Miscellaneous Tumors And Tumor-like Lesions
Benign Lesions
Giant Cell Tumor
Giant cell tumor (GCT) is a locally aggressive neoplasm characterized by richly vascularized tissue containing proliferating mononuclear stromal cells and many osteoclast-like, multinucleated giant cells randomly but evenly distributed throughout (23,58,74,82). GCT represents approximately 5% (94) to 8.6% (82) of all primary bone tumors and about 22.7% of benign bone tumors (94) and is the sixth most common primary osseous neoplasm (30).
The mononuclear component of GCT consists of two types of cells, fibroblast-like and histiocyte/monocytic-like stromal cells (4,78,139). Roessner et al. (78) also demonstrated that only fibroblast-like stromal cells were able to incorporate [3H]-thymidine, thus representing the proliferating neoplastic cell pool. Recently, Wulling et al. (100,101) provided evidence from immunohistochemistry (IHC), tissue culture experiments, and molecular biology studies that the proliferating neoplastic stromal cells in GCT are derived from mesenchymal stem cells. In vitro, these stromal cells of GCT can still differentiate into osteoblasts, chondroblasts, and adipocytes. In addition, Atkins et al. demonstrated that these stromal cells, similar to osteoblasts, express factors necessary for osteoclast formation and differentiation (osteoclast differentiation factor/ODF or receptor activator of nuclear factor kappa B ligand/RANKL), leading to the hypothesis that GCT is a mesenchymal stem cell–derived tumor with the ability to attract monocytic precursors (the second mononuclear component in GCT) and to induce their transformation to osteoclastic giant cells (6,100). In cytogenetic studies, telomeric associations (end-to-end fusions of apparently intact chromosomes) involving chromosomes 11p, 13p, 14p, 15p, 19q, 20q, and 21p have been identified as the most commonly occurring chromosomal aberration (16,74).
Other structural chromosomal abnormalities have also been detected in single cases (57). In an analysis of 12 cases including primary, recurrent, metastatic, and malignant GCTs, Rao et al. (73) observed loss of heterozygosticy (LOH) of 1p, 3p, 5q, 9q, 10q, and 19q, whereas LOH of 17p and 9p occurred only in pulmonary metastases of GCTs (73). However, the possible
roles of these findings in the evolution of GCT are not clearly understood.
roles of these findings in the evolution of GCT are not clearly understood.
In a study of c-myc expression (a transcription factor involved in cell proliferation and in cell growth and differentiation) in GCTs, Gamberi et al. (32) demonstrated a strong correlation between c-myc overexpression at the mRNA and protein levels and the occurrence of lung metastases.
Clinical Presentation
GCT occurs almost exclusively after skeletal maturity, when the growth plates are obliterated, and patients are usually between the ages of 20 and 40 years, with a female preponderance (2:1). At least 60% of cases arise in long bones and almost all extend to the articular end (29). Those unusual cases with presentation in the metaphysis are seen in skeletally immature patients (46,71). The most common skeletal sites are the distal femur, the proximal tibia, the distal radius, the proximal humerus, and the sacrum (23). Rarely, the tumor arises in other sites, such as the bones of hands and feet, the vertebral bodies, the ribs (14,18,42,89,94), the scapula (3), the ischium (83), and the skull (48) (Fig. 7-1). Very uncommonly, GCT may be multifocal (69,85,96), with a reported frequency of 0.04% to 1% (22,58,91,94). The majority of multifocal GCTs are associated with Paget disease of bone (31,41,72), and most have a predilection for the skull, face (43), and ilium (82). Multifocal GCTs in patients who do not exhibit Paget disease usually occur in the bones of the hand (7,8,21) and characteristically affect a slightly younger age group (27,98).
The symptoms most commonly reported include pain of increasing intensity, with local swelling and tenderness of the affected area. Limitation of motion of the adjacent joint is also present in many patients (82). Only in exceptional cases is a pathologic fracture the first sign of a GCT. Although a histologic grading system exists, the clinical behavior of GCT cannot be accurately predicted on the basis of histologic, clinical, or radiologic features (11,12,15,28). Recurrences after surgical curettage and bone grafting are common [reported recurrence rates range from 12% to 50% (40)].
Imaging
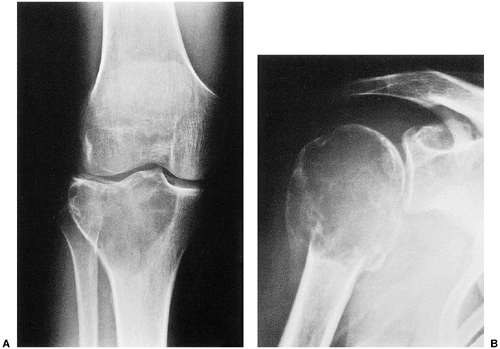
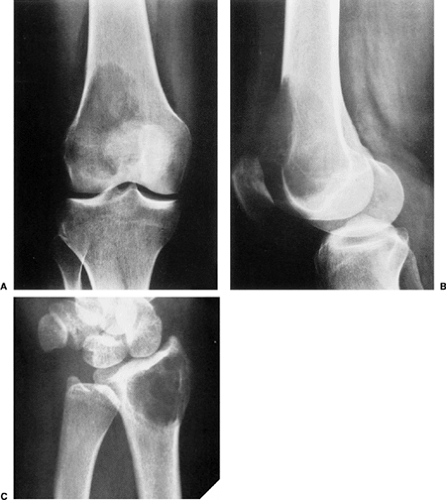
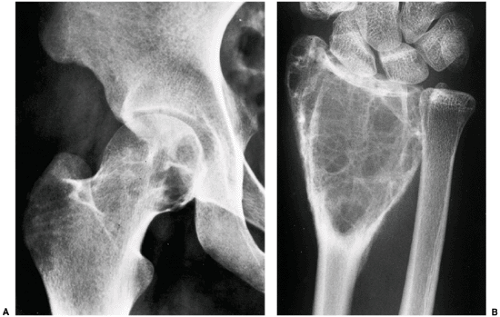
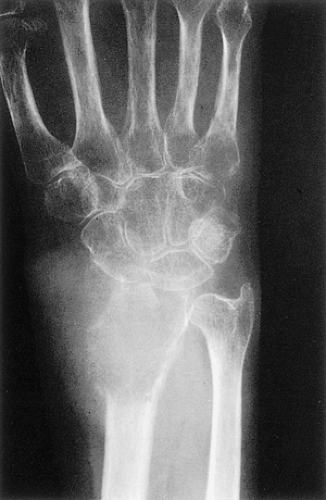
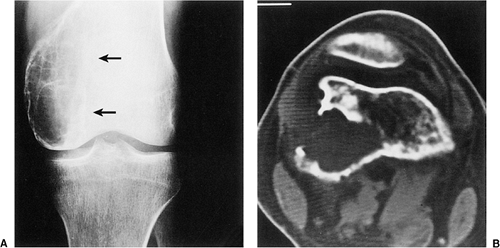
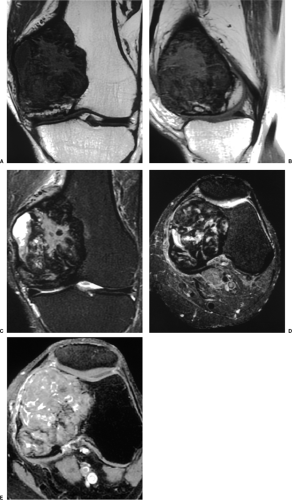
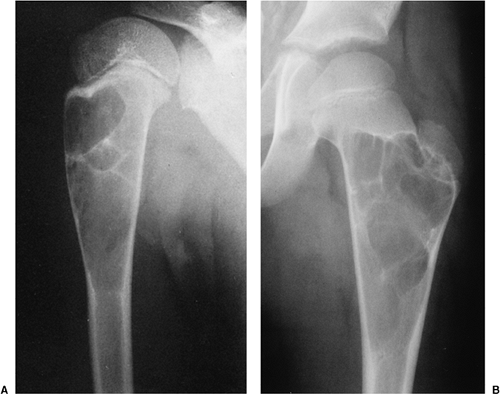
The characteristic radiographic features of GCT are those of a purely osteolytic lesion with a geographic type of bone destruction (50). The lesion is radiolucent, frequently expansive, and eccentrically located, without marginal sclerosis but usually with well-defined borders (Figs. 7-2 and 7-3). Internal trabeculations or pseudotrabeculations are occasionally present (82) (Fig. 7-4), most likely representing a nonuniform growth of the tumor (62). There is usually no periosteal reaction. Attempts have been made to assess the aggressiveness of GCT on the basis of radiologic criteria. According to some investigators (60), nonaggressive tumors exhibit a prominent trabeculation and no cortical expansion or soft tissue mass. Conversely, aggressive tumors exhibit a lack of trabeculation, and expansion or destruction of the cortex and soft tissue mass are present (Fig. 7-5).
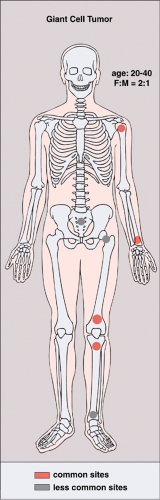 Figure 7-1 Giant cell tumor: skeletal sites of predilection, peak age range, and male-to-female ratio. |
Conventional tomography, particularly trispiral or hypocycloidal, reveals the osseous margins of the lesion and the status of the cortex more clearly than radiographs. Arthrotomography is effective for the evaluation of tumor invasion through the subchondral plate and articular cartilage (39). When a soft tissue mass is present, computed tomography (CT) or magnetic resonance imaging (MRI) examination may be particularly helpful (24,56,75,90). CT may outline the tumor extent and better delineate areas of cortical destruction (Fig. 7-6). The attenuation coefficient of unenhanced GCT averaged 44 Hounsfield units, similar to measurements of other noncalcified bone lesions (39). However, enhancement after infusion of a contrast agent is striking according to Hudson et al. (39). Conversely, deSantos and Murray (25) did not observe a significant contrast enhancement.
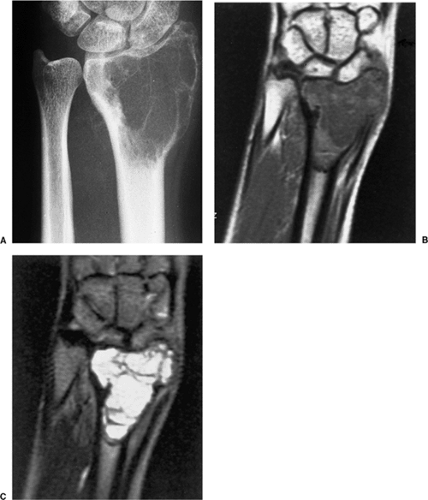
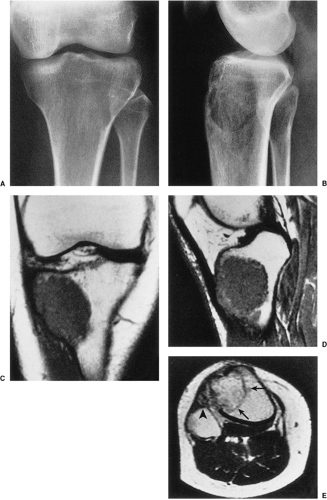
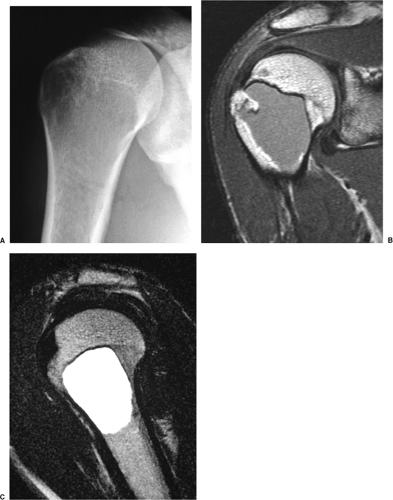
On MRI, like most bone tumors, GCT exhibits a low to intermediate signal intensity on T1-weighted images and a high signal on T2-weighted sequences (Fig. 7-7). The intramedullary portion of tumor is best seen on T1 weighting, whereas its extraosseous component is more clearly observed on T2-weighted images (13,38) (Fig. 7-8). After intravenous injection of gadolinium, heterogeneous enhancement of the tumor is observed (Fig. 7-9). Some reports in the literature indicate that certain instances of GCT containing large amounts of hemosiderin may show different MRI characteristics (2). Aoki et al. (1) found in these instances a markedly decreased signal on both T1- and T2-weighted sequences and, in one case, the extraosseous tumor extension appeared as a signal void area on MR imaging. MRI is also effective in demonstrating subchondral breakthrough and extension of tumor into the adjacent joint (38). Fluid levels in the tumor have been reported (45,75) and are considered by some investigators to be secondary to an aneurysmal bone cyst (ABC) component (39,45).
Radionuclide bone scan rarely provides additional information because the degree of tracer uptake does not correlate with the histologic grade of the tumor (39,53,68,95). However, scintigraphy may help in detection of multiple foci if a multicentric GCT is clinically
suspected. In a study by Hudson et al. (39), these investigators found in 49% of cases an abnormal uptake pattern resembling a doughnut: intense uptake around the periphery with relatively little activity in the central portion of the tumor. They contended that this appearance was due to uptake of the bone-seeking radiopharmaceutical agents predominantly by reactive new bone or by hyperemic bone around the tumor, with the tumor tissue retaining little tracer. Occasionally, an increased tracer activity can be detected across the adjacent joint (49). This phenomenon may be due to increased blood flow and to increased bone turnover secondary to disuse osteoporosis (86).
suspected. In a study by Hudson et al. (39), these investigators found in 49% of cases an abnormal uptake pattern resembling a doughnut: intense uptake around the periphery with relatively little activity in the central portion of the tumor. They contended that this appearance was due to uptake of the bone-seeking radiopharmaceutical agents predominantly by reactive new bone or by hyperemic bone around the tumor, with the tumor tissue retaining little tracer. Occasionally, an increased tracer activity can be detected across the adjacent joint (49). This phenomenon may be due to increased blood flow and to increased bone turnover secondary to disuse osteoporosis (86).
Although 5% to 10% of GCTs undergo malignant degeneration (or are malignant from their inception) (10,36,54), malignant GCT is unaccompanied by additional or specific radiologic characteristics and therefore cannot be diagnosed radiographically.
Histopathology
The histologic features of GCT include a dual population of fibrocytic or monocytic mononuclear stromal cells and of giant cells that are usually uniformly distributed throughout the tumor (Fig. 7-10). Morphologically, the giant cells somewhat resemble osteoclasts and, like all resorptive giant cells, exhibit marked acid phosphatase activity (5). However, they are much larger than normal osteoclasts and are not apposed to bone surfaces and therefore do not possess a ruffled border (88). The cells are round, oval, or fusiform and vary considerably in shape owing to their capacity for ameboid motility. In general, they exhibit large nuclei with little chromatin and very few inconspicuous nucleoli (74,82). The number of nuclei may vary widely but is usually 20 or more. Giant cells never exhibit mitotic activity. Areas of hemorrhage, and occasional groups of foam cells and of hemosiderin-laden macrophages, are frequently present (30).
In typical areas, the tumor matrix contains only sparse amounts of collagen. On histologic study, silver staining reveals a network of reticulin fibers that surrounds the individual cells but does not penetrate the
giant cells (82). The tumor is rich in newly formed capillaries, with walls composed of only a single endothelial layer. Occasionally the vascular channels appear to be directly lined with tumor cells (82). This may be related to the production of proteases [urokinase-type plasminogen activator (u-PA) and matrix metalloproteinases (MMPs), which are capable of degrading proteins of the extracellular matrix] by the cells in GCT that are involved in vascular invasion (33,84,93,102). Elevated u-PA expression appears to be associated with increased tumor aggressiveness (33). Bone or osteoid formation can be seen especially at sites of previous pathologic fracture or curettage and, occasionally, surrounding a focus of recurrent tumor in the soft tissue. Vascular invasion has been observed in the tumor periphery and even in the surrounding soft tissues. However, no studies have demonstrated a correlation with clinical behavior or prognosis. Mitoses are always present and do not indicate malignancy in GCT. However, atypical mitoses are not usually found and, if present, should raise the suspicion of malignancy.
giant cells (82). The tumor is rich in newly formed capillaries, with walls composed of only a single endothelial layer. Occasionally the vascular channels appear to be directly lined with tumor cells (82). This may be related to the production of proteases [urokinase-type plasminogen activator (u-PA) and matrix metalloproteinases (MMPs), which are capable of degrading proteins of the extracellular matrix] by the cells in GCT that are involved in vascular invasion (33,84,93,102). Elevated u-PA expression appears to be associated with increased tumor aggressiveness (33). Bone or osteoid formation can be seen especially at sites of previous pathologic fracture or curettage and, occasionally, surrounding a focus of recurrent tumor in the soft tissue. Vascular invasion has been observed in the tumor periphery and even in the surrounding soft tissues. However, no studies have demonstrated a correlation with clinical behavior or prognosis. Mitoses are always present and do not indicate malignancy in GCT. However, atypical mitoses are not usually found and, if present, should raise the suspicion of malignancy.
A fibroxanthomatous pattern is sometimes observed in GCT with only a few scattered giant cells. The presence of these lipoid-bearing foam cells, particularly in the vicinity of necrotic foci and with association of fibrous tissue, may indicate spontaneous regression and healing of the tumor (43) or, as others have suggested, a less aggressive type of tumor (82). When this pattern predominates, the GCT may be misdiagnosed as benign fibrous histiocytoma. Some authorities, however, consider the latter lesion to represent merely the end stage of a burned-out GCT (55).
Attempts to correlate the histologic appearance of the tumor with its biological behavior have proven unreliable and have now been abandoned (35). Histologic classification of GCT into three grades, corresponding to the number and size of giant cells and the degree of pleomorphism of the mononuclear cells (grade III being frankly malignant histologically), has been attempted in the past (44). In this historical classification, grade I tumor is characterized by a dense layer of huge giant cells with up to 100 or more nuclei, almost covering the tumor tissue proper of mononuclear cells. Grade II tumor exhibits a considerable reduction in the size and number of giant cells, and the mononuclear cells may be pleomorphic to some degree. In grade III tumors, the giant cells are further reduced in number and there is an increase in pleomorphic mononuclear cells. All three grades may be characterized by extended fields with spindle cells and practically no giant cells. At present, most investigators agree that grading of GCT is without practical value because prediction of the evolution of a GCT based on its histologic features is impossible (26,82,94), and histologic grading has not been shown to correlate with local recurrence rate or the incidence of pulmonary metastases (19,35,52,74).
Malignant Giant Cell Tumor
Although GCT is usually benign, malignant tumors have been reported (17,64,77). However, one must be very cautious in making the diagnosis of malignant GCT. Several reported cases of “primary” malignant GCT later proved to be sarcomas rich in multinucleated giant cells of the osteoclast type, such as telangiectatic osteosarcomas, giant cell–rich osteosarcomas, fibrosarcomas, or malignant fibrohistiocytomas (82). Dahlin and Unni believe that primary malignant GCT is extremely unusual and that most of these cases actually represent another type of malignancy arising within GCT (94). Nevertheless, malignant transformation may occur spontaneously, after surgery (103), or after radiation therapy. McGrath (59) divided malignant GCT into three types: (a) primary, in which the tumor was malignant from the onset (de novo); (b) evolutionary, in which there was malignant transformation of originally benign GCT spontaneously or after multiple surgical resections for recurrent lesions, or after a long latency period; and (c) secondary, developing as a result of malignant transformation of an initially benign tumor after radiation therapy (59). In the latter group, the majority of sarcomas that occur are fibrosarcomas, malignant fibrous histiocytomas, or osteosarcomas (82). Exceedingly rare cases represent the so-called dedifferentiated GCT, in which there is the concomitant presence of a typical GCT of bone juxtaposed to a sarcoma [such as a malignant fibrous histiocytoma (61)]. Under the heading “Malignancy in giant cell tumor” the World Health Organization (WHO) only discriminates between primary malignancy, i.e., sarcomas arising in a GCT, or secondary malignancy, i.e., sarcomas arising at the site of a previously documented GCT (17). The diagnosis of malignant GCT requires either a frankly sarcomatous change in the lesion or a sarcoma arising in the previous site of a treated GCT (94). The histologic diagnosis of malignant GCT is established only when the stromal cells are frankly sarcomatous, as indicated by pleomorphism, atypia, and high mitotic activity (80,103). In addition, areas of typical benign GCT should be present in the tumor at the time of diagnosis (primary malignant GCT), or in tissues taken previously from the same lesion (secondary malignant GCT) (41,94). Histologic grading of GCT in an attempt to predict local recurrence and clinical prognosis has generally met with little success. Moreover, there are reports of completely benign-appearing GCT that were followed by distant metastases to the lung, having the histologic characteristics of classic GCT (47,52,66,76,92,94,99).
Differential Diagnosis
Radiology
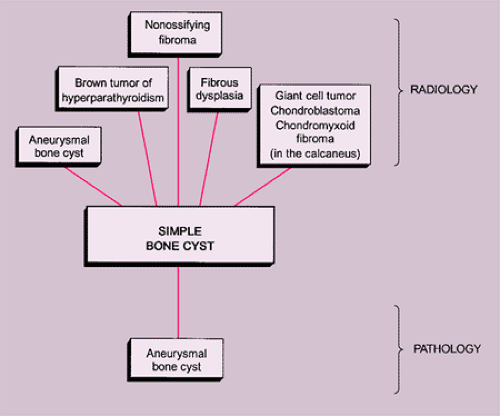
Various lesions may be mistaken for GCT and, conversely, GCT can mimic other lesions that affect the articular end of a bone (87). Primary ABC rarely affects the articular end of a bone and occurs in a younger age group. However, after obliteration of the growth plate at skeletal maturity, this lesion may extend into the subarticular region of a long bone, becoming indistinguishable from a GCT. Occasionally, if
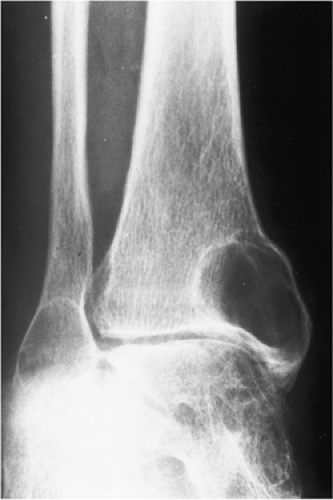
a fluid-fluid level is demonstrated either on CT or MRI examination, this feature is more consistent with ABC (75). However, it should be noted that ABC may sometimes coexist with other lesions, among them the GCT. The so-called solid ABC, also referred to as a giant cell reparative granuloma (GCRG) when affecting the articular end of bone, may have the same radiologic characteristics as a conventional GCT (9,67,70). Benign fibrous histiocytoma, because of its frequent location at the end of a long bone, may appear identical to a GCT. However, as mentioned earlier, some investigators consider benign fibrous histiocytoma to be an end stage of a “burned-out” GCT (55). Brown tumor of hyperparathyroidism is yet another lesion that radiologically can mimic GCT. However, the former lesion is usually accompanied by other skeletal manifestations of hyperparathyroidism, such as osteopenia, cortical or subperiosteal resorption, resorptive changes at the distal phalangeal tufts, or loss of the lamina dura of the teeth (53). Occasionally, an unusually large intraosseous ganglion may be mistaken for GCT, although the former lesion invariably exhibits a sclerotic border (Fig. 7-11). Some malignant lesions, such as chondrosarcoma, may extend into the articular end of the bone and, particularly without radiographically identified calcifications, may closely mimic GCT. Myeloma and a lytic metastasis occupying subchondral segments of bone can usually be distinguished from GCT without much difficulty (the older age group in which the latter malignancies usually occur is a helpful hint), although at times the radiographic differences between the lesions may not be so obvious (Fig. 7-12A, B). Finally, on rare occasions, fibrosarcoma, malignant fibrous histiocytoma, and fibroblastic osteosarcoma, because of their purely lytic radiographic presentation, may exhibit some similarities to GCT.
a fluid-fluid level is demonstrated either on CT or MRI examination, this feature is more consistent with ABC (75). However, it should be noted that ABC may sometimes coexist with other lesions, among them the GCT. The so-called solid ABC, also referred to as a giant cell reparative granuloma (GCRG) when affecting the articular end of bone, may have the same radiologic characteristics as a conventional GCT (9,67,70). Benign fibrous histiocytoma, because of its frequent location at the end of a long bone, may appear identical to a GCT. However, as mentioned earlier, some investigators consider benign fibrous histiocytoma to be an end stage of a “burned-out” GCT (55). Brown tumor of hyperparathyroidism is yet another lesion that radiologically can mimic GCT. However, the former lesion is usually accompanied by other skeletal manifestations of hyperparathyroidism, such as osteopenia, cortical or subperiosteal resorption, resorptive changes at the distal phalangeal tufts, or loss of the lamina dura of the teeth (53). Occasionally, an unusually large intraosseous ganglion may be mistaken for GCT, although the former lesion invariably exhibits a sclerotic border (Fig. 7-11). Some malignant lesions, such as chondrosarcoma, may extend into the articular end of the bone and, particularly without radiographically identified calcifications, may closely mimic GCT. Myeloma and a lytic metastasis occupying subchondral segments of bone can usually be distinguished from GCT without much difficulty (the older age group in which the latter malignancies usually occur is a helpful hint), although at times the radiographic differences between the lesions may not be so obvious (Fig. 7-12A, B). Finally, on rare occasions, fibrosarcoma, malignant fibrous histiocytoma, and fibroblastic osteosarcoma, because of their purely lytic radiographic presentation, may exhibit some similarities to GCT.
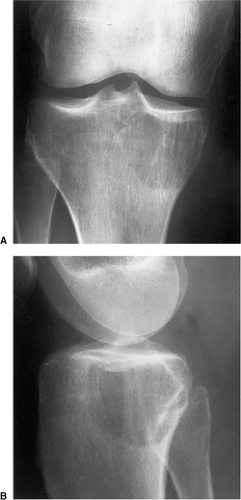 Figure 7-12 Metastasis. A, B: Osteolytic metastasis from a renal cell carcinoma into the articular end of the tibia in this 42-year-old woman has an appearance of a giant cell tumor. |
Multicentric GCT should be differentiated from osteolytic metastases, myeloma, brown tumor of hyperparathyroidism, and multicentric GCRG (20,34,37,51,70).
Pathology
Diagnosis of GCT, with its typical histology and cytology of giant cells, usually does not create a problem. The characteristic radiologic picture of an expansile, eccentric lytic lesion in the articular end of a long tubular bone adds to the security of the histologic diagnosis. For a tumor formerly considered as grade II or III, diagnosis has been and is still difficult. In such cases several lesions must be considered in the differential diagnosis. In the epiphysis, chondroblastoma has in the past been considered as a cartilage-containing GCT. However, it can now be easily differentiated from GCT by the presence of cleaved or indented nuclei of the mononuclear cells, “chicken-wire”-like calcifications, and cartilage islands that are only exceedingly rarely observed in GCT. Another giant cell–containing epiphyseal lesion, benign fibrous histiocytoma, which some authors consider to be a burned-out GCT (55), may create diagnostic difficulties because GCT may contain areas of storiform and cellular fibrous tissue intermingled with a few giant cells in a pattern exactly like that of benign fibrous histiocytoma. However, the latter tumor does not exhibit broad sheets of polygonal mononuclear cells with heavy interspersal of giant cells, which is a pattern diagnostic of GCT. It is possible for certain epiphyseal lesions to be arbitrarily diagnosed as GCT or as benign fibrous histiocytoma (30). Matsuno (55) reclassified three of five epiphyseal lesions with the latter diagnosis as being GCT. Clear cell chondrosarcoma is fairly easy to distinguish from GCT because it contains clusters of large clear cells (see Chapter 3).
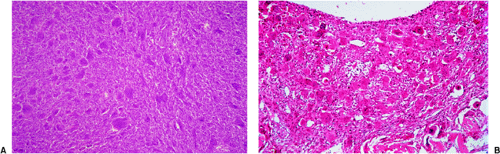
If the giant cell–containing lesion is located in the metaphysis and the growth plate is still open, there are several possibilities in the differential diagnosis. Nonossifying fibroma and, with a lower probability, fibrous cortical defect can be considered, but because of their giant cells arranged in small groups and exhibiting a resorption activity, and the more or less marked cartwheel-like pattern of the spindle-cell stroma, the histiocytic character of these lesions becomes obvious. Both lesions may be characterized by groups of foam cells. Microscopic fields within GCT may contain spindle cells, fibrosis, and clumps of foamy macrophages that cannot be histologically distinguished from nonossifying fibroma (30). In most cases these changes are only focal and the typical GCT pattern predominates. ABC can be differentiated because of its more or less large spaces bordered by flat undifferentiated cells and containing blood, as well as the presence of smaller and not evenly distributed giant cells, whereas in a true GCT the giant cells are larger and contain more nuclei (Fig. 7-13). However, it should be kept in mind that GCT, like many other tumors, may be associated with secondary ABC formation, so that constituents of both lesions may be found. Differentiation is particularly difficult in so-called solid ABC, also known as GCRG (9,63,97). This tumor contains giant cells, often in large numbers, and has a solid component that resembles the spindle-cell variant of GCT. Its smaller size and the clustered arrangement of the giant cells may assist in the differential diagnosis. The tissue of GCT usually contains less fibrosis and is largely composed of sheets of characteristic mononuclear cells (30). GCRG, on the other hand, is more fibrotic, and hemorrhage and hemosiderin deposition are more prominent. Both lesions may exhibit foci of reactive bone formation, but this is more commonly seen in GCRG (30). Chondromyxoid fibroma is usually not a diagnostic problem because large areas of the tumor possess typical myxoid characteristics. Brown tumor of hyperparathyroidism contains numerous giant cells, but these are smaller than in GCT and, instead of being uniformly distributed, are usually arranged in groups (Fig. 7-14).
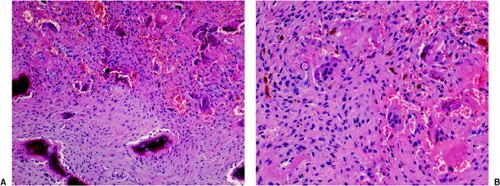 Figure 7-14 Brown tumor of hyperparathyroidism. A: The loose connective tissue with a group of osteoclasts (upper right corner) is bordered by immature bone trabeculae made up of woven bone and lined by dense seams of osteoblasts (bottom) (hematoxylin and eosin, original magnification ×100). B: At high magnification there is an obvious difference between these giant osteoclasts seen in hyperparathyroidism and giant cells of a giant cell tumor (compare with Fig. 7-10) (hematoxylin and eosin, original magnification ×200). |
The differential diagnosis must also consider giant cell–containing malignant tumors. A particular problem is that of osteosarcoma with prominent giant cells (so-called giant cell–rich osteosarcoma), which emphasizes the fact that GCT is frequently a diagnosis of exclusion. The histologic examination of some osteosarcomas reveals that they consist predominantly of large sheets of giant cells, many or most of which are histologically bland. Production of osteoid may be minimal, detectable only by high-power microscopy as fine strands that surround a population of pleomorphic, mononuclear stromal cells. These cells may be undetectable at low power. However, on higher power examination the tissue consists of scattered hyperchromatic cells exhibiting many atypical mitotic figures. When radiographic studies suggest the presence of matrix production, a permeative growth pattern, a lesion not extending to the articular cartilage, or a lesion in a bone with an active growth-plate, giant cell–rich osteosarcoma should be suspected and a very careful histologic examination is warranted (30). By the same token, the giant cell–rich variant of malignant fibrous histiocytoma should be included in the differential diagnosis, particularly when irregular arrangement of the usually smaller giant cells and pleomorphism and hyperchromasia of their nuclei are present. Telangiectatic osteosarcoma may be difficult to exclude unless special consideration is given to the conspicuous pleomorphism of the tumor tissue and the high number of blood-filled spaces. In all three of these malignant tumors, the usual location in the metaphysis may be of help in differentiation from GCT, which is usually located in the articular end of bone.
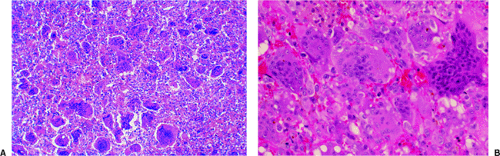
GCT may occasionally be confused with metastatic carcinoma containing giant cells. These lesions may contain large numbers of giant cells, indistinguishable from those of GCT. The thyroid, breast, and pancreas (79) are the most common primary sites for these giant cell–rich metastatic lesions. IHC should demonstrate the epithelial quality of the mononuclear carcinoma cells and, in the case of thyroid carcinoma, may show evidence of thyroglobulin production.
As can be seen from this discussion, tumors and tumor-like lesions containing considerable numbers of giant cells are quite common (Table 7-1). Each of these must be taken into account and must be differentiated from true GCT when a giant cell–containing lesion is under investigation. As shown in Table 7-1, the giant cells are considered to have different functions. However, with the exception of tumor giant cells, which are formed by and belong to the tumor tissue proper, all are derived from the monohistiocytic branch of hematogenic stem cells and are very close relatives, with corresponding enzymatic pathways involved in their resorptive activity.
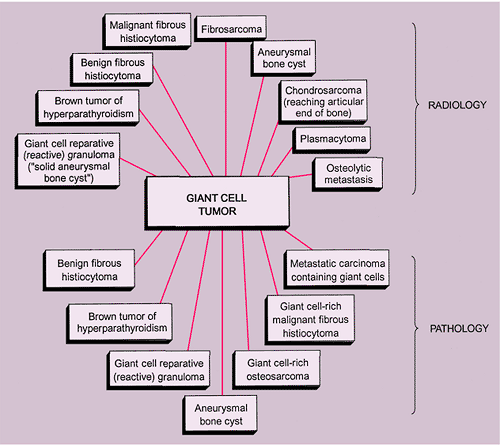
The radiologic and pathologic differential diagnosis of GCT is depicted in Figure 7-15.
Simple Bone Cyst
The simple bone cyst (SBC), also called unicameral bone cyst, is a tumor-like lesion of unknown cause, attributed to a local disturbance of bone growth (110,114,122,145). Although the pathogenesis is still unknown (130,131), the lesion appears to be reactive or developmental rather than to represent a true neoplasm (30). SBC consists of a solitary cavity lined by a membrane of variable thickness and filled with a clear yellow fluid (105). It represents approximately 3% of all primary bone lesions (111).
Until now, only two SBCs have been examined cytogenetically, revealing complex clonal rearrangements involving chromosomes 4, 6, 8, 16, 21, and 12 in one
case, and a translocation, (16;20)(p11.2;q13), in the second case (138,143). In case one, examination of the fourth recurrence by polymerase chain reaction (PCR) revealed a TP53 mutation (144).
case, and a translocation, (16;20)(p11.2;q13), in the second case (138,143). In case one, examination of the fourth recurrence by polymerase chain reaction (PCR) revealed a TP53 mutation (144).
Table 7-1 Giant Cell Tumor and Lesions Containing Different Forms of Giant Cells (GC) | ||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Clinical Presentation
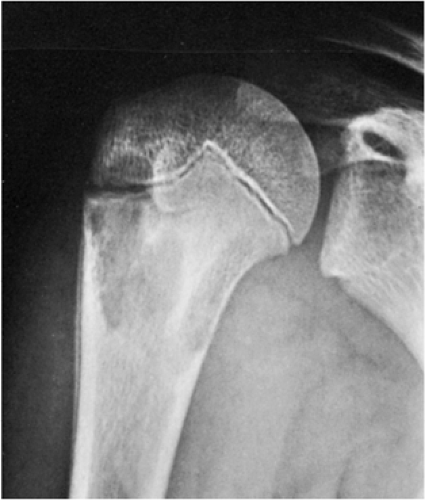
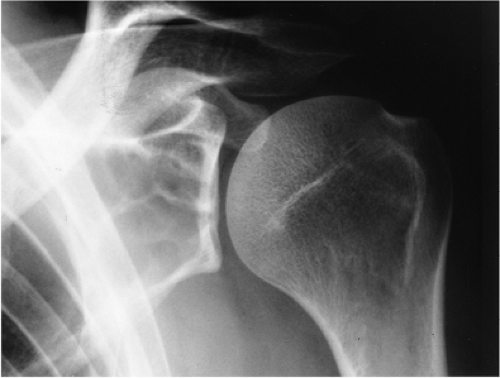
The SBC is more common in males (3:1) and is usually detected during the first 2 decades of life (116). About 65% of these cysts occur in teenagers and an additional 20% in the first decade of life (30,135). The vast majority of SBCs are located in the proximal diaphysis of the humerus and the femur, especially when they occur in patients younger than 17 years (122,134). The symptoms include pain, swelling, or stiffness at the nearest joint (109). A pathologic fracture is often the first sign of the lesion (115,120). In fact, this is the most common complication of SBC and occurs in about 66% of cases. In older patients, the incidence of involvement of atypical sites, such as the calcaneus, talus, and ilium, rises significantly (117,135) (Fig. 7-16). In these sites the lesion is usually asymptomatic and is discovered by accident.
Imaging
Radiographically, the appearance of an SBC is that of a centrally located, well-circumscribed, radiolucent lesion with sclerotic margins. The lesion is located within the metaphysis or diaphysis of a long bone, abutting or being remote from the cartilaginous growth plate (Fig. 7-17). Epiphyseal extension is unusual (112,118).
The cortex is frequently thinned and cortical expansion may be present, but, unlike the ABC, the width of the SBC does not exceed the width of the neighboring growth plate (116). A periosteal reaction is absent unless there has been a pathologic fracture, and this feature distinguishes it from ABC, in which there is almost invariably some degree of periosteal response. Diagnosis is best based on conventional radiographs; conventional tomography and CT are used only exceptionally in equivocal cases.
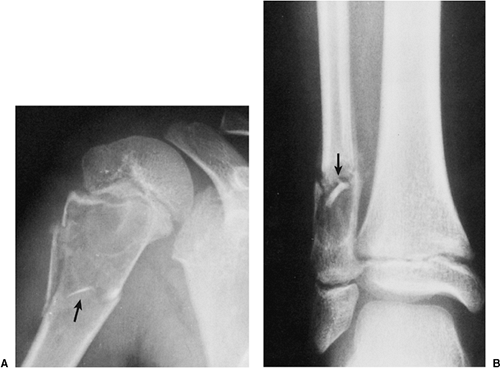
Occasionally (in 20% of cases in the experience of Struhl et al.), one can identify a characteristic “fallen fragment” sign (128,141). This represents a piece of fractured cortex that is displaced into the interior of the lesion, indicating that the lesion is either hollow or is filled with fluid, as most SBCs are (Fig. 7-18). A variant of this phenomenon is the “trap door” sign (116) [also called a “forme fruste” of the fallen fragment by Reynolds (137)], in which the fragment remains attached to the periosteum but folds inward at the fracture site and floats in the fluid. It is important to obtain multiple views of the cyst to accurately identify this sign because a pathologic fracture through a solid lesion can also produce fragments that appear intramedullary on one view but are in fact adjacent to the outer cortex or are located in the soft tissue. Radiographs taken in both the erect and the recumbent position (133), or the observation of a movement of the fallen fragment under fluoroscopy, may demonstrate the free movement of the fragments within the cyst. These signs permit differentiation of SBC from radiographically similar radiolucent lesions containing solid fibrous or
cartilaginous tissue, such as ABC, fibrous dysplasia, nonossifying fibroma, and enchondroma.
cartilaginous tissue, such as ABC, fibrous dysplasia, nonossifying fibroma, and enchondroma.
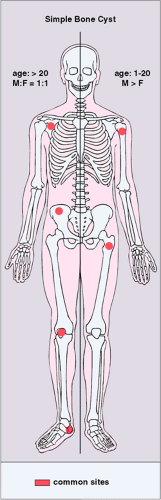 Figure 7-16 Simple bone cyst: skeletal sites of predilection, peak age range, and male-to-female ratio. The left half of the skeleton shows sites of occurrence seen in an older patient population. |
CT may be helpful when the appearance of an SBC is atypical or when the lesion is located in the pelvis. This modality may also assist in the identification of a fallen fragment sign (141). Attenuation values of 15 to 20 Hounsfield units have been reported in the center of fluid-filled cysts (108,117). Skeletal scintigraphy may demonstrate an increased peripheral uptake around the cyst and a decreased central activity, although this appearance is not specific for the SBC (120). Some cysts may be scintigraphically normal. MRI of a cyst shows the signal characteristics of fluid: a low to intermediate signal on T1-weighted images and a bright, homogeneous signal on T2 weighting (116) (Figs. 7-19 and 7-20).
Histopathology
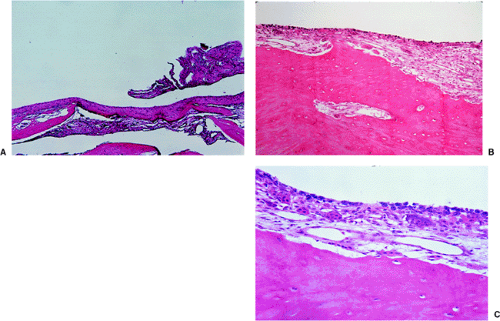
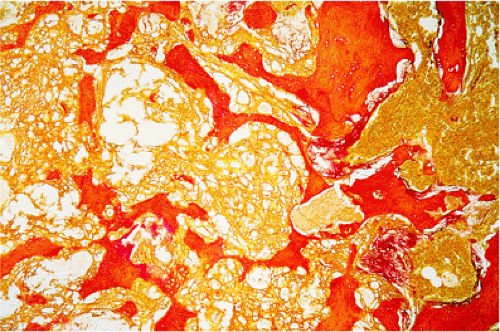
Histologically, SBC is a diagnosis of exclusion. A vigorous surgical curettage, if the lesion is not simply injected, yields almost no solid tissue, but the walls of the cavity may show remnants of fibrous tissue or, occasionally, a flattened single-cell lining (Fig. 7-21). Granulation tissue, containing hemosiderin deposits and small lymphocyte infiltrates, is often present, along with scattered osteoclasts (30). Chemical examination of the fluid usually identifies an elevated alkaline phosphatase level (139). Furthermore, a cloudy-appearing, cell-free material is often found in the loose connective tissue surrounding the cysts (121). With stains for collagen, such as van Gieson stain, the material stains strongly and reveals loose and irregularly arranged fibers. It therefore cannot be fibrin, which it is considered by some authors to represent on the basis of hematoxylin and eosin (H&E) staining (Fig. 7-22).
Differential Diagnosis
Radiology
In a long bone, the main differential diagnosis is an ABC. The primary differences are that SBC is a centric solitary lesion, with minimal expansion, invariably lacks periosteal reaction, and never extends into the soft tissues. In contrast, an ABC is almost invariably an eccentric lesion, with a significant blown-up appearance, and is always accompanied by a solid periosteal reaction (usually in the form of a buttress) (see Figs. 7-31, 7-32, 7-33 and 7-34).
Fibrous dysplasia may mimic SBC, but usually it lacks trabeculation and exhibits a ground-glass, smoky appearance (see Fig. 4-35A). If a pathologic fracture occurs and the fallen fragment sign can be demonstrated, a diagnosis of a SBC is usually confirmed.
Nonossifying fibroma can usually be distinguished by its eccentric location and its well-defined, usually thick, sclerotic margin (see Fig. 7-44).
Brown tumor of hyperparathyroidism may at times appear similar to SBC, particularly if it is located in the proximal humerus or proximal femur. In this situation, one should look for other features of hyperparathyroidism, such as osteopenia and subcortical resorption. Bone abscess likewise may occasionally mimic SBC, particularly when it is located in sites of SBC predilection such as the proximal humerus or the proximal femur. However, the presence of a periosteal reaction and the frequent extension of the former lesion beyond the boundaries of a growth plate are important features indicating a bone abscess (Fig. 7-23).
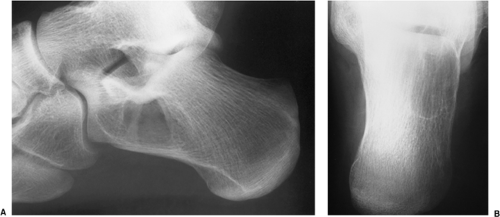
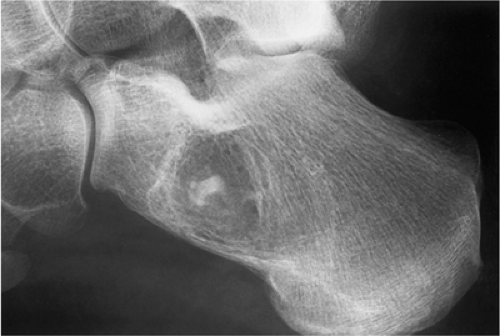
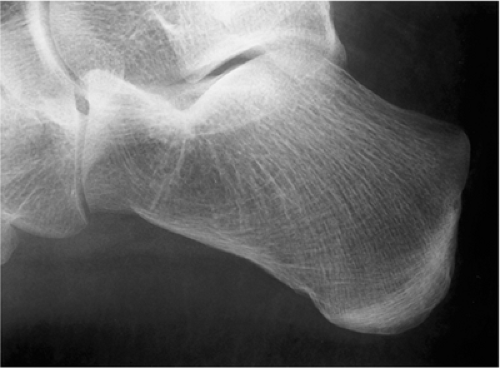
Exceptionally rare, an intraosseous ganglion may resemble SBC (Fig. 7-24).
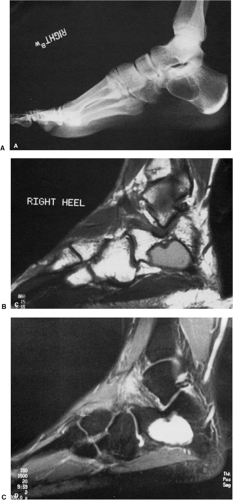
In the calcaneus, SBC is characteristically located at the base of the calcaneal neck on the lateral aspect of the bone, within a particular anatomic structure poor in trabeculae called Ward triangle. Most of the cysts in this location have a characteristic shape: the anterior margin is usually straight and vertically oriented, and the posterior border is typically curvilinear, paralleling the trabeculae in the posterior portion of the bone (104) (Fig. 7-25A, B). In this location, SBC must be differentiated from a bone infarct, an ABC, and several other benign bone tumors (106,136). Here are some
helpful hints. A bone infarct often exhibits central calcifications, which are never present in an SBC (Fig. 7-26). ABC of the calcaneus is more expansive than SBC. The margin may be either poorly defined or sharp and thinly sclerotic (125) and is usually located toward the plantar and posterior aspect of the bone (107,119), although occasionally it may be found within Ward triangle. In the latter location it probably develops secondary to other lesions, such as bone infarct or an intraosseous hemorrhage. Chondroblastoma of the calcaneus is usually a subarticular lesion and is located in the subtalar region. It may exhibit a sclerotic, lobulated margin. Seven percent of chondroblastomas located in the calcaneus present with punctate calcifications (126). It is important to remember that Ward triangle normally contains fatty marrow, and this feature has been interpreted as an intraosseous lipoma by several authors (113,124,129). These pseudotumors are usually
found by serendipity, as they are not symptomatic (123,140,142) (Fig. 7-27).
helpful hints. A bone infarct often exhibits central calcifications, which are never present in an SBC (Fig. 7-26). ABC of the calcaneus is more expansive than SBC. The margin may be either poorly defined or sharp and thinly sclerotic (125) and is usually located toward the plantar and posterior aspect of the bone (107,119), although occasionally it may be found within Ward triangle. In the latter location it probably develops secondary to other lesions, such as bone infarct or an intraosseous hemorrhage. Chondroblastoma of the calcaneus is usually a subarticular lesion and is located in the subtalar region. It may exhibit a sclerotic, lobulated margin. Seven percent of chondroblastomas located in the calcaneus present with punctate calcifications (126). It is important to remember that Ward triangle normally contains fatty marrow, and this feature has been interpreted as an intraosseous lipoma by several authors (113,124,129). These pseudotumors are usually
found by serendipity, as they are not symptomatic (123,140,142) (Fig. 7-27).
Pathology
SBC is a diagnosis of exclusion, and pathologists must rely on the radiologic examination. In a long tubular bone the diagnosis is usually readily made. Most problems occur when the lesion is located in the calcaneus. In this instance, a bone infarct must be included in the differential diagnosis (127). The presence of characteristic microscopic features of dead bone marrow is diagnostic for the latter (Fig. 7-28).
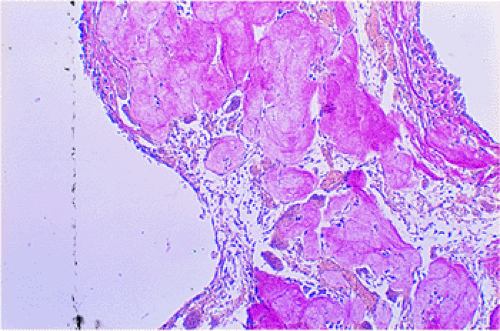
If the membrane of the SBC contains giant cells of the osteoclastic type, ABC may be a consideration. However, fairly characteristic of SBC is the finding of focal deposits of an amorphous, fibrillar, cloudy eosinophilic material that stains strongly for collagen with special stains. This material resembles osteoid and may undergo calcification or even ossification (132).
The radiologic and pathologic differential diagnosis of SBC is depicted in Figure 7-29.
Aneurysmal Bone Cyst
The term aneurysmal bone cyst was first used by Jaffe and Lichtenstein (122) to describe two examples of blood-filled cysts in which tissue from the cyst wall contained conspicuous spaces, areas of hemosiderin deposition, giant cells, and occasional bone trabeculae. In a subsequent publication, Jaffe (176) chose the designation aneurysmal bone cyst as a descriptive term for this lesion to emphasize the blown-out appearance.
Although the cause of this lesion is unknown (159), alterations in local hemodynamics related to venous obstruction or arteriovenous fistula are believed to play an important role (155,191). Some investigators believe that the lesion is caused by a trauma (130,160,189,198). Dahlin and McLeod (162) believe that it may be similar to and related to reactive nonneoplastic processes, such as GCRG or traumatic reactions observed in periosteum and bone. ABC constitutes between 2.5% and 6% of the primary lesions of bone with an incidence of 1.4 per million individuals (26,175,180). The lesion may arise de novo in bone, in which case no recognizable preexisting lesion can be demonstrated in the tissue, or it may be associated with various benign (e.g., GCT, osteoblastoma, chondroblastoma, chondromyxoid fibroma, fibrous dysplasia) and malignant (e.g., osteosarcoma, fibrosarcoma, or chondrosarcoma) lesions (156,177,185,192). The concept of ABC as a secondary phenomenon occurring in a preexisting lesion has been proposed by several investigators (178,181,186,187). Some investigators, however, regard ABC as a reparative process, probably the result of trauma or a tumor-induced anomalous vascular process (168,188). The typical ABC affects the medullary portion of a long bone (183). ABCs localized to the cortex (151) (see Fig. 7-36A) or in a subperiosteal location are unusual (158,202). Malignant transformation of ABC is extremely rare (179), and it is still a controversial issue. However, recent studies and cytogenetic findings permit the assumption that at least some ABCs are neoplastic in nature. A reciprocal translocation t(16;17) (q22;p13) has been detected in classic ABCs, as well as in solid extraosseous variants (163,169,197). Other chromosomal alterations have also been described, often involving chromosome 16 or 17 (149,152). Oliveira et al. (194,195) demonstrated that the translocation t(16;17) (q22;p13) initiates a fusion transcript in which the promoter region of the osteoblast cadherin 11 gene CDH11 (cloned from human osteosarcoma cell lines and involved in neoplastic processes) fuses with the entire sequence of the ubiquitin protease USP6 (which may
be involved in invasion), leading to upregulation of USP6 by the highly active CDH11 promoter. In a molecular cytogenetic study, Althof et al. (149) detected abnormalities of the 17p13.2 break-point region by fluorescent in situ hybridization (FISH) techniques, even in karyotypically normal ABCs, indicating that the t(16;17) (q22;p13) translocation may be useful for differential diagnostic exclusion of other tumors, e.g., telangiectatic osteosarcoma. In a comparative analysis of 52 primary and 17 secondary ABCs (associated with GCT, chondroblastoma, osteoblastoma, or fibrous dysplasia), CDH11 and USP6 rearrangements have not been detected in secondary ABCs, which may indicate that secondary ABCs are biologically different (reactive) lesions (196).
Although the cause of this lesion is unknown (159), alterations in local hemodynamics related to venous obstruction or arteriovenous fistula are believed to play an important role (155,191). Some investigators believe that the lesion is caused by a trauma (130,160,189,198). Dahlin and McLeod (162) believe that it may be similar to and related to reactive nonneoplastic processes, such as GCRG or traumatic reactions observed in periosteum and bone. ABC constitutes between 2.5% and 6% of the primary lesions of bone with an incidence of 1.4 per million individuals (26,175,180). The lesion may arise de novo in bone, in which case no recognizable preexisting lesion can be demonstrated in the tissue, or it may be associated with various benign (e.g., GCT, osteoblastoma, chondroblastoma, chondromyxoid fibroma, fibrous dysplasia) and malignant (e.g., osteosarcoma, fibrosarcoma, or chondrosarcoma) lesions (156,177,185,192). The concept of ABC as a secondary phenomenon occurring in a preexisting lesion has been proposed by several investigators (178,181,186,187). Some investigators, however, regard ABC as a reparative process, probably the result of trauma or a tumor-induced anomalous vascular process (168,188). The typical ABC affects the medullary portion of a long bone (183). ABCs localized to the cortex (151) (see Fig. 7-36A) or in a subperiosteal location are unusual (158,202). Malignant transformation of ABC is extremely rare (179), and it is still a controversial issue. However, recent studies and cytogenetic findings permit the assumption that at least some ABCs are neoplastic in nature. A reciprocal translocation t(16;17) (q22;p13) has been detected in classic ABCs, as well as in solid extraosseous variants (163,169,197). Other chromosomal alterations have also been described, often involving chromosome 16 or 17 (149,152). Oliveira et al. (194,195) demonstrated that the translocation t(16;17) (q22;p13) initiates a fusion transcript in which the promoter region of the osteoblast cadherin 11 gene CDH11 (cloned from human osteosarcoma cell lines and involved in neoplastic processes) fuses with the entire sequence of the ubiquitin protease USP6 (which may
be involved in invasion), leading to upregulation of USP6 by the highly active CDH11 promoter. In a molecular cytogenetic study, Althof et al. (149) detected abnormalities of the 17p13.2 break-point region by fluorescent in situ hybridization (FISH) techniques, even in karyotypically normal ABCs, indicating that the t(16;17) (q22;p13) translocation may be useful for differential diagnostic exclusion of other tumors, e.g., telangiectatic osteosarcoma. In a comparative analysis of 52 primary and 17 secondary ABCs (associated with GCT, chondroblastoma, osteoblastoma, or fibrous dysplasia), CDH11 and USP6 rearrangements have not been detected in secondary ABCs, which may indicate that secondary ABCs are biologically different (reactive) lesions (196).
Clinical Presentation
ABC is seen predominantly in childhood, and 76% of cases occur in patients younger than 20 years (211). There is a slight female preponderance (200,209). The metaphysis of the long bones is the site of predilection, although the diaphysis, the flat bones, the short tubular bones (150), and even the spine may be involved (176) (Fig. 7-30). The pelvis accounts for about 50% of all flat bone lesions (212). When the spine is affected, the lesion is usually situated in the posterior elements (neural arch) (162,176,182), but the vertebral body may also be affected (209). In this location, ABC may occasionally cross the intervertebral disk to involve more than one vertebra (175). Lesions in a spinal location often produce clinical symptoms by compression of adjacent structures (the spinal cord or nerve roots) or as a result of a pathologic fracture (190,191). The most common symptoms in lesions located in the long tubular bones include pain and local swelling (161,167,190).
Imaging
The radiographic hallmark of ABC is a multicystic, eccentric expansion (blow-out) of the bone, with a buttress or thin shell of periosteal response (Fig. 7-31; see also Figs. 7-32, 7-33 and 7-34). In the short tubular bones the lesion may appear more central, destroying the entire shaft of the bone (166,172). The lesion exhibits a geographic type of bone destruction with a narrow zone of transition and often a sclerotic margin (165,205) (Fig. 7-32). Sometimes ridges or trabeculations are observed within the lesion (Fig. 7-35). Rarely, internal calcifications may be present (204). A soft tissue extension is produced by bulging of the periosteum. This periosteal envelope may or may not be visible on radiography. The outer shell of bone may be partially absent (172) but, if seen, the diagnosis can frequently be ascertained. In the vertebral column the posterior elements are usually affected. Although radiographs are usually sufficient to evaluate the lesion, other modalities, such as radionuclide bone scan, CT, and MRI, can be of further assistance (Fig. 7-36).
CT is superior to radiography in defining these lesions, particularly in areas in which bone anatomy is complex (e.g., the pelvis). The cortical or periosteal shell surrounding ABC and its soft tissue extension, as
well as the sharp interface between the extraosseous mass and the adjacent tissue planes, are well demonstrated with this technique (172,203). CT may also show ridges in the bony walls, described on radiographs as trabeculation or septation (172), and corresponding to the ridges found pathologically (Fig. 7-37). Attenuation coefficient values (Hounsfield units) range from 20 to 78. Fluid-fluid levels can also be demonstrated (119,171,173,174). These fluid levels are believed to represent the sedimentation of red blood cells and serum within the cystic cavities (153,210). To demonstrate this phenomenon, the patient must remain motionless for at least 10 minutes before scanning, and imaging must be performed in a plane perpendicular to the fluid levels.
well as the sharp interface between the extraosseous mass and the adjacent tissue planes, are well demonstrated with this technique (172,203). CT may also show ridges in the bony walls, described on radiographs as trabeculation or septation (172), and corresponding to the ridges found pathologically (Fig. 7-37). Attenuation coefficient values (Hounsfield units) range from 20 to 78. Fluid-fluid levels can also be demonstrated (119,171,173,174). These fluid levels are believed to represent the sedimentation of red blood cells and serum within the cystic cavities (153,210). To demonstrate this phenomenon, the patient must remain motionless for at least 10 minutes before scanning, and imaging must be performed in a plane perpendicular to the fluid levels.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree