Immunotherapy is now recognized as a viable option for patients with metastatic melanoma. The field of immunotherapy now offers treatments with the potential for a long-term cure. As the field moves forward, studies will focus on improving the response rates with new immunotherapy agents or novel treatment combinations.
Key points
- •
Immunotherapy agents have been demonstrated to improve survival option for patients with metastatic melanoma.
- •
The field of immunotherapy now offers treatments with the potential for a long-term cure.
- •
As the field moves forward, studies will focus on improving the response rates with new immunotherapy agents or novel treatment combinations.
History of immunotherapy
The idea of enhancing the immune response to reduce cancer growth can be traced back to Dr William Coley’s work at New York Cancer Hospital more than a century ago. Dr Coley initially noted tumor regression in a patient who developed a postoperative infection. The patient had recurrent cheek sarcoma, underwent a partial excision of his tumor, and was cured of the remaining tumor after he developed a wound infection with the bacteria Streptococcal pyogenes . Coley concluded that the immune response to the bacteria played an integral role in fighting the cancer. He then inoculated with bacteria his next 10 patients who had tumors that could not be surgically excised successfully. This approach had several problems, given that he saw a range of responses; some patients failed to develop an infection, whereas others developed too strong of an infection that it proved fatal. However, he did find that the patients who had an immune reaction to the bacteria, including fever and inflammation, experienced tumor reduction. He subsequently tested the effect of injecting a vaccine with killed bacteria into the tumors, otherwise known as Coley’s toxin , to stimulate an immune response without risking fatal infection, and found that he was able to cause complete regression of cancer in some patients. Unfortunately, much of this work was abandoned for years after his death as a result of the discovery of radiation and cytotoxic chemotherapy.
However, over the past few decades, the field of immunotherapy has been reborn. Careful preclinical studies have enhanced the understanding of the immune system and ways to promote antitumor immunity, most specifically with the use of adoptive T cells, which are specific for the cancer itself, and immune checkpoint blockade. Prospective randomized trials have demonstrated the efficacy of immune checkpoint blockade in the treatment of melanoma, and the field continues to move forward at a rapid pace. Together these treatments have resulted in a cure for many patients and have changed the way in which metastatic melanoma is treated. This article focuses on the immune system, immunotherapy treatments for melanoma, and the new treatments in development that show potential for improved success.
History of immunotherapy
The idea of enhancing the immune response to reduce cancer growth can be traced back to Dr William Coley’s work at New York Cancer Hospital more than a century ago. Dr Coley initially noted tumor regression in a patient who developed a postoperative infection. The patient had recurrent cheek sarcoma, underwent a partial excision of his tumor, and was cured of the remaining tumor after he developed a wound infection with the bacteria Streptococcal pyogenes . Coley concluded that the immune response to the bacteria played an integral role in fighting the cancer. He then inoculated with bacteria his next 10 patients who had tumors that could not be surgically excised successfully. This approach had several problems, given that he saw a range of responses; some patients failed to develop an infection, whereas others developed too strong of an infection that it proved fatal. However, he did find that the patients who had an immune reaction to the bacteria, including fever and inflammation, experienced tumor reduction. He subsequently tested the effect of injecting a vaccine with killed bacteria into the tumors, otherwise known as Coley’s toxin , to stimulate an immune response without risking fatal infection, and found that he was able to cause complete regression of cancer in some patients. Unfortunately, much of this work was abandoned for years after his death as a result of the discovery of radiation and cytotoxic chemotherapy.
However, over the past few decades, the field of immunotherapy has been reborn. Careful preclinical studies have enhanced the understanding of the immune system and ways to promote antitumor immunity, most specifically with the use of adoptive T cells, which are specific for the cancer itself, and immune checkpoint blockade. Prospective randomized trials have demonstrated the efficacy of immune checkpoint blockade in the treatment of melanoma, and the field continues to move forward at a rapid pace. Together these treatments have resulted in a cure for many patients and have changed the way in which metastatic melanoma is treated. This article focuses on the immune system, immunotherapy treatments for melanoma, and the new treatments in development that show potential for improved success.
The immune system
The human immune system comprises 2 main branches, innate and adaptive immunity, which work together to form a fast and effective response to a pathogen. Innate immunity involves nonspecific mechanisms to fight all foreign invaders. These cells are normally the first responders to an insult because they are always present in individuals and do not need time to develop. The first components of the innate immune response are anatomic barriers, such as the skin, which serves as a barrier to prevent the entry of microbes, and mucous membranes, which trap foreign organisms to remove them from the body; and physiologic barriers, such as the low pH of the stomach, which kills most organisms that are ingested. The main cells involved in the innate immune response are (1) mast cells, which cause blood vessel dilation to increase flow to the injured area, and the release of chemokines, proteins that attract other immune cells to the area; (2) macrophages, which are large cells that phagocytose (engulf and digest) the bacteria and secrete immunostimulatory proteins, also referred to as cytokines ; (3) neutrophils, which are the most abundant circulating white blood cell (WBC), and phagocytose bacteria or dead cells; (4) basophils and eosinophils, which secrete immunostimulatory proteins; and (5) natural killer cells, which destroy damaged or abnormal cells, including tumor cells, and are recognized by their lack of certain proteins, called major histocompatibility complex (MHC) class I, which are on the surface of all normal cells.
On the other hand, the adaptive immune system generates a highly specific response, which depends on the exact pathogen that has invaded. It takes several days after the initial exposure to generate a notable response. Adaptive immunity exhibits memory and, on subsequent exposures to the same antigen, is able to generate a faster and stronger response. The main cells of the adaptive immune response are B and T cells. Each B cell recognizes and binds to a specific antigen, causing the B cell to replicate and differentiate into plasma cells, which secrete antibodies specific for the antigen that then circulate in the blood and bind to the pathogen, leading to its elimination.
There are multiple types of T cells, including helper T cells, cytotoxic T cells, and regulatory T cells (Treg). In general, T cells require an interaction with other cells, called antigen-presenting cells (APCs), to be activated. When activated with antigen, an APC will bind to a T cell with a receptor (TCR) specific for that APC-antigen complex, providing the initial T cell stimulatory signal, or “signal one.” For T cells to be fully activated, a second costimulatory signal is required, which is also known as “signal two.” The best-characterized costimulatory interaction is between CD80 or CD86 on APC and CD28 on the surface of T cells. Once both signals occur simultaneously, the T cell is able to respond, secrete factors that activate other T cells, and differentiate into the different types of T cells. Helper T cells secrete proteins that activate other immune cells, including B cells, cytotoxic T cells, and macrophages. Cytotoxic T cells are able to kill damaged or infected cells and cancer cells. Treg cells are a subset of lymphocytes that have a role in attenuating the immune response to help prevent excessive immune activation, and alterations in these cells are important in the pathogenesis of cancers and autoimmune conditions.
Immunotherapy and melanoma
Melanoma is a highly aggressive cancer that is resistant to most available chemotherapy agents. An estimated 76,000 new cases of melanoma were diagnosed in the United States in 2014, with more than 9700 melanoma-related deaths. Although melanoma accounts for less than 5% of all skin cancers, it is responsible for 75% of skin cancer deaths. The median survival for patients diagnosed with metastatic melanoma historically is only 6 to 9 months. However, in the past few years, several exciting new therapies have been shown to improve overall survival.
Localized Bacillus Calmette-Guerin Injection
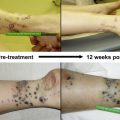
As noted, the field of immunotherapy has origins from the end of the 19th century ( Fig. 1 ). The resurgence of the field was initiated in part through the hard work of Dr Lloyd Old who, among many things, established the importance of Bacillus Calmette-Guérin (BCG), tumor necrosis factor (TNF), and described numerous tumor to antigens. BCG, a live Mycobacterium bovis vaccine, was initially created in an attempt at generating a tuberculosis vaccine, but it was also found to have antitumor properties. It was the first immunotherapy agent approved by the US Food and Drug Administration (FDA) for cancer treatment. Locally injected BCG is currently an effective treatment for early-stage bladder cancer, and BCG has also been studied in the treatment of melanoma, pioneered in part by Dr Donald Morton. Intratumoral BCG injection for melanoma was associated with high response rates for localized disease, although this was not proven to be statistically significant and was associated with unacceptable toxicity. BCG was also used in the randomized phase III study of the failed Canvaxin whole-cell vaccine in which patients were randomized to receive postoperative Canvaxin plus BCG or BCG plus placebo after tumor resection. Although the trial was discontinued because of failure to show a survival benefit with Canvaxin treatment, the higher survival seen in both arms of this trial when compared with similar patients in other studies resulted in a renewed interest in BCG as a biological agent. Recently, reduced doses of BCG in combination with other treatments, such as topical imiquimod, have demonstrated efficacy in small studies, although larger, randomized trials are still needed to evaluate the potential survival benefits of intratumoral BCG injection.
Interleukin-2
Interleukin-2 (IL-2) was approved by the FDA for the treatment of malignant melanoma in 1998. IL-2, or T-cell growth factor, is normally secreted by T cells after the binding of antigen to the TCR, resulting in stimulation and increased activity of all types of T cells. Early melanoma studies showed promising results with high-dose IL-2 treatment, resulting in the possibility of a long-term response in some patients. Unfortunately, few patients experience response to the treatment, with an overall response rate of 16% and a complete response, resulting in disappearance of all lesions, seen in only 6% of patients. Additionally, no randomized controlled studies have shown a survival benefit with IL-2 treatment compared with standard chemotherapy regimens. Furthermore, IL-2 treatment is associated with a variety of adverse events, most of which are related to increased permeability of blood vessels, resulting in low blood pressure, elevated heart rate, and extremity swelling. Other side effects include rash, fever, nausea, vomiting, diarrhea, and infection. These side effects can be managed well through adjusting the dose and administering the medication in an environment where experienced personnel can manage the side effects. However, the newer immunotherapy medications can be administered in the outpatient setting, and have shown improved efficacy in prospective randomized trials, and thus are replacing the use of IL-2 as a first-line treatment.
Immune Checkpoint Blockade
The field of immunotherapy was changed in 2011 when the FDA approved the first immune checkpoint inhibitor, ipilimumab (or Yervoy). Ipilimumab is a humanized antibody that binds to cytotoxic T-lymphocyte antigen 4 (CTLA-4) and blocks its activity. CTLA-4 is normally expressed on the surface of T cells after activation, and functions to turn off the T-cell response to prevent an excessive immune reaction. CTLA-4 binds to CD80 or CD86 on APC with a stronger bond than to CD28, displacing the costimulatory interaction and delivering a powerful negative signal to the TCR ( Fig. 2 ). Dr James Allison hypothesized that blocking this negative regulatory signal with an antibody would allow the immune response to continue and result in antitumor immunity. His preclinical work demonstrating efficacy in murine models was then moved forward into clinical trials. Studies have demonstrated that anti–CTLA-4 treatments augment tumor-specific immunity through increasing the CD8/Treg ratio in the tumor and by directly depleting Treg cells through an Fcγ receptor–dependent mechanism, resulting in a rapid loss of these inhibitory cells from the tumor environment.