TABLE 50.1 HISTOLOGIC CLASSIFICATIONS OF NEOPLASMS OR TUMOR-LIKE LESIONS OF ADRENAL CORTEX AND MEDULLA | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
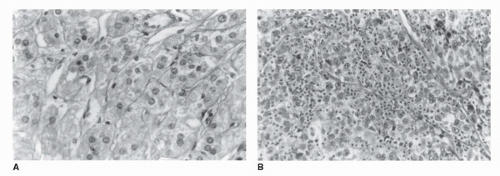
(a marker of cell proliferation) may be overexpressed in carcinomas compared to normal adrenal tissue, and benign adenomas (27). Immunostaining for chromogranin is positive in pheochromocytomas and negative in ACC. Similarly, the monoclonal antibody D11 has utility in distinguishing adrenocortical tumors from other tumors, but not benign from malignant. It therefore appears that immunohistochemical studies are useful in distinguishing adrenal neoplasms from other tumors but are of limited value in differentiating benign and malignant adrenal tumors.
TABLE 50.2 HISTOLOGIC CRITERIA OF MALIGNANCY IN ACC | ||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||
is not well characterized and its impact on long-term morbidity of patients with benign adrenal masses is unclear. The reader is referred to an article by Boscaro et al. (37) for a detailed review of the diagnosis and management of Cushing syndrome. A finding of hypokalemia suggests an aldosterone-secreting tumor. This suspicion should be confirmed by a plasma aldosterone concentration to plasma renin activity (PAC:PRA) ratio. A ratio >30 and a PAC of >0.5 nmol/L are highly suggestive of autonomous aldosterone production. For a detailed review of the diagnosis and management of adrenal aldosterone-secreting tumors, the reader is referred to an outstanding review (38). Elevated 24-hour urinary catecholamines are indicative of pheochromocytoma. For diagnosis of pheochromocytoma, plasma-free metanephrines (normetanephrine and metanephrine) should be measured. These combined tests have a sensitivity of 99% and specificity of 89%.
TABLE 50.3 DIFFERENTIAL DIAGNOSIS OF AN ADRENAL MASS | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
TABLE 50.4 CLINICAL FEATURES OF HYPERCORTISOLISM | ||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||
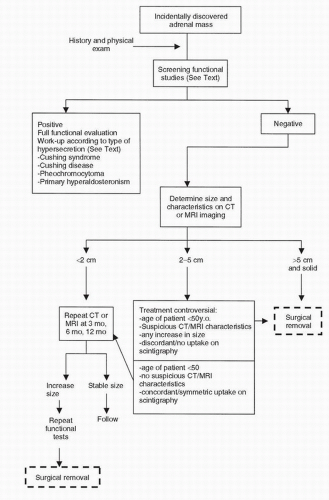
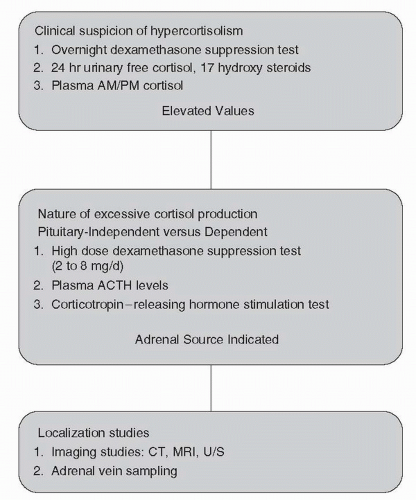 FIGURE 50.5. Nomogram illustrating the stepwise clinical evaluation of patients with suspected hypercortisolism. |
TABLE 50.5 DEXAMETHASONE SUPPRESSION TESTS: INTERPRETATION OF RESULTS | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||
4.2 years. The adrenal masses enlarged in 8% and decreased in size in 1.3%, and none of the lesions proved to be malignant.
mutations in p53 are responsible for autosomal dominant Li-Fraumeni syndrome, and 5% of such patients develop ACCs (63). Germline mutations of p53 have been found in children with ACCs without classic family history of Li-Fraumeni syndrome, as well (63). The complete loss of one insulin-like growth factor type II (IGF-II) allele, which maps to 11p15.5 locus, and a duplication of the remaining allele in association with IGF-II overexpression has been demonstrated in tumors with Beckwith-Wiedemann syndrome and in sporadic adrenocortical tumors (63,64). In a report by Gicquel et al. (65), IGF-II was overexpressed in 27 of the 29 malignant adrenocortical tumors compared to 3 of 35 benign adenomas. Carney complex is another hereditary syndrome that is associated with primary pigmented nodular adrenocortical disease in 20% to 30% of patients (66). Two chromosomal loci have been identified (2p16 and 17q22-17q24); however, no deletions at these loci have been identified, implying that the responsible gene may be an oncogene rather than a tumor- suppressor gene (66,67). Approximately 35% of patients with MEN I have adrenal nodules, and the syndrome is related to germline mutations in the tumor-suppressor menin gene (11q13). In a series of 33 patients with MEN I, 12/33 had adrenocortical tumors, with loss of constitutional heterozygosity reported in 1 patient with ACC and not in 11 patients with benign adenomas (68). The genes involved in familial adenomatous polyposis coli (APC gene) and McCune Albright syndrome (activating mutations in the GNAS1 gene) have also been investigated as possible contributors to the presence of adrenal lesions in patients with these genetic syndromes.
shown equal distributions of chromosomal gains and losses in benign and malignant adrenocortical tumors, although the specific genetic events have been different (15). Bernard et al. (71) suggested multistep tumorigenesis. They studied a patient with malignant and benign sections in a tumor. This was confirmed by molecular analysis (expressions of IGF II and allelic status of 11p15 and 17p13 loci) and CGH. Using the “candidate gene approach,” several studies have looked at putative oncogenes and tumor-suppressor genes such as p53, IGF II, APC, MEN I, and Ras; however, a low prevalence of mutations were found (72). Mutations of ACTH receptors and constitutive activations of regulatory proteins of cAMP such as G-protein-coupled receptors have also been implicated in adrenal tumorigenesis. Mutational analysis of the coding region of the receptor in 41 adrenocortical tumors, however, did not demonstrate presence of any mutation (73).
Feminization or aldosterone excess is rarely reported (76,77). Both functional and nonfunctional adrenal cancers have been described. Reviews of various case series in patients with adrenal carcinoma demonstrate that clinical presentation varies based on whether the report comes from an oncologic or endocrine clinic. Functional carcinomas are more frequent in the endocrinologic literature. In a review of 1,480 patients in which functional status was mentioned, Wooten and King (60) noted that 60% of the cases were associated with evidence of function. In large retrospective series of patients with adrenal cancers, functional tumors have been reported in 34% to 72% (78).
TABLE 50.6 STAGING SYSTEM FOR ACC | ||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree