Tumors and Tumor-like Lesions of the Joints
Benign Lesions
Synovial (Osteo)chondromatosis
Synovial osteochondromatosis (also known as synovial chondromatosis or synovial chondrometaplasia) is an uncommon benign disorder marked by the metaplastic proliferation of multiple cartilaginous nodules in the synovial membrane of the joints, bursae, or tendon sheaths (26,27,30,42). It is almost invariably monoarticular; rarely, multiple joints may be affected (40,43,44).
Clinical Presentation
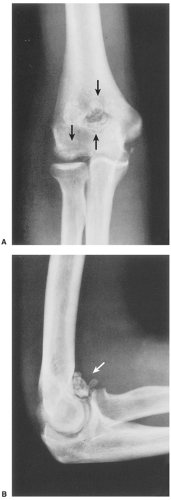
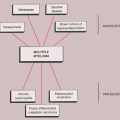
The disorder is twice as common in men as in women and is usually discovered in the third to the fifth decade (27). The knee is a preferential site of involvement, with the hip, shoulder, elbow, and ankle accounting for most of the remaining cases (6,7,12,23,39) (Fig. 9-1). Patients usually report pain and swelling. Joint effusion, tenderness, limited motion in the joint, and a soft tissue mass are common clinical findings (11).
Imaging
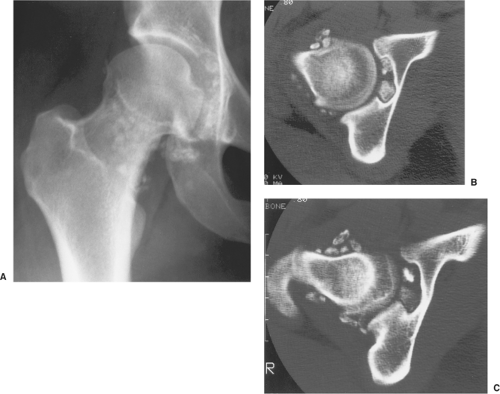
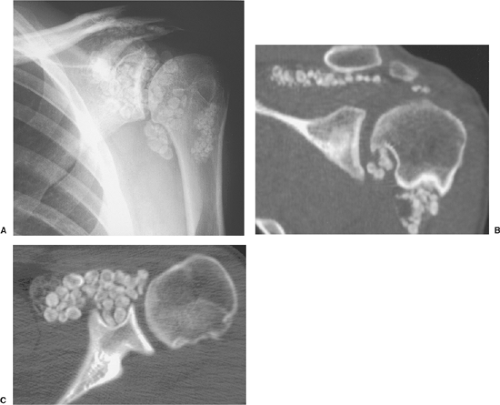
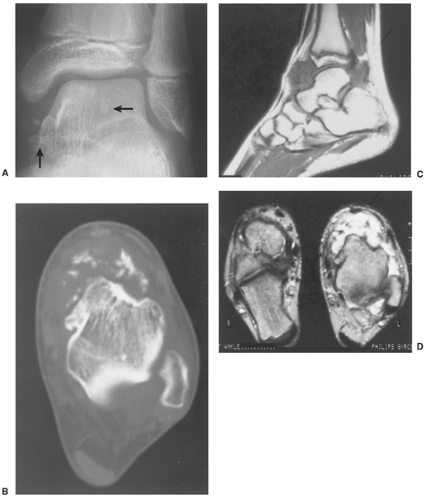
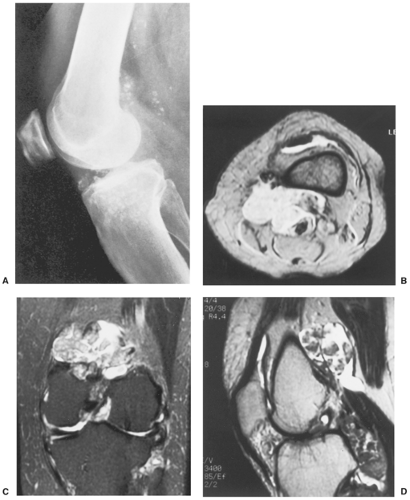
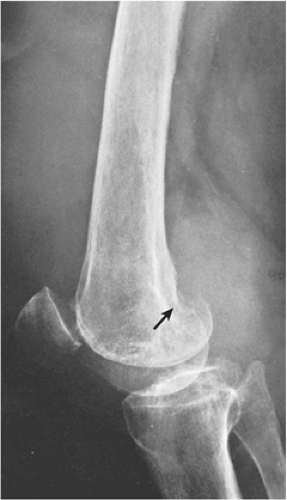
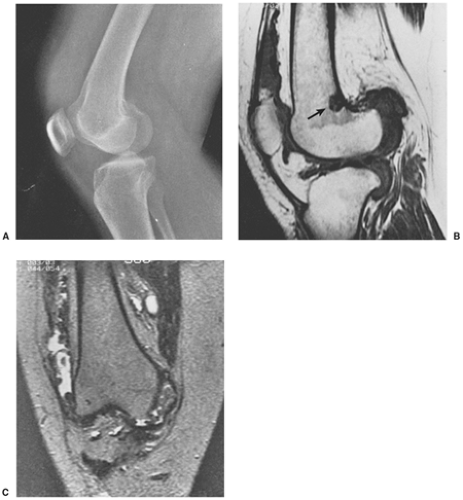
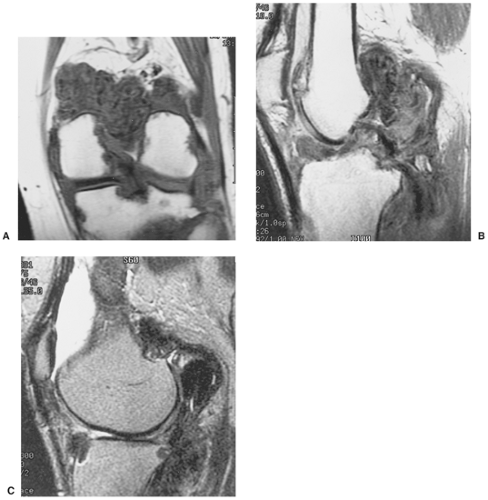
The radiographic findings depend on the degree of calcification within the cartilaginous bodies, ranging from mere joint effusion to visualization of many radiopaque joint bodies, usually small and uniform in size (31,33) (Fig. 9-2). The best proof that the bodies are indeed intraarticular is achieved by arthrography or computed tomography (CT) (3) (Figs. 9-3 and 9-4). These modalities can visualize even noncalcified bodies (13). MRI may also be helpful: calcifications can be seen as signal void on T2-weighted images against the high-signal-intensity fluid and an inflamed hyperplastic synovium (22,24,25,44,45) (Figs. 9-5 and 9-6). In addition to revealing loose bodies in the joint, these modalities may demonstrate bone erosion (34) and extracapsular extension of the lesion (38).
Histopathology
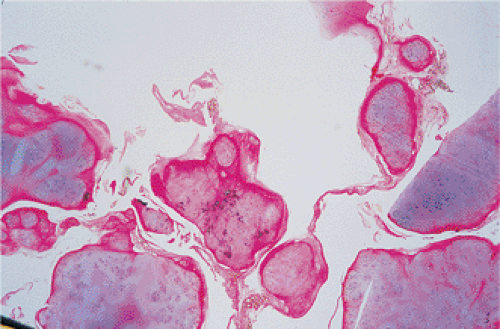
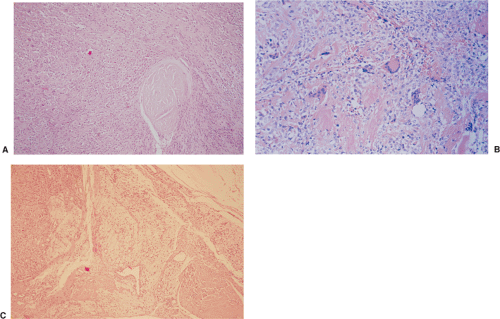
By microscopy, many cartilaginous nodules are observed as they form beneath the thin layer of cells that line the surface of the synovial membrane (12,36) (Fig. 9-7). These nodules are highly cellular, and the cells
themselves may exhibit a moderate pleomorphism, with occasional plump and double nuclei (11). Although such cytologic atypia merely indicates an actively growing cartilaginous focus, an erroneous diagnosis of malignancy may sometimes be considered (Fig. 9-8). The cartilaginous nodules, which often are undergoing calcification and endochondral ossification, may detach and become loose bodies. The loose bodies continue to be viable and may increase in size as they receive nourishment from the synovial fluid (18), but they ossify no more.
themselves may exhibit a moderate pleomorphism, with occasional plump and double nuclei (11). Although such cytologic atypia merely indicates an actively growing cartilaginous focus, an erroneous diagnosis of malignancy may sometimes be considered (Fig. 9-8). The cartilaginous nodules, which often are undergoing calcification and endochondral ossification, may detach and become loose bodies. The loose bodies continue to be viable and may increase in size as they receive nourishment from the synovial fluid (18), but they ossify no more.
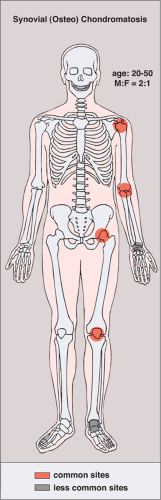 Figure 9-1 Synovial (osteo)chondromatosis: skeletal sites of predilection, peak age range, and male-to-female ratio. |
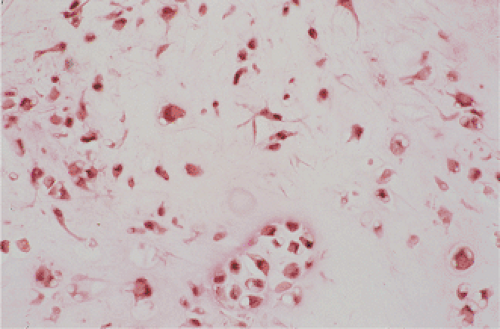
Mitoses in synovial chondromatosis are rare. However, subpopulations of cells appear to proliferate, according to immunohistochemistry (IHC) results and those of studies using image cytometry (8,32). Robinson et al. (37) also demonstrated that proliferating cells in cartilaginous nodules of synovial chondromatosis simultaneously express fibroblast growth factor receptor 3 (FGFR3) and its ligand, fibroblast growth factor 9 (FGF9), found in the synovial fluid, obviously produced by synoviocytes. This would constitute an autocrine loop that might be expected in tumor cells but not in a benign lesion. In addition, cytogenetic studies revealed clonal chromosomal aberrations with involvement of chromosome 6 (losses or rearrangements) in five of eight thus far reported cases, indicating that synovial chondromatosis may be a neoplastic rather than a metaplastic lesion (4,41). The expression of FGFR3 is regulated by sonic hedgehog (SHH), an important gene involved in embryonic development and in tumorigenesis (1,29). A negative regulator of the SHH gene, Rab23, is localized on chromosome 6p11 (10). Buddingh et al. (4) hypothesized that rearrangements of chromosome 6 may inactivate Rab23, leading to activation of SHH that, in turn, acts on FGFR3 expression. The involvement of SHH signaling in the development of synovial chondromatosis was recently demonstrated by Hopyan et al. (19) using transgenic mice defective in a transcriptional repressor for SHH.
Differential Diagnosis
Radiology
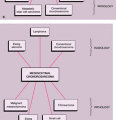
This condition should be differentiated from the secondary osteochondromatosis caused by osteoarthritis, particularly in the knee and hip joints, and from synovial chondrosarcoma, either primary (arising de novo from the synovial membrane) or secondary (due to malignant transformation).
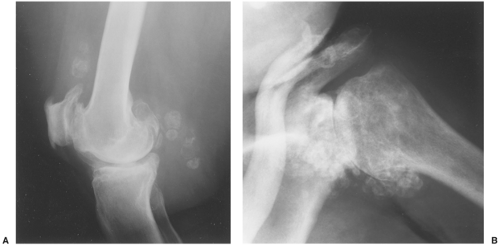
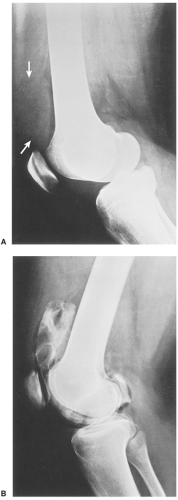
Distinguishing primary synovial osteochondromatosis from secondary osteochondromatosis usually presents no problems. In the latter condition, there is invariably radiographic evidence of osteoarthritis with all of its typical features, such as narrowing of the radiographic joint space, subchondral sclerosis, and, occasionally, periarticular cysts or cyst-like lesions (Fig. 9-9). The loose bodies are fewer, larger, and invariably of different sizes. Conversely, in primary synovial chondromatosis the joint is not affected by any degenerative changes. In some cases, however, the bone may show erosions secondary to pressure of the calcified bodies on the outer aspects of the cortex. The intraarticular bodies are numerous, small, and usually of uniform size (see Fig. 9-2).
More difficult is to distinguish synovial chondromatosis from synovial chondrosarcoma. The clinical and radiographic features have not been useful in this differentiation and are equally ineffective in distinguishing a secondary malignant lesion arising in synovial chondromatosis (2). In addition, both entities tend to have a protracted clinical course, and local recurrence is common after synovectomy for synovial chondromatosis or local resection of synovial chondrosarcoma (17). The presence of frank bone destruction rather than merely erosions, and the association of a soft tissue mass, should always raise the concern about malignancy (14) (see Fig. 9-41). Although extension beyond the joint capsule should heighten the suspicion of malignancy, some cases of synovial chondromatosis have
been reported to have an extraarticular extension (5,31).
been reported to have an extraarticular extension (5,31).
The other conditions that can radiologically mimic synovial chondromatosis include pigmented villonodular synovitis (PVNS), synovial hemangioma, and lipoma arborescens (15,16).
In PVNS the filling defects in the joint are more confluent and less distinct. Magnetic resonance imaging (MRI) may show foci of decreased intensity of the synovium in all sequences because of the paramagnetic effects of deposition of hemosiderin (18) (see Fig. 9-14). Synovial hemangioma usually presents as a single soft tissue mass. On MRI, T1-weighted images show that the lesion is either isointense or slightly higher (brighter) in signal intensity than surrounding muscles but much lower in intensity than subcutaneous fat. On T2-weighted images the mass is invariably much brighter than fat (15) (see Figs. 9-26 and 9-27). Phleboliths and fibrofatty septa in the mass are common findings that show low signal characteristics.
Lipoma arborescens is a villous lipomatous proliferation of the synovial membrane (35). This rare condition usually affects the knee joint but has occasionally been reported in other joints, including the wrist and ankle (16). The disease has been variously reported to have a developmental, traumatic, inflammatory, or neoplastic origin, but its true etiology is still unknown. The clinical findings include slowly increasing but painless synovial thickening as well as joint effusion with sporadic exacerbation. Radiographic studies reveal a joint effusion accompanied by various degrees of osteoarthritis. MRI is diagnostic, showing frond-like masses arising from the synovium that have the signal intensity of fat on all imaging sequences (see Fig. 9-30). Histologic examination demonstrates complete replacement of the subsynovial tissue by mature fat cells and the formation of proliferative villous projections.
Pathology
From the histopathologic standpoint it may be very difficult to distinguish synovial chondrosarcoma from synovial chondromatosis (20,21,28). The histopathologic distinction between primary and secondary malignant synovial lesions and synovial chondromatosis has been a matter of dispute. Occasional foci of increased cellularity showing hyperchromatic atypical cells, consistent with grade 1 chondrosarcoma, should not be considered sufficient evidence for a malignant change in synovial chondromatosis. However, evidence of aggressive growth (invasion), arrangement of atypical chondrocytes in sheets, spindling of cells in the periphery, presence of mitoses, myxoid change of the matrix, and lack of attachment of a lesion to the synovial lining support the diagnosis of malignancy (2,9) (see Fig. 9-43).
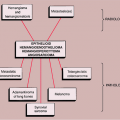
The radiologic and pathologic differential diagnosis of synovial chondromatosis is depicted in Figure 9-10.
Pigmented Villonodular Synovitis
PVNS is a locally destructive fibrohistiocytic proliferation, characterized by many villous and nodular synovial protrusions (11), which affects joints, bursae, and tendon sheaths (46,77,109). PVNS was first described by Jaffe, Lichtenstein, and Sutro (94) in 1941, who used
this name to identify the lesion because of its yellow-brown, villous, and nodular appearance. The yellow-brown pigmentation is due to excessive deposits of lipid and hemosiderin. This condition can be diffuse or localized. When the entire synovium of the joint is affected and there is a major villous component, the condition is referred to as diffuse PVNS. When a discrete intraarticular mass is present, the condition is called localized PVNS (84,88,110) or (in the European literature) pigmented giant cell tumor of articulations. When the process affects the tendon sheaths, it is called localized giant cell tumor of the tendon sheaths or nodular tenosynovitis (69,98,110,111,127,143). The diffuse form usually occurs in the knee, hip, elbow, or wrist and accounts for 23% of cases (103). The localized nodular form is often regarded as a separate entity. It consists of a single polypoid mass attached to the synovium. Nodular tenosynovitis is most often seen in the fingers and is the second most common soft tissue tumor of the hand, exceeded only by the ganglion (133). In the new revised classification of soft tissue tumors (2002), the World Health Organization (WHO) classifies localized intra- and extraarticular lesion as giant cell tumor of sheath (72), whereas diffuse intra- and extraarticular forms are categorized as diffuse-type giant cell tumor (keeping PVNS as a synonym) (71).
this name to identify the lesion because of its yellow-brown, villous, and nodular appearance. The yellow-brown pigmentation is due to excessive deposits of lipid and hemosiderin. This condition can be diffuse or localized. When the entire synovium of the joint is affected and there is a major villous component, the condition is referred to as diffuse PVNS. When a discrete intraarticular mass is present, the condition is called localized PVNS (84,88,110) or (in the European literature) pigmented giant cell tumor of articulations. When the process affects the tendon sheaths, it is called localized giant cell tumor of the tendon sheaths or nodular tenosynovitis (69,98,110,111,127,143). The diffuse form usually occurs in the knee, hip, elbow, or wrist and accounts for 23% of cases (103). The localized nodular form is often regarded as a separate entity. It consists of a single polypoid mass attached to the synovium. Nodular tenosynovitis is most often seen in the fingers and is the second most common soft tissue tumor of the hand, exceeded only by the ganglion (133). In the new revised classification of soft tissue tumors (2002), the World Health Organization (WHO) classifies localized intra- and extraarticular lesion as giant cell tumor of sheath (72), whereas diffuse intra- and extraarticular forms are categorized as diffuse-type giant cell tumor (keeping PVNS as a synonym) (71).
Both the diffuse and the localized forms of villonodular synovitis usually occur as a single lesion, mainly in young and middle-aged individuals of either sex (67,119). One of the most characteristic findings in PVNS is the ability of the hyperplastic synovium to invade the subchondral bone, producing cysts and erosions. Although the etiology is unknown (78) and is often controversial (86,87), some investigators have suggested an autoimmune pathogenesis (57). Trauma also is a suspected cause, because similar effects have been produced experimentally in animals by repeated injections of blood into the knee joint (133,148). Some investigators have suggested a disturbance in lipid metabolism as an etiologic factor (82,91). It has also been postulated by Jaffe et al. (94) that these lesions may represent an inflammatory response to an unknown agent, and by Stout and Lattes (138), and later by Ray et al. (128) that they are true benign neoplasms. Although the latter theory was supported by pathologic studies indicating that the histiocytes present in PVNS may function as facultative fibroblasts (116) and that foam cells may derive from histiocytes (73), relating PVNS to a benign neoplasm of fibrohistiocytic origin (86), it was presumed that these findings did not constitute definite proof that PVNS is a true neoplasm but rather a special form of a chronic proliferative inflammation process, as has already been postulated by Jaffe et al. (94). However, the most recent cytogenetic investigations have shed a new light on this subject. Clonal aberrations have been described in both localized and diffuse forms of PVNS, presenting a near- or pseudo-diploid karyotype and rearrangements of chromosome 1p, preferentially at 1p11-p13 (122,132). Aneuploid forms (in 3 of 7 diffuse-type tenosynovial giant cell tumors of tendon sheaths and 1 of 15 in diffuse type tenosynovial giant cell tumor of the knee joint) have
been described in DNA flow cytometric studies (47,54). In addition, Berger et al. (54) demonstrated chromosomal gains in 5 of 15 cases by CGH, preferentially at 22q, 16p, and 16q. These data are in favor of a neoplastic origin of PVNS. Gains of chromosomes 5 and 7 have been reported only in diffuse forms of tendon sheath giant cell tumors, indicating possible differences between localized and diffuse types (71,117). However, because at least gains of chromosomes 5 and 7 have been reported in osteoarthritis and rheumatoid arthritis, the significance of these findings remains to be determined (68,114).
been described in DNA flow cytometric studies (47,54). In addition, Berger et al. (54) demonstrated chromosomal gains in 5 of 15 cases by CGH, preferentially at 22q, 16p, and 16q. These data are in favor of a neoplastic origin of PVNS. Gains of chromosomes 5 and 7 have been reported only in diffuse forms of tendon sheath giant cell tumors, indicating possible differences between localized and diffuse types (71,117). However, because at least gains of chromosomes 5 and 7 have been reported in osteoarthritis and rheumatoid arthritis, the significance of these findings remains to be determined (68,114).
Clinical Presentation
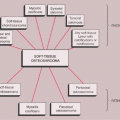
Clinically, PVNS is a slowly progressive process that manifests as mild pain and joint swelling with limitation of motion (48,78,137,144,149). Occasionally, increased skin temperature is noted over the affected joint (73). The knee joint is most commonly affected, and 66% of patients present with a bloody joint effusion (97). In fact, the presence of a serosanguinous synovial fluid in the absence of a history of recent trauma should strongly suggest the diagnosis of PVNS (86). The synovial fluid contains elevated levels of cholesterol (59,121), and fluid reaccumulates rapidly after aspiration (89). Other joints may be affected, including the hip, ankle, wrist, elbow, and shoulder (49,50,58,66,124,131). There is a 2:1 predilection for females. Patients range from 4 to 60 years of age, with a peak incidence in the third and fourth decades (11,113) (Fig. 9-11). The duration of symptoms can range from 6 months to as long as 25 years (51,70,105,106).
Although a few “malignant” PVNS have been reported in the literature (55,99), this diagnosis is still debatable. Recently, attention has been drawn to the extraarticular form of diffuse PVNS, also referred to as diffuse-type giant cell tumor. This condition is characterized by the presence of an infiltrative, extraarticular mass with or without involvement of the adjacent joint (100). This presentation of PVNS creates a real diagnostic challenge for both radiologist and pathologist because its extraarticular location, invasion of the bones, and more varied histologic infiltrative pattern may suggest malignancy (100,134).
Imaging
Radiography reveals a soft tissue density in the affected joint, frequently interpreted as joint effusion. However, the density is greater than that of a simple effusion, and it reflects not only a hemorrhagic fluid but also lobulated synovial masses (Fig. 9-12). A marginal, well-defined erosion of subchondral bone with a sclerotic margin may be present [incidence reported from 15% (61) to 50% (74)], usually on both sides of the affected articulation (75,150). Narrowing of the joint space has also been reported (86). In the hip, multiple cyst-like or erosive areas involving non-weight-bearing regions of the acetabulum, as well as the femoral head and neck, are characteristic (65). Calcifications are encountered only in exceptional cases (52).
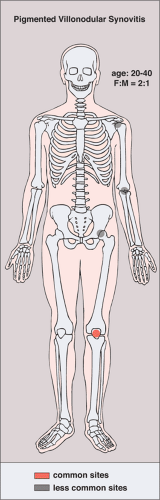 Figure 9-11 Pigmented villonodular synovitis: skeletal sites of predilection, peak age range, and male-to-female ratio. |
Arthrography reveals multiple lobulated masses with villous projections, which appear as filling defects in the contrast-filled suprapatellar bursa (Fig. 9-13). CT effectively demonstrates the extent of the disease (60,102). The increase in iron content of the synovial fluid results in high Hounsfield values, a feature that can help in the differential diagnosis (129). MRI is extremely useful in making a diagnosis (53,56,107,126,135,136,139,141,146) because on T2-weighted images the intraarticular masses demonstrate a combination of high-signal-intensity areas, representing fluid and congested synovium, interspersed with areas of intermediate to low signal intensity, secondary to random distribution of hemosiderin in the synovium (Fig. 9-14) (76,96). In general, MRI shows a low signal on both T1- and T2-weighted images because of hemosiderin deposition and thick fibrous tissue (24,120) (Fig. 9-15). In addition, within the mass, signals consistent with fat can be noted, which are due to clumps of lipid-laden macrophages (93). Other MRI findings include hyperplastic synovium and occasionally bone erosions. Administration of gadolinium in the form of Gd-DTPA
leads to a notable increase in overall heterogeneity, which tends toward an overall increase in signal intensity of the capsule and septae (93). This enhancement of the synovium allows it to be differentiated from the fluid invariably present, which does not enhance. Apart from its diagnostic effectiveness, MRI also is useful in defining the extent of the disease (92,112).
leads to a notable increase in overall heterogeneity, which tends toward an overall increase in signal intensity of the capsule and septae (93). This enhancement of the synovium allows it to be differentiated from the fluid invariably present, which does not enhance. Apart from its diagnostic effectiveness, MRI also is useful in defining the extent of the disease (92,112).
Histopathology
On histologic examination, PVNS reveals a tumor-like proliferation of the synovial tissue. A dense infiltration of mononuclear histiocytes is observed, accompanied by plasma cells, lymphocytes, and variable numbers of resorptive giant cells (Fig. 9-16A, B). The villi are made up of a matrix that contains collagen and reticulin fibers, with a varied cell population. The villi are also covered by hyperplastic and reactive-appearing synovial cells that may contain a large amount of hemosiderin (11). The synovial layer merges gradually with the underlying cell infiltrate that occupies the central core of the nodules (11). The stroma of the nodules contains variable amounts of collagen fibers and foci of large, epithelioid histiocytic cells that exhibit a macrophagic activity for hemosiderin and lipids (mainly cholesterol) (61,118) (Fig. 9-16C). Between these areas giant cells are present, some of which contain up to 50 nuclei (61,74). Dilatation of the blood vessels is often observed, and the histiocytic elements may contain variable amounts of hemosiderin or lipids (150). Mitotic figures are occasionally seen in the stromal and synovial cells (11). Electron microscopic studies have demonstrated that the histiocytic cell population of
PVNS is not uniform but contains two basic cell types: (a) fibroblasts that produce collagen and proteoglycan and (b) macrophages that contain hemosiderin and lipids (85,86,130).
PVNS is not uniform but contains two basic cell types: (a) fibroblasts that produce collagen and proteoglycan and (b) macrophages that contain hemosiderin and lipids (85,86,130).
The pathologic spectrum of PVNS parallels the clinical features. Over time, the cellularity of the synovium diminishes and the degree of fibrosis increases (74). As the stroma becomes more fibrous, the numbers of foamy macrophages decrease and they may eventually disappear. Lesser numbers of lymphocytes and plasma cells remain, often in a perivascular distribution. Hemosiderin also persists, although much of it usually disappears (74).
Differential Diagnosis
Radiology
The most common diagnostic possibilities include hemophilia, synovial chondromatosis, synovial hemangioma, osteoarthritis, rheumatoid arthritis, and tuberculous arthritis (7,63,83,108,123). MRI is very effective in the differential diagnosis because it can reveal the presence of hemosiderin deposition in PVNS (76) (see Fig. 9-14C), whereas neither monoarticular rheumatoid arthritis nor synovial hemangioma clearly exhibits this feature. Some investigators, however, postulate that both hemophilia and synovial hemangioma may be associated with an intraarticular bleeding, and hence the hemosiderin deposition (119). Detection of diffuse hemosiderin clumps, synovial irregularity and thickening, and distention of the synovial sac usually suggest the diagnosis of PVNS (140).
Synovial chondromatosis may manifest with pressure erosions of the bone similar to those of PVNS, but it can be distinguished by the presence of multiple joint bodies, calcified or uncalcified (18) (see Fig. 9-6). Synovial hemangioma is usually associated with the formation of phleboliths (15). Synovial sarcoma tends to have a shorter T1 and longer T2 on MRI compared with PVNS, and when calcifications are present the latter diagnosis
can be excluded. Lipoma arborescens has a very characteristic appearance on MRI, with its signal compatible with fatty tissue and its typical frondlike synovial projections (see Fig. 9-30). In addition, there is very little or no enhancement of the mass after administration of gadolinium (16,90).
can be excluded. Lipoma arborescens has a very characteristic appearance on MRI, with its signal compatible with fatty tissue and its typical frondlike synovial projections (see Fig. 9-30). In addition, there is very little or no enhancement of the mass after administration of gadolinium (16,90).
Hemophilia, unlike PVNS, usually affects multiple joints. The growth disturbances typical of this disorder are not a feature of PVNS. Tuberculous arthritis, like PVNS, favors the knee joint and the hip and tends to be monoarticular. On the radiographs, characteristic subchondral cysts and articular erosions are present, with relative preservation (at least in the early stages) of the joint space. However, the most consistent radiographic finding of tuberculosis arthritis is severe juxtaarticular osteoporosis, which is not seen in PVNS.
Monoarticular osteoarthritis (degenerative joint disease) commonly occurs in an older population. Osteoarthritis affecting large joints, such as the knee and hip, may be characterized by cyst-like bony lesions that resemble those of PVNS. The presence of juxtaarticular soft tissue masses is helpful in distinguishing PVNS from osteoarthritis and the former usually exhibits a lack of joint space narrowing. The formation of hypertrophic spurs that often occurs in degenerative joint disease is lacking in PVNS (74). Differentiation between rheumatoid arthritis and PVNS is sometimes more difficult because this arthropathy causes bony erosions secondary to the formation of a synovial pannus and to a chronic elevation of intraarticular pressure, which is a similar mechanism in both conditions (74). The lack of hypertrophic bone changes in rheumatoid arthritis constitutes another similarity to PVNS. Again, periarticular osteoporosis and the occasional formation of so-called rice bodies in rheumatoid arthritis serve as important differentiating points. Clinical information is also crucial in this situation.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree