Urinary Diversions and Reconstructions
▪ 25A Cutaneous Urinary Diversions
G. Joel DeCastro
Michael C. Large
Gary D. Steinberg
INTRODUCTION
In 1852, Simon reported the first use of bowel in the construction of a urinary diversion (1). This first operation, which entailed bilateral anastomosis of the ureters into the rectum of a bladder exstrophy patient, resulted in failure due to reflux of fecal matter into the upper tracts. Subsequent early attempts to prevent reflux and infection included anastomosis of the ureters into the hepatobiliary system and duodenum, as well as transplanting the trigone with ureters in situ to the sigmoid colon (2-4). Since then, there have been multiple techniques and modifications described of increasing complexity and sophistication (5). Indeed, the 20th century has been described as “the century of urinary diversion” (6). Each of these modifications has sought to minimize upper tract reflux while improving urinary continence. As each diversion has its own benefits and associated risks, the ultimate reconstructive operation is the result of patient, medical, and surgical judgment. In this chapter, we review incontinent and continent cutaneous diversions.
TYPES OF URINARY DIVERSIONS
When removing the bladder, the urologist must address the patient’s subsequent ability to both store and excrete urine. The ideal urinary diversion would be continent to allow for natural cycling, nonrefluxing, able to store urine at a low pressure in order to preserve the upper tracts, easy to construct and easy to survey, suitable for both genders, and metabolically stable with no increased risk of malignancy. While no urinary diversion to date meets all of these criteria, the gold standard for comparison has been the incontinent cutaneous diversion, of which the most common type is the ileal conduit (IC). The reason for its popularity is multifold: it is the simplest to construct and may be associated with less immediate postoperative morbidity when compared to other forms of diversion.
The most common type of continent urinary diversion is the orthotopic neobladder. Many patients and urologists alike consider this form of diversion to be the most desired, largely due to the fact that it minimizes changes to the patient’s body image. Accordingly, a separate chapter is devoted to this form of diversion. The next most common continent urinary diversions are catheterizable pouches that are directed to the abdominal wall. The evolution of these diversions is long and complex and is discussed in some detail in this chapter.
PATIENT SELECTION AND PREPARATION
Physiologic as well as anatomic factors can limit what bowel segments are available and what types of diversion are possible. Previous abdominal surgery may limit the amount and type of bowel available. In those with a history of abdominal radiation resulting in affected ileum, a diversion using the ascending colon (e.g., Indiana pouch), which has likely been spared from the radiation field, may be preferable. However, a history of radiation does not always preclude the use of ileum, as it does not appear to increase the risk of complications in patients undergoing IC construction (7). The ileum should be evaluated intraoperatively, and the choice of bowel segment and diversion made accordingly (8).
ICs are considered appropriate for patients with significant preoperative renal or hepatic dysfunction. Creatinine clearance of 40 to 50 mL/min with serum creatinine <2.0 mg/dL is often considered a minimum requirement for patients desiring continent diversion. In patients with serum creatinine >2.0 mg/dL, a continent diversion may still be considered if they are able to acidify urine to a pH <5.8 following ammonium chloride loading, concentrate urine to >600 mOsm/kg after water restriction, and have a glomerular filtration rate >35 mL/min with minimal proteinuria (9). Furthermore, continent diversion may be considered in patients with borderline renal insufficiency stemming from upper tract obstruction that is expected to improve following cystectomy.
In addition, catheterizable continent pouches require a certain degree of manual dexterity that must be evaluated preoperatively. Patients who need or may soon need assistance with activities of daily living may not be appropriate candidates for continent catheterizable diversions. The stomal appliances and bags used for ICs require less dexterity and need to be changed only twice per week. Emptying of the conduit bag can usually be accomplished without difficulty either by the patient or by a caretaker, while intermittent catheterization of a cutaneous diversion may require more attention and manipulation.
The role of age in selecting an appropriate urinary diversion is controversial. There are several studies demonstrating that age does not appear to independently affect the clinical outcomes of various types of diversion. Navon et al. compared the incidence of postoperative complications with the Indiana pouch in elderly (>75 years) versus younger patients and found no significant difference in early or late complications between the two groups (10).
PERIOPERATIVE MANAGEMENT
Intraoperative findings such as short mesenteric length, previous bowel resection, or significant bowel adhesions may limit the ability to perform a continent cutaneous diversion. Both the surgeon and patient must be prepared to either construct a reservoir from the intestinal segments that are available or convert to an IC. Accordingly, surgeons should be familiar with various reconstructive options, and all patients should be appropriately consented and marked preoperatively. The stomal site should be chosen carefully to avoid fat creases and
the patient’s belt line. Assistance from an enterostomal nurse can be invaluable in choosing the best site (9).
the patient’s belt line. Assistance from an enterostomal nurse can be invaluable in choosing the best site (9).
Despite the absence of much urologic literature on the matter, most surgeons employ some form of contemporary mechanical bowel preparation 1 day preoperatively (11). This usually entails an oral solution of sodium phosphate or polyethylene glycol, with some institutions also including oral antibiotics. However, several studies suggest that bowel preps do not decrease anastomotic or infectious complications (12,13). Indeed, inclusion of oral preoperative antibiotics may actually increase the risk of postoperative Clostridium difficile infection (14). At our institution, we no longer use bowel preps prior to performing any type of urinary diversion. A second-generation cephalosporin is administered 1 hour prior to skin incision. Stenting across the uretero-intestinal anastomosis should be performed regardless of the type of diversion. In a randomized prospective trial, Mattei et al. found that stenting decreased the incidence of postoperative hydroureteronephrosis, ileus, and metabolic acidosis (15).
Postoperative management is, of course, dictated by the individual patient and specific circumstances of the surgery. The authors have found that most patients do well postoperatively in surgical multispecialty floors, as long as the nursing and ancillary staffs are familiar with and comfortable managing postcystectomy patients. However, some institutions routinely keep these patients in an ICU or closely monitored care setting at least overnight, especially if the patient is elderly. While the use of nasogastric suction intraoperatively is important, its use postoperatively has been found to be associated with increased complications, prolonged gastrointestinal recovery, and length of hospitalization (16,17). For these reasons, we do not routinely use postoperative nasogastric suction.
METABOLIC CONSEQUENCES OF INTESTINAL URINARY DIVERSION
The segment of bowel utilized in the creation of a urinary diversion may carry significant metabolic consequences. In contrast to normal urothelium, the gastrointestinal mucosa is designed for absorption. The amount of urinary metabolites that are absorbed into the circulation from a urinary diversion is a function of the total surface area and contact time between the urine and intestinal mucosa. ICs, which employ the smallest surface area of bowel, are also associated with the shortest transit times, while continent diversions involve both a greater surface area and longer contact times. In order to minimize metabolic risks, preoperative evaluation of renal and hepatic function is essential.
Stomach
Given its limited surface area, the use of stomach in urinary tract reconstruction is mostly limited to composite reservoirs (18). However, there are several advantages to utilizing the stomach over other bowel segments. Its lining is the least permeable to urinary solutes, and the secretion of hydrogen chloride produces an acidified urine that helps to reduce bacteriuria (19). The resultant metabolic alkalosis appears to benefit patients with preexisting metabolic acidosis and renal insufficiency. However, acidified urine may itself lead to complications, including the hematuria-dysuria syndrome.
Jejunum
The jejunum has unique absorptive characteristics compared to other segments of small bowel. It lacks the chloride-bicarbonate exchanger present in the ileum, and its relatively permeable membrane allows for rapid efflux of water, sodium, and chloride into the lumen according to their osmotic gradients. Absorption of potassium and urea from urine, coupled with loss of sodium chloride due to its impermeability across jejunal mucosa, can result in a hypochloremic, hyperkalemic metabolic acidosis that can be life-threatening. Of note, the use of jejunum in urinary tract reconstruction is rarely associated with nutrient malabsorption (8).
Ileum and Colon
The ileum and colon are the most commonly employed intestinal segments in urinary diversion. The ileum has a unique absorptive role: the majority of bile acids, fat-soluble vitamins, and folic acid are absorbed here. As a result, removal of >100 cm of ileum can lead to bile acid malabsorption with resultant steatorrhea and vitamin deficiency (20,21). In addition, impaired vitamin B12 absorption in the distal ileum may lead to megaloblastic anemia and the irreversible neurological deficits associated with dorsal column demyelination. Patients who experience symptoms related to fat malabsorption can be treated with cholestyramine, while those with megaloblastic anemia can be treated with vitamin B12 replacement (22).
Hyperchloremic, hypokalemic metabolic acidosis secondary to ammonium chloride reabsorption is the most common metabolic abnormality associated with urinary diversion using either ileum or colon. In patients undergoing IC diversion, metabolic acidosis is reported in 13% to 17% of patients (8,23-25). In part as a result of longer urinary storage times, the incidence of metabolic acidosis in continent cutaneous diversion is higher, with estimates of approximately 24% in patients with an Indiana pouch (23). It should be emphasized that the risk of metabolic acidosis is largely dependent on the patient’s baseline renal function. In a recent study, Kristjansson et al. found that in patients with a GFR >55 mL/min, the increased acid load created by urinary diversion can be sufficiently counterbalanced to avoid severe acidosis (26). Lastly, in urinary diversions that incorporate the ileocecal valve in order to create a continent catheterizable mechanism, chronic diarrhea, and even some degree of fecal incontinence, have been reported as persistent side effects (27).
INCONTINENT CUTANEOUS DIVERSIONS
Ileal Conduit
An isolated ileal diversion was first reported by Seiffert, but only after Bricker’s publication in 1951 did it gain wider acceptance (28,29). At our institution, IC creation is performed based upon patient preference or for those with renal insufficiency (creatinine clearance <40 mL/min), hepatic disease, bulky T4 disease, markedly impaired dexterity, or significant comorbidities that necessitate abbreviated anesthetic time. In comparison to continent diversion, ICs are simpler to construct, resulting in significantly shorter operative times.
Studies comparing the rates of postoperative complications between ICs and continent diversion have reported mixed results. In a comparison of 20 patients undergoing IC diversion to 19 with continent cecal reservoir, Davidsson et al. reported no significant difference in metabolic abnormalities at 15 years after surgery (30). Both groups were found to have a mild metabolic acidosis with respiratory compensation. Using the Nationwide Inpatient Sample, Gore et al. found that the incidence of immediate postoperative complications was not associated with the type of urinary diversion used (31).
However, the researchers were only able to include complications assessed during the initial hospitalization for surgery, thereby excluding any readmissions and associated diagnoses. In contrast, Nieuwenhuijzen et al. reported on the incidence of perioperative complications after various types of urinary diversion (23). They found that late complications occurred in 39% of patients undergoing IC diversion, compared to 63% of patients with an Indiana pouch. Similarly, Knap et al. found that patients undergoing continent diversion were at higher risk of suffering complications requiring cystectomy-related surgical procedures in the future (32). However, they found no difference in perioperative mortality between the two groups.
However, the researchers were only able to include complications assessed during the initial hospitalization for surgery, thereby excluding any readmissions and associated diagnoses. In contrast, Nieuwenhuijzen et al. reported on the incidence of perioperative complications after various types of urinary diversion (23). They found that late complications occurred in 39% of patients undergoing IC diversion, compared to 63% of patients with an Indiana pouch. Similarly, Knap et al. found that patients undergoing continent diversion were at higher risk of suffering complications requiring cystectomy-related surgical procedures in the future (32). However, they found no difference in perioperative mortality between the two groups.
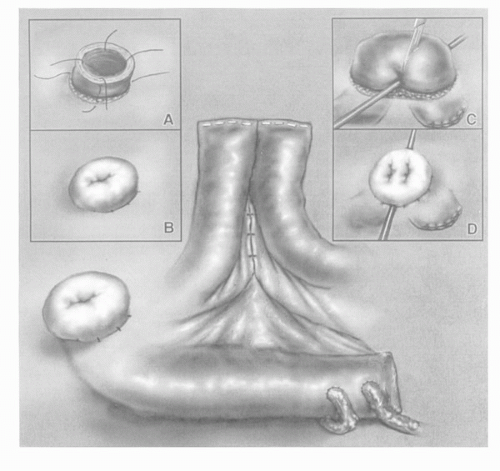
The modern IC consists of a straight segment of distal ileum taken 15 cm proximal to the ileocecal junction in order to minimize malabsorption of bile acids and vitamin B12 (Fig. 25A.1). The length of bowel needed for the conduit will vary based on mesenteric length, patient body habitus, and available ureteric length. The segment of bowel chosen may be adjusted to ensure adequate vascular supply, as best evaluated intraoperatively with transillumination of the mesentery. Bowel anastomosis is most commonly performed using a stapler, leaving the isolated conduit inferior to the anastomosis. The ileal segment may be irrigated, and its proximal end is closed with a two layer Lembert or Parker-Kerr suture. Using a nonabsorbable stapler for closure of the conduit may lead to stone formation (33).
The left ureter is tunneled through an avascular portion of the sigmoid mesocolon, and the site for its anastomosis is sharply excised in full-thickness fashion at the proximal portion of the conduit. The ureter is spatulated medially. A mucosa-to-mucosa, end-to-side, and tension-free anastomosis is performed with interrupted, absorbable 4-0 sutures, although continuous runs may be substituted. At the halfway point of completing the anastomosis, most surgeons place ureteral stents or feeding tubes for postoperative drainage. Upon completion, the conduit is irrigated in retrograde fashion to ensure a watertight anastomosis.
Following the second ureteroileal anastomosis, the preoperatively marked circular skin site is incised and carried down to the anterior rectus fascia. A cruciate fascial incision is made no larger than two fingers in width, and the conduit is grasped with a Babcock clamp and passed through to the skin. Four interrupted sutures incorporate deep conduit serosa followed by dermis in order to evert the stoma. Some surgeons incorporate fascia in the everting sutures.
In obese patients or those with short mesenteries, a loop end urostomy, or Turnbull stoma, may be fashioned over a temporary support bar. Several studies have demonstrated a reduced incidence of stomal stenosis associated with the Turnbull, although at the cost of an increased risk of developing a parastomal hernia (34,35). Additional maneuvers for gaining conduit length include separating the distal bowel from its mesentery, although this may increase the risk of ischemia and mucosal sloughing. The sigmoid mesentery tunnel may also be widened, and the angle at which the left ureter traverses the sigmoid mesocolon can be manipulated. Some have described creating an ileocecal conduit prior to isolating the conduit limb in order to gain even further length, although this has the disadvantage of removing the ileocecal valve from gastrointestinal continuity.
Colon Conduit
The colon conduit is an alternative for patients with a history of extensive pelvic radiation or inflammatory bowel disease in whom the ileum is identified as damaged and not usable in
a urinary diversion. A transverse colon conduit is ideal in the patient undergoing total pelvic exenteration, as it avoids the necessity of a bowel anastomosis. In addition, the creation of a nonrefluxing ureterointestinal anastomosis is simplified by tunneling the ureter beneath the taenia of the colon.
a urinary diversion. A transverse colon conduit is ideal in the patient undergoing total pelvic exenteration, as it avoids the necessity of a bowel anastomosis. In addition, the creation of a nonrefluxing ureterointestinal anastomosis is simplified by tunneling the ureter beneath the taenia of the colon.
Unique to conduit constructions are stomal complications, the incidence of which may be as high as 15% (36). These include parastomal hernias, stenosis, and prolapse. Risk factors for stomal complications include high BMI, especially in elderly patients. A series of 30 patients with history of pelvic radiation who underwent transverse colon conduit diversion reported no cases of bowel or urinary anastomotic leaks; stable or improved renal function in 80%; and a reoperation rate of 20% (37). A large, prospective randomized trial comparing a two-layer sewn to a stapled colon to colon anastomosis found no difference in complication rates (38). In this study, the time needed for completion of the anastomosis was reduced by approximately 10 minutes when stapled, but rates of leak and fistula formation were comparable (39). For patients undergoing ileal or gastric resection, the incidence of bowel obstruction may be as high as 10%, and for colonic resections 5% (24).
As patients live longer lives after radical cystectomy, it is important to be aware of the long-term sequelae of urinary diversion. Madersbacher et al. examined the long-term incidence of complications in 131 patients who underwent IC diversion between 1971 and 1995 (40). At a median follow-up of 96 months, they found that 66% of patients developed a complication related to the IC. Stomal complications, including parastomal hernia, stenosis, and recurrent bleeding or skin irritation, occurred in 24% of patients. Bowel-related complications occurred in 24%, with obstruction being the most common (12% of patients). Upper tract complications also appear to be somewhat common on long-term follow-up, especially in patients with specific comorbidities. At a median follow-up of 91 months, Yang et al. found that of 100 evaluable patients, 14% were found to have radiologic changes in the upper tracts, and 10% developed abnormal serum creatinine levels (41). However, all of these patients had specific comorbidities that may have contributed to the above changes, including diabetes, chronic pyelonephritis, and ureteroileal anastomotic stricture.
Ureterostomy/Pyelostomy
Direct anastomosis of the ureter or renal pelvis to the skin avoids the need for a bowel conduit. This technique is largely reserved for pediatric patients when used as a temporary diversion until more definitive bladder reconstruction can be attempted. In addition, it is sometimes used for palliation in patients with extensive pelvic malignancy. Complications resulting from these diversions include stenosis of the ureter at the anastomotic site, as well as significant cutaneous irritation from contact with urine.
CONTINENT CUTANEOUS DIVERSIONS
Catheterizable Pouches
Since Gilchrist first introduced the concept of a continent cutaneous pouch in the 1950s using the cecum as a reservoir and the ileocecal valve as a continence mechanism, there has been a marked evolution in this form of diversion (42). The differences between the various techniques are significant: some, like the double-T pouch, are surgically complex and accessible only to experienced reconstructive surgeons, while others employ more common techniques available to many urologists.
Improvements on the catheterizable pouch have focused on two main aspects: prevention of reflux into the upper tracts, and establishing an effective continence mechanism. Surgeons have long sought to create an effective antireflux mechanism at the uretero-intestinal anastomosis in order to prevent the transmission of elevated pressures and bacteria into the upper tracts. However, many of the techniques employed have been found to be associated with an increased risk of anastomotic strictures (43,44). In addition, the actual benefit of preventing bacteriuria in the upper tracts is unclear, and, as a result, there has been a recent trend toward creating refluxing anastomoses (45).
Any catheterizable pouch must include an effective continence mechanism that prevents leakage of urine while still allowing easy catheterization at regular intervals. The type of diversion chosen, and the segment of bowel employed, in large part determines the type of continence mechanism that may be used. One of the most important breakthroughs in ensuring continence came not in the form of a new surgical technique, but rather in the realization that the high intraluminal pressures created by peristalsis could be disrupted. The concept of detubularization had been reported by Goodwin et al. in the 1950s, and in the 1960s, Ekman and Kock applied it to the construction of an ileostomy (46,47). Essentially all present-day continent urinary diversions involve detubularization of the chosen bowel segment, with subsequent closure into a Heineke-Mikulicz configuration.
There are several postoperative complications specific to catheterizable pouches. The most common are associated with the stoma itself, and usually present as inability to catheterize. Wiesner et al. recently compared the incidence of channel-related complications in 401 cutaneous ileocecal pouch diversions using either an instussuscepted ileal nipple or an in situ, submucosally embedded appendix. Thirty-six percent of patients developed a stomal complication that required intervention. Stomal stenosis was most common, occurring in 17% of patients with an ileal nipple and 32% of patients with an appendiceal channel (48). The authors reported that most of these complications were addressed with endoscopic procedures without need for extensive surgical revision. Similar results have been reported by Gerharz et al. (49,50).
Continence Mechanisms in Catheterizable Pouches
There are four general strategies for achieving continence in catheterizable pouches that have been developed over the last several decades: appendiceal tunneling; use of an intussuscepted “nipple” or “flap” valve; the hydraulic ileal valve; and techniques that incorporate the ileocecal valve.
Appendiceal tunneling was first described by Mitrofanoff in 1980 and involves tunneling the in situ or transposed appendix beneath the taenia of the adjacent cecum (51). Its main advantage is that it is a relatively simple technique that does not require specialized surgical skills (9). However, there are also various disadvantages (6). The appendiceal blood supply from the appendicular artery is not robust, so great care must be taken to preserve it when dissecting the mesoappendix. In addition, the appendix may be absent or of insufficient length or diameter. Appendiceal catheterization should be performed multiple times intraoperatively to ensure that there is no obstruction or kinking. Short appendiceal length can be overcome using the “Mitchell technique” in which a portion of the adjacent cecum is incorporated into the efferent limb (52). A variant of appendiceal tunneling has been described by Montie that involves detubularizing a piece of small intestine and tunneling it into the colon (53).
The Kock Pouch
A second continence mechanism involves the intussusception of a segment of small bowel, as first applied by Ashken et al. (6,54). Contemporary versions of this technique involve the creation of an intussuscepted “nipple valve” as first described by Kock, or, more recently, the “flap valve” as employed in the T-pouch by Stein et al. (55). Although originally described for orthotopic neobladders, these techniques have been extended to continent cutaneous reservoirs.
The Kock pouch was first described as a continent ileostomy in 1971, and adapted as a urinary diversion in 1982 (56,57). The technique involves intussuscepting a piece of small bowel (the “nipple”) into a low-pressure reservoir to create both the antireflux and continence mechanisms. It is considered a technically complex diversion associated with a significant learning curve (9). In addition, complications with the nipple valve mechanism, such as slippage and ischemic damage leading to difficulty catheterizing, have been reported in up to 26% of patients in high-volume centers (58). As a result, this technique has not been widely adapted (9). However, it maintains an important historical role by demonstrating that a continent catheterizable stoma may be constructed while preserving the ileocecal valve. The Kock pouch was in large part responsible for the increased interest and developments in continent urinary diversion over the past several decades.
The Double-T Pouch
As a response to the complications associated with the nipple valve, Skinner et al. at the University of Southern California created the “T” mechanism involving the construction of a flap valve (55). First described by Abol-Eneim, the flap valve utilizes a serous-lined extramural tunnel instead of the segment of intussuscepted bowel used for the nipple valve (59). Skinner originally used the flap-valve technique as an antireflux mechanism in the afferent limb of orthotopic neobladders (the “T Pouch”) (55). He later adapted the same technique to create a continence mechanism in the efferent limb as well, resulting in the double-T pouch (60). While the functional outcomes of the double T-pouch were good, the technical complexity of the case led to very long operating times, especially in obese patients (61). As a result, this technique has not been widely adapted.
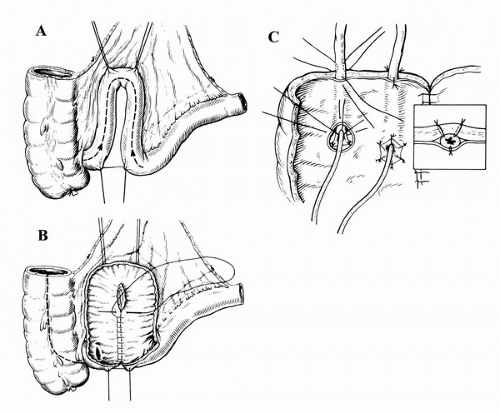
The Mainz Pouch
Since first being described in 1983, the mixed augmentation ileum ‘n zecum (Mainz) pouch has undergone multiple modifications (62). In general, the Mainz pouch involves isolating and detubularizing the cecum and right colon along with two ileal segments (Fig. 25A.2). The antireflux mechanism consists of tunneling the ureters submucosally into the cecum. The continence mechanism has been described with various
techniques, including implantation of an alloplastic stomal prosthesis, ileal intussusception, an appendiceal stoma, and the bowel-flap valve (63,64). The two latter techniques are most widely used at present. The flap valve technique involves intussusception of the ileum through the ileocecal valve with stabilization accomplished by applying two rows of staples (63). This is similar to a Kock valve, with the intussuscepted ileum serving a similar function as the nipple system. Alternatively, the use of an appendiceal stoma provides a convenient pathway for catheterization. For patients without an adequate appendix, several substitutions have been described (65,66). Long-term follow-up using both an intussuscepted valve and the appendix has been reported, with the latter demonstrating better results (49,64).
techniques, including implantation of an alloplastic stomal prosthesis, ileal intussusception, an appendiceal stoma, and the bowel-flap valve (63,64). The two latter techniques are most widely used at present. The flap valve technique involves intussusception of the ileum through the ileocecal valve with stabilization accomplished by applying two rows of staples (63). This is similar to a Kock valve, with the intussuscepted ileum serving a similar function as the nipple system. Alternatively, the use of an appendiceal stoma provides a convenient pathway for catheterization. For patients without an adequate appendix, several substitutions have been described (65,66). Long-term follow-up using both an intussuscepted valve and the appendix has been reported, with the latter demonstrating better results (49,64).
The Hydraulic Ileal Valve
A third type of continence mechanism described for catheterizable pouches involves the creation of a hydraulic ileal valve. First described by Benchekroun in 1975, this technique involves the inward folding of a 14-cm long intestinal loop on itself along with its mesentery, thereby apposing mucosal surfaces (67). As the reservoir fills, urine slowly causes compression of the mucosal surface, with resultant apposition of the serosal edges of the valve. Continence has been reported to be as high as 88% using this technique (67). However, at 5 years, up to 90% of patients have reported complications requiring revision, with stomal stenosis being the most common (68).
More commonly used examples of hydraulic valves involve incorporating the ileocecal valve as a continence mechanism. Such surgical techniques date back to the early 1900s when Verhoogen et al. used it to separate urine from feces (69). The technique of tapering and/or imbricating the terminal ileum and incorporating the ileocecal valve evolved directly from the original Gilchrist procedure, where imbrication and plication of the ileocecal valve, in combination with tapering of the adjacent ileum, served as the continence mechanism (42). This technique uses already existing anatomical structures without need for extensive reconfiguration.
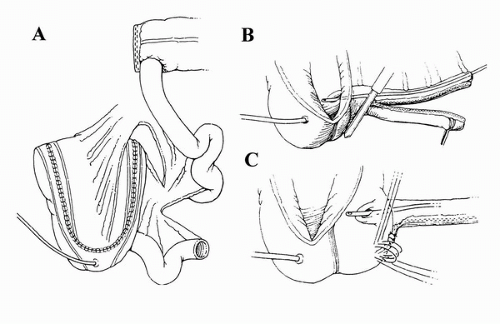
Presently, there are several types of continence mechanisms and diversions that incorporate the ileocecal valve, with the most common being the Indiana pouch. The Indiana pouch has undergone multiple modifications over the years. In 1987, Rowland et al. were the first to describe a cecoileal reservoir, where a buttressed ileocecal valve provided a continence mechanism through which intermittent catheterization could be carried out (70). As originally described, the right colon was detubularized and the segment reconfigured using an ileal or sigmoid patch. Reinforcement of the valve was achieved by plicating the entire length of the ileal segment using two rows of imbricating sutures, thereby providing a secure continence mechanism. The ureters were tunneled along the taenia in order to prevent reflux.
We also detubularize the entire right colon along its antimesenteric border, making sure to preserve the ileocecal valve. We then fold it upon itself in a Heineke-Mikulicz configuration (Fig. 25A.3). An ileal segment approximately 10 cm in length is then isolated. Some authors recommend removing the appendix, though in our experience this has not been necessary. The technique of ileocecal buttressing has been described in various ways, including taking purse-string sutures around the valve and apposing Lemberts along the terminal ileum (71). Before being brought out to the skin stoma, the efferent ileal limb can be tapered by imbrication over a 14-French catheter, though we prefer to use a GIA stapler to remove excess ileum. The continence mechanism for the Indiana pouch is the result of various factors, including the ileocecal valve, ileal peristalsis, and resistance from ileal segment plication (72).