In-transit melanoma is an uncommon pattern of recurrence, but presents unique management challenges and opportunities for treatment. The clinical presentation usually involves from 1 to more than 100 small subcutaneous or cutaneous nodules, ranging from submillimeter to multiple centimeters in diameter. Regional chemotherapy techniques are a mainstay of treatment of patients without systemic disease spread. Future applications of regional therapy are likely to involve combination therapy with cytotoxic agents and novel immune modulators. Regional therapy provides distinct opportunities for the treatment of unresectable disease, and offers a unique platform for investigation of novel therapeutics in early-stage clinical trials.
Key points
- •
In-transit disease is a challenging pattern of recurrence to manage, occurring in up to 10% of patients with melanoma.
- •
Regional therapy, by isolated limb infusion or hyperthermic isolated limb perfusion, offers treatment options for patients with nonresectable disease.
- •
Regional therapies are generally well tolerated, and offer complete response rates of 25% to 50%, although long-term responses are often not durable.
- •
Future management of in-transit melanoma is likely to involve combination therapies, joining cytotoxic regional therapy and systemic immune-modulating drugs such as anti–cytotoxic T-lymphocyte antigen 4 (CTLA-4) and anti-programmed cell death 1 surface protein molecule agents.
Introduction
In-transit melanoma occurs in up to 10% of patients with melanoma, and is a pattern of recurrence that presents unique management challenges and opportunities for treatment. In-transit disease is defined as tumor deposits that usually occur somewhere between the primary lesion and its draining regional lymph node basin. Although often associated with distant metastases, the presence of in-transit disease is an independent adverse prognostic factor. Unique treatment modalities, in the form of regional chemotherapy, are often necessary because this pattern of recurrent disease is often not amenable to surgical resection.
Introduction
In-transit melanoma occurs in up to 10% of patients with melanoma, and is a pattern of recurrence that presents unique management challenges and opportunities for treatment. In-transit disease is defined as tumor deposits that usually occur somewhere between the primary lesion and its draining regional lymph node basin. Although often associated with distant metastases, the presence of in-transit disease is an independent adverse prognostic factor. Unique treatment modalities, in the form of regional chemotherapy, are often necessary because this pattern of recurrent disease is often not amenable to surgical resection.
Background
Incidence
In-transit disease is uncommon, occurring in less than 10% of patients diagnosed with melanoma and accounting for 12% to 22% of melanoma recurrences. The presence of positive nodal disease significantly increases the risk of developing in-transit disease, with estimates suggesting risks as high as 31% when at least 3 positive nodes are present. Although initial disease stage seems to be the most important factor in predicting in-transit recurrences, lesion location may also play a role, with higher rates in the lower extremities versus upper extremities. Early observations also suggested an association between surgical lymphadenectomy and the development of in-transit disease, presumably caused by lymphatic trapping in which outflow obstruction of the draining lymphatic system led to stasis and trapping of tumor deposits. However, in recent larger studies, neither lymphadenectomy nor sentinel lymph node biopsy were associated with an increased risk of developing in-transit metastases.
Biology
Although the true underlying biology of in-transit melanoma is unknown, it is thought to be related to small tumor emboli disseminating along the path of lymphatic drainage from the primary tumor to its draining nodal basin. These migrating tumor cells are thought to become ensnared in the draining dermal and subdermal lymphatics, eventually progressing to clinically detectable lesions. Although tumor deposits becoming trapped along the lymphatic drainage remains the most likely biological explanation, other mechanisms have been suggested, including hematogenous spread, similar to that of distant metastases. Supporters of this alternative theory argue that, if the lymphatic concept is true, wider margins during primary excision would be expected to include a higher proportion of trapped occult cells and lead to superior clinical outcomes, which is not consistent with current observations. However, the hematogenous theory is not supported by the significant differences in long-term survival observed for patients with stage III versus stage IV disease.
Nomenclature
Several different terms have been used in the literature to describe what is likely to be the same underlying oncologic process. In the past, terms such as satellitosis, locoregional recurrence, and in-transit disease have each been used to describe various clinical findings. Satellitosis was usually defined as a locoregional recurrence that was located within either 2 cm of the excision scar or 5 cm of the initial lesion, whereas the term in-transit disease was reserved for a recurrence occurring at greater distances from the initial lesion or scar. Because such lesions all likely reflect tumor deposits proliferating along paths of lymphatic drainage, it has more recently become apparent that distance from the primary lesion to the site of locoregional recurrence does not carry meaningful prognostic value. Consequently, the most recent American Joint Committee on Cancer (AJCC) staging system for melanoma does not distinguish between traditional satellitosis and in-transit lesions, with both being designated as N2 or N3 disease, depending on regional node status. To address the ongoing ambiguity arising from nomenclature issues, many authorities no longer use the term satellitosis, instead referring to all regional nonnodal metastatic disease as in-transit melanoma.
Patient evaluation overview
Presentation
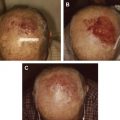
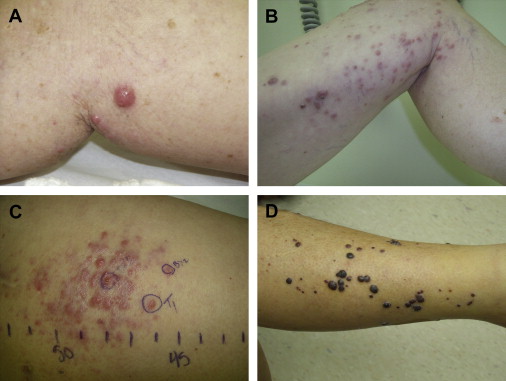
By definition, in-transit melanoma represents locoregionally advanced disease, and is typically discovered months to years after the initial surgical excision of the primary lesion, with a disease-free interval to recurrence ranging from 12 to 16 months. The clinical presentation usually involves from 1 to more than 100 small subcutaneous or cutaneous nodules, although it can be variable ( Fig. 1 ). Individual lesions can range from submillimeter to multiple centimeters in diameter, and may take the form of superficial cutaneous (also called epidermotropic) or deeper subcutaneous nodules. For disease located in an extremity, the lesions are often clustered in proximity to the primary lesion, but can also involve the entire extremity extending from the primary tumor to its regional lymphatic basin. For truncal disease, the pattern of distribution can be even more inconsistent, with potentially extensive tumor burden depending on the location of the primary melanoma.
Evaluation
The work-up of patients with in-transit melanoma starts with a thorough skin examination, and in patients with an unclear diagnosis and suspicious lesions it is our practice to use a 3-mm to 5-mm punch biopsy for small superficial lesions and fine-needle aspiration biopsy for larger lesions to obtain pathologic confirmation of melanoma. All major nodal basins should be examined for the presence of clinically involved nodes, and preoperative cross-sectional imaging should be performed to evaluate for distant metastatic spread. In patients who have disease isolated to an extremity and are candidates for general anesthesia, regional chemotherapy should be considered as a first-line therapy.
Dosing
Drug doses for regional therapy are typically based on limb volume, either measured directly or imputed. For hyperthermic isolated limb perfusions (HILPs), melphalan doses of 10 mg/L for the lower limb and 13 mg/L for the upper limb are typically used. For isolated limb infusion (ILI), slightly lower doses are used; typically 7.5 mg/L for the lower limb and 10 mg/L for the lower limb, both usually with the addition of 50 to 100 μg/L of actinomycin-D.
Multiple techniques to determine the optimal dose for regional therapy have been developed, with the simplest methods relying on patient body weight and/or ideal body weight, offering simple and fast calculations. In recent years such techniques have largely been abandoned, because they have been shown to produce substantial variability compared with more accurate limb volume measurements. The original methods described by Wieberdink and colleagues for estimating limb volume relied on a method of measuring water displacement from the submerged limb. Although accurate and still used, this method is hampered by logistical issues related to the nature of the water reservoir apparatus.
An alternative method that has more recently gained popularity is calculation based on limb circumference. In this approach, circumference measurements are taken at standardized intervals (usually 1.5 cm) along the length of the extremity. By calculating the volume of each theoretic slice based on the limb circumference at that level, and summing across the limb, highly accurate estimates of limb volume can be obtained. This method offers distinct advantages compared with water displacement techniques, in that it is inexpensive, does not require specialized equipment or facilities, and can be performed at the bedside and on immobile or otherwise functionally impaired individuals.
Although numerous techniques for limb volume calculation have been described, further adjustment is often required, particularly in the setting of obesity in order to avoid excessively large doses of chemotherapy being delivered to limbs that have a disproportionately large amount of fat. Although there remains some debate regarding the role of weight-based dose adjustment for ILI, in our experience, multiplying the calculated limb volume by the quotient of ideal body weight (IBW) and actual body weight to obtain an IBW-adjusted limb volume has helped minimize unnecessary toxicity without compromising clinical response. Furthermore, recent work by Podleska and colleagues has confirmed that this method gives lean limb volume estimates that correlate extremely well with no-fat computed tomography scans, which digitally subtract subcutaneous fat tissue.
Regional chemotherapy
History, Concept, and Overview
The origins of limb infusion can be traced back to 1950, when Klopp and colleagues discovered that intra-arterial infusion of nitrogen mustards proved substantially more effective than intravenous administration. However, it was not until 1958 that Creech and colleagues first described what is now considered limb perfusion, adopting advances in cardiopulmonary bypass to isolate and oxygenate the relevant area and administer high-dose intra-arterial chemotherapy. A decade later, Stehlin added hyperthermia to the treatment, with improved results.
The fundamental concept of regional chemotherapy for in-transit melanoma entails vascular isolation of the affected limb, followed by high-dose chemotherapy delivery at doses up to 20 times larger than can be tolerated systemically. Because regional therapy requires isolation of the affected limb compartments, the major inflow and outflow vessels to the area of interest must be accessed and cannulated, and the treatment region must then be completely isolated from the systemic circulation, usually by means of a pneumatic or Esmarch tourniquet. The 2 general approaches to accomplishing this are HILP and ILI. Although both procedures involve regional delivery of high-dose chemotherapy, they differ in several important technical and conceptual aspects.
Isolated Limb Perfusion
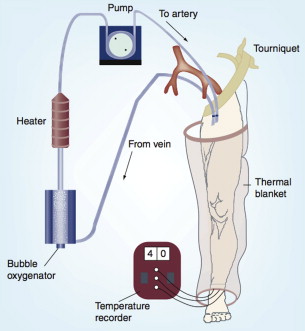
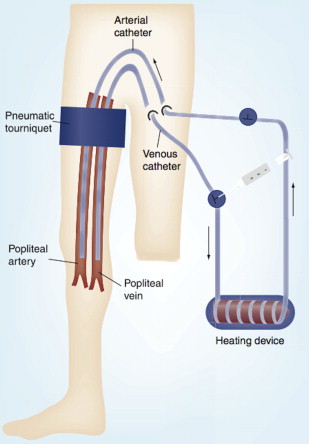
HILP as originally described by Creech and colleagues is performed under general anesthesia, and requires exposure and direct cannulation of the vasculature supplying the affected limb. An oncologic regional lymphadenectomy is typically performed concurrently, which has the added benefit of aiding vascular exposure, particularly when accessing the iliac vessels. A proximal tourniquet is then placed, and perfusion is initiated via the cannulated vessels, using a standard extracorporeal bypass circuit with membrane oxygenation to maintain physiologic limb oxygen tension and acid-base status ( Fig. 2 ). To achieve hyperthermia with a goal of 40°C, the circuit is heated and external warming blankets are placed on the involved limb. Chemotherapy is typically perfused through the limb circuit for 60 minutes, followed by a washout period of 20 to 30 minutes with crystalloids to facilitate removal of the cytotoxic agents. During the perfusion portion of the procedure, leakage of perfusate into the systemic circulation can pose substantial risks of toxicity, particularly when high-dose tumor necrosis factor alpha (TNF-α) is used in conjunction with cytotoxic therapy. In the past, such leakage was monitored by injecting intravenous fluorescein into the perfusion circuit, and watching for evidence of staining proximal to the tourniquet. More recently, administration of a radiolabeled tracer into the HILP circuit with continuous monitoring of systemic radiation exposure using a precordial gamma probe has provided a more precise method of leak detection. At the conclusion of the procedure, the cannulas are removed, the blood vessels repaired, the tourniquet is removed, and a careful vascular and musculoskeletal examination is performed.
Isolated Limb Infusion
ILI was first described by Thompson and colleagues in the mid-1990s as a less-invasive alternative to HILP. During ILI, the target limb vessels are cannulated using percutaneously placed catheters inserted with the aid of fluoroscopy. Similar to HILP, the limb is isolated using an external tourniquet, and is then wrapped in heating blankets. In contrast with HILP, ILI is performed without extracorporeal perfusion and oxygenation, and the perfusate is manually circulated using a syringe and 3-way stopcock ( Fig. 3 ). As a result, the limb becomes markedly acidotic and hypoxic during the procedure, which some clinicians have suggested augments the effectiveness of the melphalan chemotherapy. Although warming blankets and a heat exchanger are typically used, limb temperatures observed during ILI are typically lower than those seen during HILP, and often do not exceed 38.5°C to 39.0°C.

Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree