical utility. In CvCa, the low sensitivity and the lack of exclusivity of their expression by CvCa cells have made several of these proteins unattractive as screening or early detection biomarkers. However, following diagnosis, they display some potential in patient management in regards to possible disease staging, prognostication, treatment monitoring, and early detection of disease relapse. Of the numerous serum biomarkers including SCCA, CYFRA 21-1, CA125, CA15-3, CEA, IL6, TNF-α, VEGF, hsCRP, IAP, and autoantibodies, SCCA, CA125, and CYFRA 21-1 have been extensively evaluated in CvCa patients (Table 16.1).
Table 16.1
Percentage of CvCa patients with elevated serum proteins at defined cutoff values
Serum protein | Cutoff values | Frequency of elevated levels (%) |
|---|---|---|
SCCA | 1.5 ng/ml | 88 |
2.5 ng/ml | 60 | |
CYFRA 21-1 | 1.0 ng/ml | 42 |
3.5 ng/ml | 52 | |
CA125 | 16 U/ml | 52 |
35 U/ml | 49 |
16.4.4.1 Serum SCCA as CvCa Biomarker
SCCA is the most extensively evaluated and a potentially promising biomarker for the management of cervical SCC. While in general serum SCCA is not evaluated for diagnostic purposes, an attempt was made to use it as an early detection biomarker of precancerous cervical lesions. Thus, in comparison to CEA and TPA, a lower cutoff value of 0.55 ng/ml for SCCA achieved the best diagnostic sensitivity of 93 % for the detection of CINI–III [20].
Serum SCCA and Clinicopathologic Variables of CvCa Patients
Serum levels of SCCA are associated with a number of clinical and pathologic variables of CvCa. The serum levels are elevated in 28–88 % of patients with SCC of the uterine cervix. Although the upper range appears promising for its utility as an early detection biomarker, the variable range due to study design and also use of different cutoff values (1.5–2.5 ng/ml) for disease prediction makes it unattractive for such purposes.
While all squamous cells can express and release SCCA, the circulating levels depend partly on the degree of cellular differentiation and possibly death, because it is released in a passive process. Keratinizing and large cell non-keratinizing CvCa cells release more SCCA than non-keratinizing small CvCa cells. However, various clinicopathologic variables of CvCa are associated with the pretreatment serum levels. These features include FIGO stage, histologic grade, tumor size, depth of cervical stromal invasion, lympho-vascular space status, parametrial involvement, and lymph node metastasis.
In a large cohort of 401 patients, very high levels of SCCA (>10 ng/ml) were associated with lymph node enlargement on CT scans, and SCCA levels were independent predictors of lymph node metastasis in a cohort of 653 women with SCC of the cervix [21, 22]. In CvCa patients inclusive of patients with adenocarcinoma and adeno-squamous carcinomas, the PPV for lymph node invasion increased with increasing levels of serum SCCA. At a cutoff value of 2 ng/ml, the sensitivity and PPV were 58.1 % and 51.4 %, respectively, but the PPV increased to 70 % and 100 % at serum cutoff values of 4 ng/ml and 8.6 ng/ml, respectively [23]. Thus, the risk of lymph node involvement increases with rising SCCA levels. The risk increases 8.4-fold in patients with levels >4 ng/ml compared to patients with lower levels. In stage IB2—IVA cervical SCC patients, the median SCCA level was 6 ng/ml, and increasing levels significantly correlated with para-aortic lymph node involvement (p = 0.045) and tumor diameter (p < 0.05) [24]. The increasing levels with advancing disease stage may be that metastasis is associated with increasing expression of SCCA in order to overcome apoptosis, and the increasing release could be accounted for by increasing necrotic cell death.
The surgery for stage IB1 patients with parametrial involvement includes the extensive procedure of parametrectomy. Thus, prior staging knowledge helps with surgical planning. In a series of 115 patients with 15.7 % diagnosed with parametrial disease spread, MRI-based tumor diameter of ≥2.5 cm (OR, 9.9), tumor volume ≥5000 mm3 (OR, 13.3), as well as serum SCCA ≥ 1.5 ng/ml and CA125 ≥ 35 U/ml (OR, 5.7) were independent risk factors for parametrial involvement in multivariate analysis [25].
Serum SCCA as Prognostic Biomarker of CvCa
Whereas many studies report the association of elevated pretreatment serum SCCA levels with prognostic indices, a few have failed to observe these associations. The risk of death increased in patients with levels >4.5 ng/ml compared to those with ≤1.3 ng/ml, and pretreatment levels were significantly predictive of PFS (p < 0.026) and OS (p < 0.001) in univariate analyses [26, 27]. In the large cohort (779) analyses by Yuan et al., of stage Ib–IIb patients, elevated pretreatment levels were equally associated with poor prognosis in univariate (but not multivariate) analyses [28]. In patients who received radiotherapy, pretreatment levels >10 ng/ml were significantly associated with worse outcome in multivariate analysis [21]. Strauss et al., observed that even levels >3 ng/ml were independent predictors of recurrence-free survival and OS in stage Ia2–IIb SCC patients treated by radical hysterectomy [29].
SCCA may still be useful for prognostication under certain circumstances. For example, in 75 patients with recurrent disease in whom SCCA levels were increased, an association with shorter post-recurrence survival was realized, and this was an independent prognostic factor in multivariate analysis. In this cohort of patients, SCCA levels <14.0 ng/ml were associated with better survival in those treated with radiotherapy or surgery than those treated with chemotherapy or supportive care. Patients with levels ≥14.0 ng/ml did not benefit from salvage therapy [30]. Also in stage IB1–IIB cervical SCC patients who received neoadjuvant chemotherapy, decreased pretreatment and posttreatment SCCA ≤3.5 ng/ml was associated with better 3 year DFS and OS. In multivariate analysis, posttreatment levels were strong independent predictor of OS (p = 001) and DFS (p = 0.012).
Serum SCCA as Treatment Monitoring Biomarker of CvCa
Circulating levels of SCCA may potentially predict response to various treatment regimens of CvCa patients. In patients with locally advanced CvCa, SCCA levels >5 ng/ml were associated with poor response to chemotherapy in multivariate analysis [31]. Moreover, pretreatment levels predicted response to neoadjuvant chemotherapy in patients with advanced stage disease. Levels also decreased in a majority of patients after treatment. In 72 patients, 60 % had pretreatment levels of ≥2.8 ng/ml, but following radiotherapy treatment, only 2 % had serum SCCA levels above this level in the complete response group, and 13 % of those who achieved partial remission [32]. At 2–3 months follow-up after radiotherapy, persistently elevated levels were strong predictor of treatment failure in association with the presence of distant metastasis [21]. Serum SCCA normalization following radiotherapy or chemoradiotherapy was associated with better recurrence-free survival as evidenced by the 74.3 % vs. 5.65 survival in patients with normalized vs. persistent levels [33].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree