As the incidence of melanoma and the number of melanoma survivors continues to rise, optimal surveillance strategies are needed that balance the risks and benefits of screening in the context of contemporary resource use. Detection of recurrences has important implications for clinical management. Most current surveillance recommendations for melanoma survivors are based on low-level evidence with wide variations in practice patterns and an unknown clinical impact for the melanoma survivor.
Key points
- •
Current surveillance practices for melanoma are based on low-level evidence with unknown clinical impact.
- •
Surveillance for melanoma recurrence is most frequently based on preferences of patient and provider.
- •
Serial routine surveillance imaging has demonstrated limited evidence for detecting recurrent melanoma at a time in which it is treatable.
Introduction
Contemporary surveillance guidelines for cancer survivors are low-level, category 2A to 2B recommendations (ie, “based upon lower-level evidence, there is uniform consensus [category 2A] or consensus [category 2B] that the intervention is appropriate”) and therefore heavily depend on expert opinion. Even the handful of tumor types for which surveillance recommendations have been rigorously studied lack category 1 (ie, “based upon high-level evidence, there is uniform consensus that the intervention is appropriate”) surveillance recommendations. As an example, seven clinical trials have evaluated various surveillance regimens for patients with surgically treated colorectal cancer and yielded mixed results. Subsequent meta-analyses of these results have suggested improvements in overall survival (but not disease-specific survival) in the setting of intensive surveillance. In contrast, several well-designed randomized studies evaluating surveillance strategies of varying intensities for women with treated breast cancer have shown no survival benefit for intensive surveillance compared with less intensive strategies. Still, controversy regarding breast cancer surveillance exists, and surveillance practice patterns vary widely.
From a practical perspective, the frequency and intensity of follow-up for cancer survivors are determined by the resources available and the preferences of the patient in conjunction with a provider’s specific preferences. These factors have increasingly important implications as the number of cancer survivors in the world increases. Because of improvements in the detection of early stage melanoma at a time when adequate local treatment is potentially curative, 5-year relative survival rates for patients with melanoma now exceed 90%, which means that more people are living longer after the diagnosis of what was once a frequently deadly cancer. However, in the absence of evidence-based follow-up guidelines, the question is how can clinicians best manage melanoma cases to detect disease recurrence while it is still treatable?
Half of all patients treated for melanoma have a recurrence. Of these recurrences, approximately 50% are in the regional lymph nodes, 20% are local recurrences, and 30% arise at distant sites. Although most recurrences develop in the first 2 to 3 years after treatment, some late recurrences more than 10 years after treatment are well documented, particularly for patients who initially had early stage melanoma. In a retrospective study of more than 7100 patients with early stage melanoma, Crowley and Seigler reported that the overall rate of recurrence 10 years after the diagnosis of the primary was 2.4%. Surgical resection is generally performed for local and regional recurrences, with good survival outcome, and metastasectomy for distant recurrences in very carefully selected patients has demonstrated survival benefits.
In designing optimal surveillance strategies, clinicians must focus on the risk of early recurrence but must also consider the risk of late recurrences within the context of a patient’s changing risk over time. As an example, in a retrospective study of 340 patients with stage III melanoma, Romano and colleagues found that most local and regional recurrences were detected by physical examination alone, whereas patients with distant recurrences most frequently presented with symptoms. Routine computed tomography (CT) imaging detected asymptomatic recurrences in 25% of all patients studied, often within 3 years of the original melanoma diagnosis. In this study, the incidence of a first-time distant recurrence was 5% or less after 32 months, 40 months, and 21 months for patients with stage IIIA, IIIB, and IIIC disease, respectively, leading the authors to conclude that routine CT imaging as a surveillance method would have low yield beyond those time points.
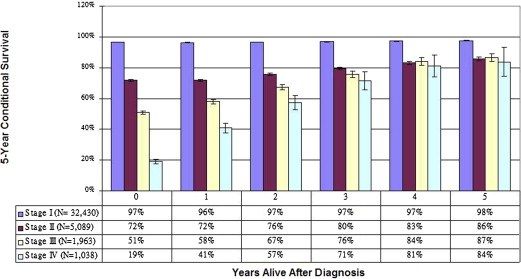
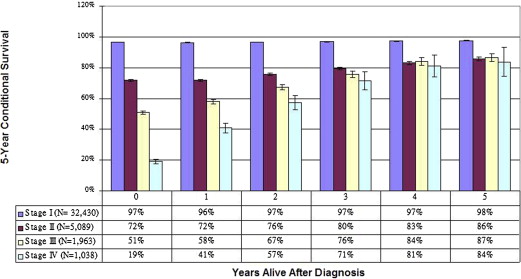
Importantly, because cancer survival estimates are heavily influenced by early cancer deaths, the estimates may not accurately reflect long-term outcomes for patients who survive to a certain point after the original diagnosis. As an alternative approach to predicting long-term survival, conditional survival analysis calculates the changing risk of death over time. For patients with all stages of melanoma, conditional survival studies have demonstrated that survival estimates improve dramatically as survival time increases, such that eventually, the original stage at diagnosis is no longer a significant predictor of ongoing survival ( Fig. 1 ). These two competing concepts—indolent disease with the potential for late recurrences but in light of known improvements in cancer survival as time from original treatment increases—make optimal melanoma surveillance a complex challenge for patients and clinicians.
To address these challenges, current guidelines for surveillance in patients with melanoma published by the National Comprehensive Cancer Network emphasize more frequent visits with more intensive stage-specific surveillance imaging early in the posttreatment phase, when the risk of recurrence is greatest. As the disease-free interval increases, office visits and imaging should become less frequent, but annual skin examinations for all stages of primary disease should continue for the rest of the patient’s life. Annual skin examinations for life, particularly for patients with early stage, low-risk melanoma, are a logical surveillance strategy based on recurrence and survival patterns; however, recommendations become less clear for patients with locally advanced stage III melanoma. Beyond annual skin examinations, the current recommendations for patients with stage III melanoma range from conventional x-ray radiography of the chest to a complex positron emission tomography (PET)/CT scan at time intervals ranging from 3 to 12 months.
The data available to aid in the development of appropriate stage-specific surveillance recommendations are limited in scope and value. The broad generalities in surveillance recommendations very likely reflect uncertainties in modern resource use and a limited understanding of the effects of different surveillance strategies on patient quality of life and cancer survival. Toward elucidating these uncertainties and enhancing the understanding of the effects of surveillance strategies on quality of life and survival for patients with melanoma, this article reviews issues key to designing surveillance strategies, including early detection, surveillance evaluation modalities, surveillance effectiveness, variation in current surveillance practices, surveillance costs, and other practice implications.
Introduction
Contemporary surveillance guidelines for cancer survivors are low-level, category 2A to 2B recommendations (ie, “based upon lower-level evidence, there is uniform consensus [category 2A] or consensus [category 2B] that the intervention is appropriate”) and therefore heavily depend on expert opinion. Even the handful of tumor types for which surveillance recommendations have been rigorously studied lack category 1 (ie, “based upon high-level evidence, there is uniform consensus that the intervention is appropriate”) surveillance recommendations. As an example, seven clinical trials have evaluated various surveillance regimens for patients with surgically treated colorectal cancer and yielded mixed results. Subsequent meta-analyses of these results have suggested improvements in overall survival (but not disease-specific survival) in the setting of intensive surveillance. In contrast, several well-designed randomized studies evaluating surveillance strategies of varying intensities for women with treated breast cancer have shown no survival benefit for intensive surveillance compared with less intensive strategies. Still, controversy regarding breast cancer surveillance exists, and surveillance practice patterns vary widely.
From a practical perspective, the frequency and intensity of follow-up for cancer survivors are determined by the resources available and the preferences of the patient in conjunction with a provider’s specific preferences. These factors have increasingly important implications as the number of cancer survivors in the world increases. Because of improvements in the detection of early stage melanoma at a time when adequate local treatment is potentially curative, 5-year relative survival rates for patients with melanoma now exceed 90%, which means that more people are living longer after the diagnosis of what was once a frequently deadly cancer. However, in the absence of evidence-based follow-up guidelines, the question is how can clinicians best manage melanoma cases to detect disease recurrence while it is still treatable?
Half of all patients treated for melanoma have a recurrence. Of these recurrences, approximately 50% are in the regional lymph nodes, 20% are local recurrences, and 30% arise at distant sites. Although most recurrences develop in the first 2 to 3 years after treatment, some late recurrences more than 10 years after treatment are well documented, particularly for patients who initially had early stage melanoma. In a retrospective study of more than 7100 patients with early stage melanoma, Crowley and Seigler reported that the overall rate of recurrence 10 years after the diagnosis of the primary was 2.4%. Surgical resection is generally performed for local and regional recurrences, with good survival outcome, and metastasectomy for distant recurrences in very carefully selected patients has demonstrated survival benefits.
In designing optimal surveillance strategies, clinicians must focus on the risk of early recurrence but must also consider the risk of late recurrences within the context of a patient’s changing risk over time. As an example, in a retrospective study of 340 patients with stage III melanoma, Romano and colleagues found that most local and regional recurrences were detected by physical examination alone, whereas patients with distant recurrences most frequently presented with symptoms. Routine computed tomography (CT) imaging detected asymptomatic recurrences in 25% of all patients studied, often within 3 years of the original melanoma diagnosis. In this study, the incidence of a first-time distant recurrence was 5% or less after 32 months, 40 months, and 21 months for patients with stage IIIA, IIIB, and IIIC disease, respectively, leading the authors to conclude that routine CT imaging as a surveillance method would have low yield beyond those time points.
Importantly, because cancer survival estimates are heavily influenced by early cancer deaths, the estimates may not accurately reflect long-term outcomes for patients who survive to a certain point after the original diagnosis. As an alternative approach to predicting long-term survival, conditional survival analysis calculates the changing risk of death over time. For patients with all stages of melanoma, conditional survival studies have demonstrated that survival estimates improve dramatically as survival time increases, such that eventually, the original stage at diagnosis is no longer a significant predictor of ongoing survival ( Fig. 1 ). These two competing concepts—indolent disease with the potential for late recurrences but in light of known improvements in cancer survival as time from original treatment increases—make optimal melanoma surveillance a complex challenge for patients and clinicians.
To address these challenges, current guidelines for surveillance in patients with melanoma published by the National Comprehensive Cancer Network emphasize more frequent visits with more intensive stage-specific surveillance imaging early in the posttreatment phase, when the risk of recurrence is greatest. As the disease-free interval increases, office visits and imaging should become less frequent, but annual skin examinations for all stages of primary disease should continue for the rest of the patient’s life. Annual skin examinations for life, particularly for patients with early stage, low-risk melanoma, are a logical surveillance strategy based on recurrence and survival patterns; however, recommendations become less clear for patients with locally advanced stage III melanoma. Beyond annual skin examinations, the current recommendations for patients with stage III melanoma range from conventional x-ray radiography of the chest to a complex positron emission tomography (PET)/CT scan at time intervals ranging from 3 to 12 months.
The data available to aid in the development of appropriate stage-specific surveillance recommendations are limited in scope and value. The broad generalities in surveillance recommendations very likely reflect uncertainties in modern resource use and a limited understanding of the effects of different surveillance strategies on patient quality of life and cancer survival. Toward elucidating these uncertainties and enhancing the understanding of the effects of surveillance strategies on quality of life and survival for patients with melanoma, this article reviews issues key to designing surveillance strategies, including early detection, surveillance evaluation modalities, surveillance effectiveness, variation in current surveillance practices, surveillance costs, and other practice implications.
Benefits of early detection
For patients who have undergone treatment of primary melanoma, early detection of a local recurrence has important implications. An isolated local recurrence in a patient with favorable features can be treated with repeat wide local excision, with good oncologic outcomes. For these patients, long-term prognosis is not adversely affected by the local recurrence if it is detected and treated early, and 5-year survival continues to be a function of primary tumor thickness. For those whose disease relapses in the regional lymph node basins, prognosis depends on the tumor burden at the time of detection, but early identification of regional recurrences and subsequent treatment with surgical resection can increase survival time compared with late identification of recurrences that present on clinical examination or with development of symptoms. In the recently published final report from the landmark Multicenter Selective Lymphadenectomy Trial-1, Morton and colleagues reported that for patients with intermediate-thickness melanoma, those who underwent nodal observation only and subsequently developed a clinically evident nodal recurrence had 5- and 10-year melanoma-specific survival rates of 57.5% and 41.5%, respectively, whereas patients with positive sentinel lymph node metastases found through biopsy and treated at original presentation had a 10-year melanoma-specific survival of 62.1%. The benefit of early detection of regional lymph node recurrences, therefore, highlights the importance of detecting them at a time when appropriate therapy still can be administered with curative intent.
For patients who develop distant melanoma recurrences, the appropriate therapeutic approach is highly debated. Because the lungs are the most common site of distant, metastatic melanoma involvement, many patients undergo imaging studies aimed at detecting “treatable” distant recurrences. For patients with melanoma with pulmonary involvement, the 5-year overall survival is 4%. For the 12% to 25% of patients who are candidates for surgical resection of their metastatic melanoma, 5-year survival may be modestly improved and is 30% in some studies.
In a retrospective analysis of patients enrolled in the Multicenter Selective Lymphadenectomy Trial-1, Howard and colleagues estimated that up to 50% of patients with stage IV melanoma may be candidates for surgical treatment of their metastatic disease. In that review, the median survival time was significantly longer for patients undergoing surgical resection of their metastatic disease (15.8 months) with or without systemic medical therapy than for patients who did not undergo surgery (6.9 months). The survival benefit was significantly greater among patients with a longer disease-free interval whose metastatic disease was isolated to one or two organ sites. Therefore, although patient selection bias likely plays a large role in retrospective reports of survival after surgery for distant disease recurrence, a carefully selected subset of patients with advanced-stage melanoma would likely benefit from early detection of treatable metastatic disease and subsequent surgical intervention.
Because the early detection of melanoma recurrences has a potential survival benefit for a select population of patients, optimal surveillance strategies that can impact clinical care must be defined. The primary objective of surveillance should be to detect local, regional, and distant recurrences at a time when intervention can still improve survival. Note, however, that for the increasing numbers of melanoma survivors in the United States, no available data suggest that disease control, survival, or quality of life are significantly improved with routine surveillance imaging studies. Patients with local and regional recurrences, most of whom are diagnosed clinically, have favorable 5-year survival, as high as 80%. Distant metastatic disease, however, which is usually diagnosed with routine oncologic surveillance imaging or imaging ordered as a result of symptom presentation, has uniformly poor 5-year survival once diagnosed (20% or less). These data lead to the question of whether more intensive surveillance to detect recurrences before distant disease develops would translate to improved patient survival.
Surveillance evaluation modalities
Clinical examination effectively diagnoses local recurrences in more than 50% of patients with treated melanoma in whom recurrent melanoma or second primary tumors develop. In a study of 1062 patients with early stage melanoma (I-II), self-detection and clinical physical examination identified 95% of all recurrences at a median of 17 months following a sentinel lymph node biopsy with negative results. A prospective database analysis of 118 patients with stage II or III melanoma found that 67% of recurrences were diagnosed through self-detection or symptomatic presentation. An additional 26% of recurrences were found by clinical examination on routine follow-up. A review found that 18% of 38 patients with stage III melanoma developed a recurrence at a median disease-free interval of 25 months; most these recurrences (57%) were identified by the patient or physician in a clinical examination.
Regional lymph node basins can be effectively evaluated using noninvasive ultrasonography. Studies have demonstrated that the sensitivity of ultrasonographic evaluation of the lymph node basins is 87% to 99%, with a specificity of 74% to 99% when the tumors within the lymph node are greater than 1 mm. To determine the effect of ultrasonographic surveillance in the detection of regional recurrences in patients with treated melanoma, a meta-analysis using data from a systematic review of four major medical indices was completed by Xing and colleagues. For this analysis, patient-level data were extracted from the 74 studies deemed eligible using quality assessment and were subsequently modeled using Bayesian statistics to yield findings related to the sensitivity, specificity, and diagnostic odds ratio for imaging of the regional lymph nodes and of distant metastases using ultrasonography, CT, PET, and PET/CT, with confidence intervals (CIs) to define the posterior probability distribution for interval estimation. Ultrasonography had the highest sensitivity (60%; 95% CI, 33%–83%), specificity (97%; 95% CI, 88%–99%), and diagnostic odds ratio (42; 95% CI, 8.08–249.8) for the surveillance of regional lymph nodes. Therefore, ultrasonography is superior to physical examination alone for the detection of regional lymph node recurrences ; as such, ultrasonography has been incorporated into many international guidelines for the follow-up of patients with melanoma. The same study found that for the surveillance of distant metastases, PET/CT had the highest sensitivity (80%; 95% CI, 53%–9%3), specificity (87%; 95% CI, 54%–97%), and diagnostic odds ratio (1675; 95% CI, 226.5–15,920). Summary findings for this study appear in Table 1 .
| Location and Imaging Method | % Median Sensitivity (95% Confidence Interval) | % Median Specificity (95% Confidence Interval) | Median Diagnostic Odds Ratio (95% Confidence Interval) |
|---|---|---|---|
| Regional lymph nodes | |||
| Ultrasonography | 96 (85–99) | 99 (95–100) | 1675 (226–15,920) |
| CT | 61 (15–93) | 97 (70–100) | 46 (2–1354) |
| PET | 87 (67–96) | 98 (93–100) | 391 (68–2737) |
| PET/CT | 65 (20–93) | 99 (92–100) | 196 (11–4675) |
| Distant sites | |||
| CT | 63 (46–77) | 78 (58–90) | 6 (2–18) |
| PET | 82 (72–88) | 83 (70–91) | 22 (9–51) |
| PET/CT | 86 (76–93) | 91 (79–97) | 67 (20–230) |
In the only prospective study evaluating surveillance for patients with melanoma according to stage, Garbe and colleagues followed a cohort of 3008 patients with stage I-IV melanoma over a 2-year period. Patients were evaluated according to German surveillance guidelines, with frequent physical examinations (every 3 months) and routine ultrasonographic evaluation of the tumor scar and draining lymph node basins (annually for patients with stage I disease, every 6 months for those with stage II disease, and every 3–6 months for those with stage III disease). Cross-sectional imaging with CT scan or MRI was used only to evaluate suspicious clinical or ultrasonographic findings. Nearly 50% of recurrences were detected with clinical examination alone, even in patients with stage III disease. The addition of CT performed based on clinical suspicion detected an additional 27.7% of recurrences.
Surveillance effectiveness
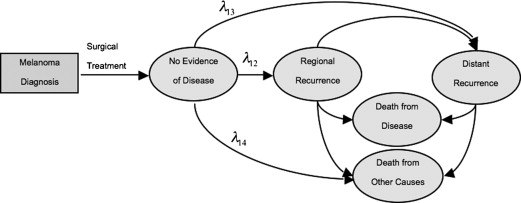
To understand the extent to which surveillance imaging translates to a survival benefit for patients with treated melanoma, Rueth and colleagues developed a decision-support model designed to evaluate the effectiveness of routine CT and PET/CT surveillance of patients with stage I-III melanoma. In this study, a probabilistic Markov model representing the stage-specific natural history of surgically treated melanoma was developed ( Fig. 2 ). The model estimates were derived from 1600 patients with melanoma who were evaluated and surgically treated at a single institution. Patient-level time-to-event data were used to determine the monthly transition probabilities, which were estimated using parametric survival models from the time of definitive local/regional treatment. The study evaluated the ability of various surveillance imaging modalities and timelines (CT or PET/CT performed every 6 or 12 months for 5 years) to detect regional or distant recurrences that could be treated with surgical resection with curative intent, using established sensitivity and specificity data for contemporary diagnostic imaging modalities including CT, PET/CT, MRI, and ultrasound.
For patients with stage I melanoma, in whom recurrence rates are low, the authors calculated that if routine surveillance imaging were performed at 12-month intervals, 362 CT scans or 249 PET/CT scans would have to be performed over the course of 5 years to diagnose one treatable recurrence in a patient with stage I disease. Furthermore, they reported that routine annual surveillance imaging for patients with stage I melanoma would result in a 5-year total of 45,296 CT scans or 45,314 PET/CT scans per 10,000 patients to detect one treatable recurrence.
In contrast, as the incidence of regional or distant recurrence increased with disease stage, so too did the number of surgically treatable recurrences detected with routine surveillance imaging increase with stage. Among the highest-risk patients (those with stage IIIC disease) the authors calculated that routine surveillance CT or PET/CT performed every 12 months would detect surgically treatable regional and distant recurrence in 6.4% and 8.4% more patients, respectively, than would annual physical examination alone. In this population, 1 of every 34 CT scans and 1 of every 26 PET/CT scans would detect a treatable recurrence.
According to these identification estimations, although more treatable recurrences were detected with the use of routine PET/CT than with CT alone, there were no differences between CT and PET/CT in the calculated 5-year disease-specific survival, regardless of disease stage. Similarly, more frequent imaging (every 6 months vs every 12 months) did not substantially increase the 5-year disease-specific survival, regardless of disease stage ( Table 2 ). The greatest survival benefit from more frequent imaging was seen for patients with stage IIIB or IIIC disease. Even in these groups, however, the difference between a less rigorous imaging strategy of CT every 12 months and a more rigorous imaging strategy of PET/CT every 6 months in terms of 5-year disease-specific survival was minimal.
| Stage/Modality | 6-mo Examination Interval | 12-mo Examination Interval | ||||
|---|---|---|---|---|---|---|
| 5-y NED (%) | 5-y DSS (%) | Average Increase in Life Expectancy (mo) | 5-y NED (%) | 5-y DSS (%) | Average Increase in Life Expectancy (mo) | |
| Stage I | ||||||
| CT | 87.7 | 91.8 | 0.3 | 87.7 | 91.5 | 0.2 |
| PET/CT | 87.7 | 91.9 | 0.4 | 87.7 | 91.5 | 0.2 |
| Stage II | ||||||
| CT | 70.5 | 77.0 | 0.9 | 70.5 | 76.2 | 0.5 |
| PET/CT | 70.5 | 77.4 | 1.1 | 70.5 | 76.3 | 0.5 |
| Stage IIIA | ||||||
| CT | 72.0 | 76.1 | 0.8 | 72.0 | 75.7 | 0.4 |
| PET/CT | 72.0 | 76.3 | 0.9 | 72.0 | 75.8 | 0.4 |
| Stage IIIB | ||||||
| CT | 48.3 | 52.9 | 1.4 | 48.3 | 52.4 | 0.7 |
| PET/CT | 48.3 | 53.4 | 1.7 | 48.3 | 52.5 | 0.7 |
| Stage IIIC | ||||||
| CT | 32.1 | 37.0 | 1.8 | 32.1 | 36.4 | 0.8 |
| PET/CT | 32.1 | 37.6 | 2.0 | 32.1 | 36.5 | 0.8 |
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree