Jack D. Sobel, Donald Kaye
Urinary Tract Infections
Bacteriuria is a frequently used term and literally means “bacteria in the urine.” The probability of the presence of infected urine in the bladder can be ascertained by quantifying the numbers of bacteria in voided urine or in urine obtained via urethral catheterization. Significant bacteriuria is a term that has been used to describe the numbers of bacteria in voided urine that usually exceed the numbers caused by contamination from the anterior urethra (i.e., ≥105 bacteria/mL). The implication was that in the presence of at least 105 bacteria/mL of urine, infection must be seriously considered and that with less than 105/mL, infection was unlikely. The 105 criterion was of significance historically. Currently it is of value only for diagnosing asymptomatic bacteriuria, which is important to treat in only limited circumstances.
Terminology
The term cystitis has been used to describe the syndrome involving dysuria, frequency, urgency, and occasionally suprapubic tenderness. However, these symptoms may be related to lower tract inflammation without bacterial infection and can be caused by urethritis (e.g., gonorrhea, chlamydial urethritis). Furthermore, the presence of symptoms of lower tract infection without upper tract symptoms by no means excludes upper tract infection, which may also be present.
Acute pyelonephritis describes the clinical syndrome characterized by flank pain, tenderness, or both, and fever, often associated with dysuria, urgency, and frequency. However, these symptoms can occur in the absence of infection (e.g., in renal infarction or renal calculus). A more rigorous definition of acute pyelonephritis is the previously described syndrome accompanied by an indication of acute infection in the kidney.
Uncomplicated urinary tract infection (UTI) refers to infection in a structurally and neurologically normal urinary tract. The generally accepted definition of complicated UTI includes infection in the presence of factors that predispose to persistent or relapsing infection, such as foreign bodies (e.g., calculi, indwelling catheters or other drainage devices); obstruction; immunosuppression; renal failure; renal transplantation; and urinary retention from neurologic disease.1,2 In addition, infection in men, pregnant women, children, and patients who are hospitalized or in health care–associated settings may be considered complicated. In the patient with complicated infection, infecting microorganisms are more likely to be resistant to antimicrobial agents. Recurrences of UTI may be relapses or reinfections. Relapse of bacteriuria refers to a recurrence of bacteriuria with the same infecting microorganism that was present before therapy was started. This is caused by the persistence of the organism in the urinary tract. Reinfection is a recurrence of bacteriuria with a microorganism different from the original infecting bacterium. It is a new infection. Reinfection may occur with the same microorganism, which may have persisted in the vagina or feces. This can be mistaken for a relapse.
The term urosepsis is commonly used to describe the sepsis syndrome caused by UTI. It includes clinical evidence of UTI plus two or more of the following: (1) temperature >38° C or <36° C; (2) heart rate >90 beats/min; (3) respiratory rate >20/min or Paco2 <32 mm Hg; (4) white blood count >12,000/mm3, <4000/mm3, or >10% band forms.
The term chronic UTI has little meaning in many patients. True chronic infection should really mean persistence of the same organism for months or years with relapses after treatment. Reinfections do not mean chronicity any more than repeated episodes of pneumonia indicate chronic pneumonia.
The term chronic pyelonephritis means different things to different authors. To some, chronic pyelonephritis refers to pathologic changes in the kidney caused by infection only. However, identical pathologic alterations are found in several other entities, such as chronic urinary tract obstruction, analgesic nephropathy, hypokalemic nephropathy, vascular disease, and uric acid nephropathy. Pathologic descriptions do not (and cannot) differentiate between the changes produced by infection versus those produced by these other entities.
Papillary necrosis from infection is an acute complication of pyelonephritis, usually in the presence of diabetes mellitus, urinary tract obstruction, sickle cell disease, or analgesic abuse. Papillary necrosis can occur in the absence of infection in some of these conditions. The necrotic renal papillae may slough and cause unilateral or bilateral ureteral obstruction. Intrarenal abscess may result from bacteremia or be a complication of severe pyelonephritis. Perinephric abscess occurs when microorganisms from the renal parenchyma or blood are deposited in the soft tissues surrounding the kidneys. Acute lobar nephronia, also called acute focal bacterial nephritis, is edema and inflammation without liquefaction of one infected kidney lobe in a patient with the clinical syndrome of acute pyelonephritis.
Pathologic Characteristics3
Acute Pyelonephritis
In severe acute pyelonephritis, the kidney is somewhat enlarged, and discrete, yellowish, raised abscesses are apparent on the surface. The pathognomonic histologic feature is suppurative necrosis or abscess formation within the renal substance.
Chronic Pyelonephritis (Chronic Interstitial Nephritis)
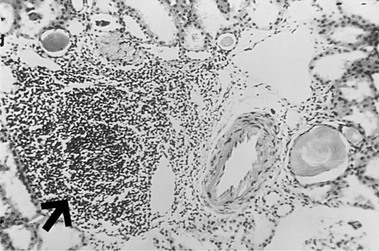
In chronic pyelonephritis, one or both kidneys contain gross scars, but even when involvement is bilateral, the kidneys are not equally damaged. This uneven scarring is useful in differentiating chronic pyelonephritis from diseases that cause symmetrical contracted kidneys (e.g., chronic glomerulonephritis). There are inflammatory changes in the pelvic wall with papillary atrophy and blunting. The parenchyma shows interstitial fibrosis with an inflammatory infiltrate of lymphocytes, plasma cells, and occasionally neutrophils (Fig. 74-1). The tubules are dilated or contracted, with atrophy of the lining epithelium. Many of the dilated tubules contain colloid casts, which suggest the appearance of thyroid tissue (“thyroidization” of the kidney). There is also concentric fibrosis about the parietal layer of Bowman’s capsule (termed periglomerular fibrosis) and vascular changes similar to those of benign or malignant arteriolar sclerosis.
Many studies have found little correlation between these pathologic findings and evidence for past or present UTI. Clearly, a better term for this pathologic entity would be chronic interstitial nephritis to encompass all the clinical states that can cause these changes. To incriminate infection as the sole cause of chronic interstitial nephritis, one needs evidence of past or present UTI and the absence of any other condition that can cause the pathologic picture of chronic interstitial nephritis. These criteria are seldom met and, even if they are, it is frequently impossible to establish whether infection is complicating interstitial nephritis of some unrecognized cause.
Papillary Necrosis Caused by Infection
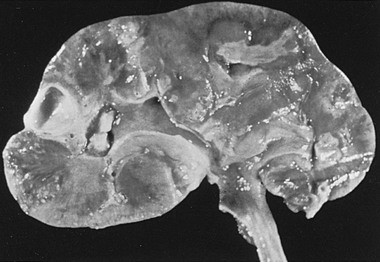
Frequently both kidneys are affected, and one or more pyramids may be involved (Fig. 74-2). The pyramids are replaced by wedge-shaped areas of yellow necrotic tissue, with the base located at the corticomedullary junction. As the lesion progresses, a portion of the necrotic papilla may break off, producing a calyceal deformity that results in a recognizable radiologic filling defect. The sloughed portion may be voided and, in some cases, can be recovered from the urine. Microscopically, edema is initially seen in the interstitium. Eventually, the lesion resembles an infarct with coagulation necrosis involving the entire pyramid. The collecting tubules are filled with bacteria and polymorphonuclear leukocytes.
Pathogenesis of Urinary Tract Infection
UTIs occur as a result of the interaction of bacterial virulence and host biologic and behavioral factors, superceding highly efficient host defense mechanisms. There are three possible routes whereby bacteria can invade and spread within the urinary tract: the ascending, hematogenous, and lymphatic pathways.
For a discussion of catheter-associated UTIs, see Chapter 304.
Ascending Route
The urethra is usually colonized with bacteria. Studies using suprapubic puncture techniques have revealed the occasional presence of small numbers of microorganisms in the urine of uninfected persons. Massage of the urethra in women and sexual intercourse can force bacteria into the female bladder. Condom use may heighten the traumatic effects. Furthermore, just one catheterization of the bladder results in UTI infection in about 1% of ambulatory patients,4 and infection develops within 3 or 4 days in essentially all patients with indwelling catheters with open drainage systems. Both the contraceptive diaphragm with nonoxynol-9 contraceptive jelly in women and the condom catheter in men have been shown to predispose to infection.5 Studies have implicated the spermicide rather than the diaphragm. Spermicides increase colonization of the vagina with uropathogens. Although the dominant Lactobacillus vaginal flora are more sensitive to nonoxynol-9 than Escherichia coli, it has not been proved that the high coliform presence in nonoxynol-9 users is caused by a loss of lactobacilli. Spermicide use also increases adherence of E. coli to vaginal epithelial cells.5 Estrogen deficiency is now recognized as a predisposing factor to recurrent UTIs in postmenopausal women because of consequent vaginal flora changes with loss of protective lactobacilli, which are replaced by coliforms and other uropathogens.6 As with younger women, recent sexual intercourse is strongly associated with incident UTIs in postmenopausal women.7 Uropathogenic E. coli (UPEC) is not infrequently shared between heterosexual sex partners.
The fact that UTI is much more common in women than in men gives support to the importance of the ascending route of infection. The female urethra is short and is in proximity to the warm, moist, vulvar and perianal areas, making contamination likely. It has been shown that the organisms that cause UTI in women colonize the vaginal introitus and the periurethral area before urinary infection results. Once within the bladder, bacteria may multiply and then pass up the ureters, especially if vesicoureteral reflux is present, to the renal pelvis and parenchyma. Animal studies have also confirmed the importance of ascending infection. If bladder bacteriuria is established after unilateral ureteral ligation, only the unligated kidney develops pyelonephritis. Finally, studies have correlated intestinal carriage of urovirulent E. coli and susceptibility to UTIs in children.8
Hematogenous Route
Infection of the renal parenchyma by bloodborne organisms clearly occurs in humans. The kidney is frequently the site of abscesses in patients with Staphylococcus aureus bacteremia or endocarditis, or both. Experimental pyelonephritis can be produced by the intravenous (IV) injection of several species of bacteria and Candida.9 However, the production of experimental pyelonephritis by the IV route with gram-negative enteric bacilli, the common pathogens in UTI, is difficult. Additional manipulations, such as the creation of ureteral obstruction, are often necessary.9 It would appear that in humans, infection of the kidney with gram-negative bacilli rarely occurs by the hematogenous route.
Lymphatic Route
Evidence for a significant role for renal lymphatics in the pathogenesis of pyelonephritis is unimpressive and consists of the demonstration of lymphatic connections between the ureters and kidneys in animals, as well as the fact that increased pressure in the bladder can cause lymphatic flow to be directed toward the kidney. Thus, it would seem that the ascending pathway of infection is the most important.
Urovirulence in Bacteria
Although UTIs are caused by many species of microorganisms, most are caused by E. coli. However, only a few serogroups of E. coli—01, 02, 04, 06, 07, 08, 075, 0150, 018ab—cause a high proportion of infections. This has led to the concept of UPEC clones, or lineages, to differentiate pathogenic populations from commensals, and clonal analysis has facilitated epidemiologic studies of the spread of antibiotic-resistant clones of E. coli and identified environmental sources of E. coli, including households.10 Certain O, K, and H serotypes also correlate with clinical severity, especially pyelonephritis. Accordingly, certain strains of E. coli are selected from the fecal flora by the presence of virulence factors that enhance both colonization and invasion of the urinary tract. Virulence factors allow evasion of host defenses and have the capability to produce disease. Cystitis and pyelonephritic E. coli isolates are genetically distinct, exhibiting differences in O, K, and H antigens. Genetic differences among uropathogens may be responsible for different clinical outcomes. Johnson and colleagues11 have confirmed that certain O, K, and H serotypes are associated with urovirulence and with the presence and expression of multiple chromosomal virulence factor determinants. Virulence is a critical determinant of clinical presentation. Recognized virulence factors include increased adherence to vaginal and uroepithelial cells,12 resistance to serum bactericidal activity, a higher quantity of K antigen in capsules (K1, K5, K12), the presence of aerobactin (iuc), cytotoxic necrotizing factor type 1 (cnf), hemolysin (hly) production, and a siderophore receptor (iroN).12 A variety of bacterial toxins have been reported, including an α-hemolysin, which inhibits protective cytokine production by bladder epithelial cells, and the importance of extracellular polysaccharide as a virulence factor has been suggested.13,14 Capsular polysaccharide contributes significantly to bacterial survival by countering lytic effects of complement and phagocytes. Induction of indoleamine 2,3-dioxygenase by UPEC attenuates innate response to uroepithelial cell invasion, facilitating colonization and establishment of infection. Genes for the various urovirulence factors are often duplicated in uropathogens and frequently linked as large, multigene, chromosomal segments called pathogenicity islands, and are absent in coliforms found in normal fecal flora.15,16 Other virulence genes for E. coli UTI include usp coding a uropathogenic specific protein and iroN coding a catechol siderophore receptor homologue.17 All uropathogens are able to use urine as a growth medium. Urine is, however, an incomplete growth medium; hence, the synthesis of one or more nutritional factors by UPEC is essential. Bacterial synthesis of guanine, arginine, and glutamine is required for optimal growth in urine.18
Adhesins
Fimbriae
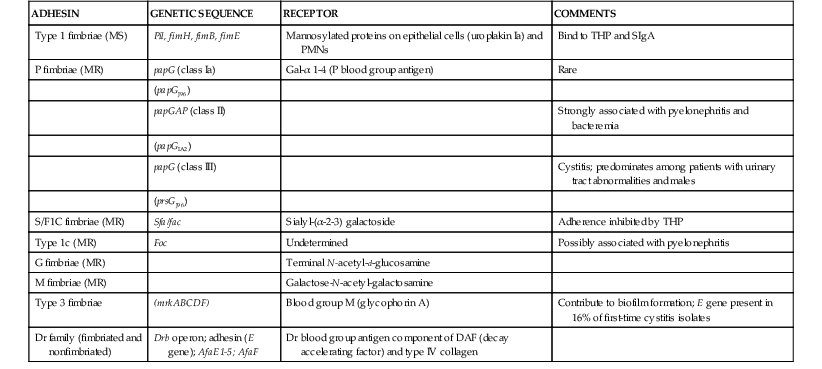
Adhesive properties of bacteria influence the selection of bacteria capable of colonizing the colon8,19 and reaching and colonizing the normal urinary tract, and they influence the anatomic level of infection in the urinary tract (Table 74-1). Accordingly, bacteria with enhanced adherence to vaginal and periurethral cells would be selected to colonize the anatomic regions adjacent to the urethral orifice. E. coli pyelonephritis isolates adhere better than E. coli cystitis isolates, and urinary isolates tend to adhere more strongly to uroepithelial cells than random fecal E. coli isolates. The adhesins of UPEC exist as filamentous surface organelles termed pili or fimbriae or as nonfilamentous proteins in the outer membrane (see Chapter 220). Numerous uropathogenic strains adhere in the absence of fimbriae.
P Fimbriae
The binding of E. coli to epithelial cell receptors containing globoseries glycosphingolipid accounts for the attachment of most strains causing kidney infection and is not inhibited by mannose; this binding is called MR for mannose resistant. Fimbriae attaching to globoseries receptors are termed P fimbriae because the receptor is a constituent of the P blood group antigen complex present in human erythrocytes and uroepithelial cells. The glycosphingolipids, synthesized by specific glycosyltransferases, are components of the glycocalyx surrounding epithelial cells and consist of an oligosaccharide moiety on the cell surface covalently linked to a lipid position embedded in the outer leaflet of the plasma membrane. Glycosphingolipases are highly specific to a given host and play an important role in determining tissue tropism for microbial pathogens and an individual host’s susceptibility to UTIs. The globoseries glycosphingolipid receptors (Gal-Gal) are distributed throughout the urinary tract, particularly in the kidney.
P fimbriae are frequently present in uropathogens; they augment the virulence of UPEC at different stages of infection, including remaining longer in the intestinal tract and spreading more efficiently to the urinary tract for purposes of colonizing and producing ascending infection.8,20 Once in the urinary tract, P–fimbriated strains adhere, persist, and, despite enhancing epithelial cytokine response, invade the kidney and induce bacteremia.20 P fimbriae invariably show the strongest association to acute disease severity with 90% or more of acute pyelonephritis-causing strains, but less than 20% of asymptomatic carriers express this phenotype. The pap gene EFG sequences encode the adhesin complex. Three molecular variants of PapG adhesin, encoded by PapG alleles I through IV, exhibit subtle receptor-binding preferences influencing the clinical outcome. Allele class II predominates among strains causing pyelonephritis and bacteremia, and class III allele is more commonly found in children and women with cystitis.21,22 P fimbriae confer enhanced ability of UPEC clones to colonize the colon and spread to the perineum. Although relatively resistant to phagocytosis by neutrophils, E. coli with P fimbriae paradoxically enhance the host inflammatory response after engaging Toll-like receptor 4 (TLR4) by inducing the elaboration of proinflammatory cytokines.23,24 Neutrophils lack receptors for P fimbriae. P-fimbriated E. coli dominates as a cause of pyelonephritis and urosepsis and especially among blood isolates; nevertheless, downregulation of P fimbriae expression may occur in the bacteria when they are in the kidney, hence facilitating parenchymal persistence.25 Limited studies have indicated that there is a potential for the development of P fimbriae–containing vaccines. In the mouse model of pyelonephritis, antibodies directed against P fimbriae that block bacterial adherence to uroepithelial cells in vitro prevent upper tract infection in vivo. With the same model, a vaccine using P fimbriae demonstrated some encouraging results; however, no progress in humans has followed.
Type I Fimbriae
Binding of E. coli to mannose-containing host epithelial receptors, glycoproteins uroplakin I and II, is universal in UPEC strains and is essential for establishment of cystitis.26 In fact, strains from cystitis patients are more likely to bind than those from pyelonephritis patients. Fimbriae attaching to mannosylated proteins via FimH subunits are the common type 1 fimbriae (pili), and attachment is inhibited in the presence of mannose (MS, mannose sensitive). Type 1 fimbriae bind mannose epitopes on secreted glycoproteins such as secretory IgA and urinary mucus, Tamm-Horsfall protein (THP), bladder uroplakin protein, integrins, and fibronectin.27 Type 1 fimbriae are encoded by the pil or fim gene cluster, including nine genes that encode structural and regulatory proteins.28 The gene fimA encodes the fimbrial subunit protein, which can be expressed independently of the fimH-encoded adhesin protein.29 The fim DNA sequences encoding type 1 fimbriae occur in most clinical isolates; consequently, epidemiologic evidence of an association between type 1 fimbriae and the site or severity of infection is more difficult to obtain. Expression of type 1 fimbriae is not especially prevalent among pyelonephritogenic strains. Clinical correlation in childhood, use of human bladder epithelial lines, and experimental animal studies have correlated type 1 fimbriae with persistence of E. coli in the urinary tract, and the use of a fimH null mutant of a type 1–positive E. coli isolate resulted in rapid elimination of the mutant from the urinary tract. Adherent bacteria multiply into biofilm-like inclusions known as intracellular bacterial communities or enter a quiescent phase for later reemergence. Organisms may be relatively protected from host immune defense, and this is hypothesized to contribute to clinical relapse. Similarly, clinical correlations in childhood urinary infections suggest that type 1 fimbriae contribute to virulence in the urinary tract when expressed in the background of a fully virulent uropathogen. Paradoxically, fimH also promotes adhesion to phagocytic cells that should presumably result in early bacterial clearance because of enhanced intracellular killing. In fact, antibody-opsonized and internalized type 1 fimbriae–bearing E. coli are rapidly killed. This is likely to occur within renal parenchyma, and hence type 1–fimbriated E. coli are programmed to shed their fimbriae on reaching the renal pelvis. In contrast, E. coli internalized only by a type 1 fimbrial mechanism survives intracellularly within the phagocyte, resulting in parasitism. Once inside the macrophage, the bacterium is safe from antibiotic assault, only to emerge later and possibly contribute to the relapse of bacteriuria. Bacterial adherence to urinary catheters is also type 1 fimbriae dependent. Type 1 fimbriae–mediated attachment to mast cells and lymphocytes results in further release of cytokines, causing cell proliferation and secretion of antibodies.
Studies have demonstrated that P and type 1 fimbriae are inversely and coordinately regulated in individual strains, explaining how invading bacteria adapt extemporaneously and sequentially to different environmental conditions prevalent in the human urogenital tract (e.g., P fimbrial expression downregulates type 1 fimbrial expression).30 Type 3 fimbriae in UPEC are thought to contribute to biofilm formation and to be important in urethral catheter infections.31
Other Adhesins
In addition to type 1 and P fimbriae, various adhesins, including S (7% of uropathogenic strains), type 1c, G, Dr fimbriae, and M and X adhesins,32 with differing molecular binding specificities and serologic properties, have been identified on UPEC and are expressed in vivo in urine (see Table 74-1). S fimbriae bind sialic acid epitopes present in renal sialylated lipoproteins.The Dr hemagglutinin family includes fimbrial and nonfimbrial adhesins. Four genes (draA,B,C,D) encoding the structural proteins and adhesins of the fimbriae have been identified. The adhesins bind to the Dr blood group antigen component of decay-accelerating factor, which is widely distributed along the urinary tract and mediates cellular invasion.33 Dr-expressing uropathogens are of relatively low invasive potential and demonstrate low multiplication rates; however, Dr-positive E. coli persists in renal infections and may play a role in chronic pyelonephritis and interstitial nephritis.32 Type 1 pili and the Dr adhesin have been linked to bladder epithelial cell invasion and intracellular persistence by UPEC. A significant number of recurrent UTIs are caused by the same bacterial strain isolated from the original infection, suggesting possible reemergence of intracellular E. coli from the underlying bladder epithelium following short-course antibiotic therapy.34
Studies with other species of bacterial uropathogens (e.g., Proteus mirabilis, Klebsiella spp.) have similarly demonstrated the significance of adherence in the pathogenesis of urinary infections. Staphylococcus aureus uncommonly causes cystitis and ascending pyelonephritis; in contrast, Staphylococcus saprophyticus is a frequent cause of lower UTIs. S. saprophyticus adheres significantly better to uroepithelial cells than S. aureus or S. epidermidis.
Expression and Selection of Virulence Factors
Evaluation of urinary isolates for virulence characteristics in the presence of underlying structural abnormalities (e.g., severe reflux) frequently fails to demonstrate the typical bacterial virulence factors. Similarly, E. coli blood isolates obtained from patients with urosepsis after bladder instrumentation often lack virulence factors. Virulence determinants are more frequently expressed by urinary isolates of E. coli obtained from women with cystitis compared with fecal isolates from healthy women. No difference in the prevalence of E. coli virulence determinants was found between subjects with first-time cystitis and those with recurrent cystitis, suggesting that host rather than bacterial factors determine the risk for recurrent infection. It has been demonstrated that E. coli isolates that cause cystitis in women using diaphragms with spermicides have fewer virulence determinants than those of nonusers, suggesting that the use of diaphragms with spermicides may allow infection with less virulent E. coli. In one study, quinolone-resistant UPEC was less virulent with decreased invasive capacity. In contrast, E. coli strains isolated from men with febrile UTI exhibited a significantly higher prevalence of virulence-associated phylogenetic groups, serotypes, and virulence genes.35 Resistant organisms have been shown to have reduced type 1 fimbriae expression and proteolytic toxin Sat elaboration.36 Trimethoprim-sulfamethoxazole, extensively used to prevent urinary infection, reduces the synthesis, expression, and adhesive function of type 1 fimbriae at concentrations well below the minimal inhibitory concentration. The importance of adherence as a virulence factor is not complete without consideration of the role of the host. A difference in receptor density and specificity linked to a difference in genetic susceptibility to infection has been proposed. In women and children with recurrent UTI, an increased avidity of bacterial attachment to vaginal, periurethral, and uroepithelial cells has been found. However, some authors have failed to corroborate these findings.
Other Virulence Factors of Uropathogenic E. coli
Certain other characteristics of bacteria may be important in the production of upper tract infection. Motile bacteria can ascend in the ureter against the flow of urine, and the toxins of uropathogenic gram-negative bacilli have been shown to decrease ureteral peristalsis and possibly contribute to the renal parenchymal inflammatory response by phagocytic cell activation.37 As a general principle, UPEC promotes a more intense inflammatory response through greater activation of the innate immune system, resulting in symptomatic urinary tract inflammation. In Proteus spp., the production of urease by infecting microorganisms has been correlated with the ability to cause pyelonephritis. The presence of K capsular antigen protects bacteria from leukocyte phagocytosis. Most uropathogenic strains produce hemolysin, which facilitates tissue invasion and causes renal tubular epithelial and parenchymal cell damage, possibly making iron available to invading E. coli. The hemolysin gene is frequently located adjacent to genes encoding for serum resistance and sialic acid–specific (S) fimbriae, but the pathogenic role of hemolysis in pyelonephritis remains controversial. Aerobactin, an iron-scavenging protein or siderophore, is present with increased frequency in uropathogenic strains of E. coli.12 Iron acquisition systems are often found in uropathogenic E. coli, presumably because conditions in the bladder are iron poor. Uropathogenic E. coli strains contain a greater number of iron acquisition systems than fecal or commensal strains, which is reflective of the adaptation to the iron-limiting urinary tract environment. Genes have been identified that encode for these virulence factors: hlya (encoding hemolysin), cnf1 (encoding cytotoxic necrotizing factor 1), and iutA (encoding aerobactin receptor). Cytotoxic necrotizing factor (CNF)-1 has been associated with UTIs and prostatitis in clinical epidemiologic studies and causes pronounced bladder inflammation in animal models.38 Iron-regulated gene homologue adhesins (Iha), an outer membrane protein encoded by iha, was recently shown to be a virulence factor in a mouse model of UTI.39 Recent studies have indicated a fitness advantage for flagellated E. coli in mouse models of UTI facilitatory colonization. Of note, E. coli strains causing asymptomatic bacteriuria appear to have lost their ability to express functional virulence associated genes.40
UPEC strains frequently downregulate responding neutrophil activity, hence evading the dominant acute immune response (i.e., suppressing neutrophil trans-epithelial movement and therefore recruitment), providing an important advantage for establishing infection. Additionally, UPEC resists phagocytic killing and dampens production of antibacterial reactive oxygen species by neutrophils. Downregulation is achieved by reduced expression of polymorphonuclear neutrophil (PMN) genes.
The greater the number of organisms delivered to the kidneys, the higher the chance of producing infection. The kidney itself is not uniformly susceptible to infection because few organisms are necessary to infect the medulla, whereas 10,000 times as many are necessary to infect the cortex. The greater susceptibility of the medulla may be caused by the high concentration of ammonia, which may inactivate complement, and by poor chemotaxis of PMNs into an area of high osmolality, low pH, and low blood flow.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree