Radiologic and Pathologic Approach to Bone Tumors
The ideal therapeutic goals for managing patients with primary or metastatic lesions can be incorporated into a triad of important factors: (a) do not overtreat a benign bone tumor; (b) do not undertreat a malignant bone tumor; and (c) do not take an incorrect biopsy approach to the lesion, because this may suggest a need for radical rather than more conservative surgery (117,133,155). Achieving these goals depends on close cooperation among the radiologist, the pathologist, and the tumor surgeon (7,75,83).
Several factors make precise diagnosis of osseous tumors difficult. Although radiographic features have a high correlation with malignancy, benignity, and sometimes even an exact histologic diagnosis, radiographic determination of these characteristics is based on statistical probabilities. Moreover, errors in the radiologic interpretation may occur. These can usually be ascribed to failure to recognize a specific pathologic finding or to misinterpretation of normal structures as pathologic. Regardless of how persuasive a radiologist’s clinical assessment of the biologic potential of a lesion may be to a pathologist colleague, any lesion, no matter how radiologically typical it appears, may represent an entirely different entity on histologic examination (68). A confident radiologic diagnosis may not outweigh the microscopic appearance of the lesion.
Both the radiologist and the pathologist play important roles by providing the diagnosis and/or differential diagnosis of a bone tumor, thus aiding the clinician in the complex process of patient management. Before a final diagnosis or differential diagnosis is made, clinical
information, radiologic imaging, and pathologic material should be carefully studied and correlated. The radiologist has the significant advantage of being able to view the three-dimensional extent of a bone tumor, whereas the pathologist can view only a small biopsy specimen of the lesion selected by the surgeon (133), which may not reflect the histology of the entire lesion. Obviously, viewing the entire resected specimen enhances the final pathologic evaluation. Many years ago, Ewing commented that “the gross anatomy (as revealed in radiographs) is often a safer guide to a correct clinical conception of the disease than the variable and uncertain nature of a small piece of tissue” (48). It is important to emphasize that the differential diagnosis contemplated by the radiologist may be identical to or different from that contemplated by the pathologist. For example, a radiologist who is evaluating a lesion that looks like an aneurysmal bone cyst must include in the differential diagnosis the possibility of telangiectatic osteosarcoma. Likewise, the pathologist who is looking at the histologic sections of the same lesion must also consider the possibility of telangiectatic osteosarcoma because both lesions have several histopathologic similarities. On the other hand, in the radiologic evaluation of a purely lytic lesion at the articular end of a long bone that looks like a giant cell tumor, plasmacytoma might enter into the differential diagnosis, whereas for a pathologist examining the same lesion (which in fact is a giant cell tumor), the differential diagnosis will obviously include other lesions that may contain giant cells. However, the pathologist would not consider plasmacytoma as one of the differential possibilities because of the completely different histologic pattern of this tumor.
information, radiologic imaging, and pathologic material should be carefully studied and correlated. The radiologist has the significant advantage of being able to view the three-dimensional extent of a bone tumor, whereas the pathologist can view only a small biopsy specimen of the lesion selected by the surgeon (133), which may not reflect the histology of the entire lesion. Obviously, viewing the entire resected specimen enhances the final pathologic evaluation. Many years ago, Ewing commented that “the gross anatomy (as revealed in radiographs) is often a safer guide to a correct clinical conception of the disease than the variable and uncertain nature of a small piece of tissue” (48). It is important to emphasize that the differential diagnosis contemplated by the radiologist may be identical to or different from that contemplated by the pathologist. For example, a radiologist who is evaluating a lesion that looks like an aneurysmal bone cyst must include in the differential diagnosis the possibility of telangiectatic osteosarcoma. Likewise, the pathologist who is looking at the histologic sections of the same lesion must also consider the possibility of telangiectatic osteosarcoma because both lesions have several histopathologic similarities. On the other hand, in the radiologic evaluation of a purely lytic lesion at the articular end of a long bone that looks like a giant cell tumor, plasmacytoma might enter into the differential diagnosis, whereas for a pathologist examining the same lesion (which in fact is a giant cell tumor), the differential diagnosis will obviously include other lesions that may contain giant cells. However, the pathologist would not consider plasmacytoma as one of the differential possibilities because of the completely different histologic pattern of this tumor.
For teaching purposes, the diagnostic approach to bone tumors is discussed separately from the radiologic and the pathologic point of view.
Radiology
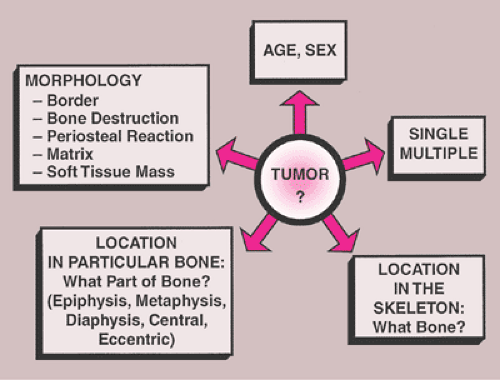
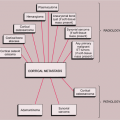
In general, the imaging of musculoskeletal neoplasms can be considered from three standpoints: detection, diagnosis and differential diagnosis, and evaluation (staging) (Fig. 1-1). Detection of a bone tumor does not always require the expertise of a radiologist. The clinical history and the physical examination are often sufficient to raise the suspicion of a tumor, although radiography is the most common means of revealing one. Skeletal scintigraphy can also pinpoint lesions, but its findings are nonspecific.
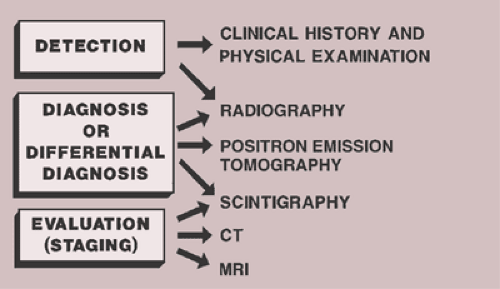 Figure 1-1 Imaging of tumors. Imaging of musculoskeletal neoplasms can be considered from three aspects: detection, diagnosis (or differential diagnosis), and evaluation. |
Despite the dramatic advances in imaging technology that have occurred in recent decades, especially the introduction of cross-sectional imaging modalities such as computed tomography (CT) and magnetic resonance imaging (MRI), radiography remains the single most important modality for establishing a diagnosis (69) and serves as the basis for differential diagnosis (37,115,136). It yields the most useful information about the location and morphology of a lesion, particularly concerning the type of bone destruction, calcifications, ossifications, and periosteal reaction. Conventional tomography, although presently rarely implemented, can be a useful diagnostic tool, particularly on those occasions when questions arise regarding cortical destruction, periosteal reaction, or mineralization of the tumor matrix. It can also detect occult pathologic fracture (1). Scintigraphy is only occasionally helpful in making a specific diagnosis but can be valuable in distinguishing, for example, multiple myeloma from similar-appearing metastases (121) or for distinguishing a benign or malignant sclerotic tumor from a bone island (70).
The imaging advances of the past decade have been most profoundly felt in the evaluation (staging) of bone tumors (46,102,144,145). The multiplanar capabilities and unsurpassed soft tissue contrast offered by CT (24,114) and MRI (10,11,103) have rendered these modalities indispensable in tumor staging (15,33,57,64,148). They have enabled radiologists to determine tumor size, location, and configuration more accurately than ever before and have facilitated demonstration of intra- and extramedullary extension of tumors and the relationship of tumors to individual muscles, muscle compartments, fascial planes, and neurovascular bundles, as well as to neighboring joints and organs (12,13,189). MRI, in fact, affords more accurate anatomic staging than any other imaging method (19,20,32). Nevertheless, it is rarely helpful in differentiating benign from malignant tumors, despite attempts to identify signal intensity patterns, the appearance of tumor margins, the presence of edema, and neurovascular bundle involvement as markers for this essential determination (76,93).
Other techniques also play roles in evaluation of bone tumors. These include scintigraphic techniques using technetium (Tc-99), gallium citrate (Ga-67), and indium (In-111)–labeled white blood cells, as well as single photon emission computed tomography (SPECT), among others (12,13,47,55,92,121,189). Because these imaging methods use radiopharmaceutical labeling, they can provide information about the pathophysiologic function of bone and surrounding soft tissues, as well as the size and location of a lesion (120).
Most recently the application of positron emission tomography (PET) for diagnosis of musculoskeletal neoplasms gained a worldwide acceptance (2,21,104,182). PET differs from other single-photon radionuclide scans in its ability to correct for tissue attenuation signal loss
and its relatively uniform spatial resolution (52). This technique contributes unique information regarding metabolism of musculoskeletal lesions (51) based on observations of high glycolysis rates in malignant tissues (186). In particular, whole-body 18F-labeled 2-fluoro-2-deoxyglucose (18FDG)-PET scanning is a valuable adjunct in identifying primary, recurrent, and metastatic cartilage malignancies (52).
and its relatively uniform spatial resolution (52). This technique contributes unique information regarding metabolism of musculoskeletal lesions (51) based on observations of high glycolysis rates in malignant tissues (186). In particular, whole-body 18F-labeled 2-fluoro-2-deoxyglucose (18FDG)-PET scanning is a valuable adjunct in identifying primary, recurrent, and metastatic cartilage malignancies (52).
Arteriography is used mainly to map out bone lesion and to assess the extent of disease. It is also used to demonstrate the vascular supply of a tumor and to localize vessels suitable for preoperative intraarterial chemotherapy as well as to demonstrate the area suitable for open biopsy, because the most vascular parts of a tumor contain the most aggressive components. Occasionally, arteriography can be used to demonstrate abnormal tumor vessels, corroborating findings revealed by radiography. In selected cases, this technique may help to differentiate osteoid osteoma from bone abscess.
Ultrasonography only rarely contributes information useful in differential diagnosis of malignant and benign musculoskeletal tumors (18).
Radiography
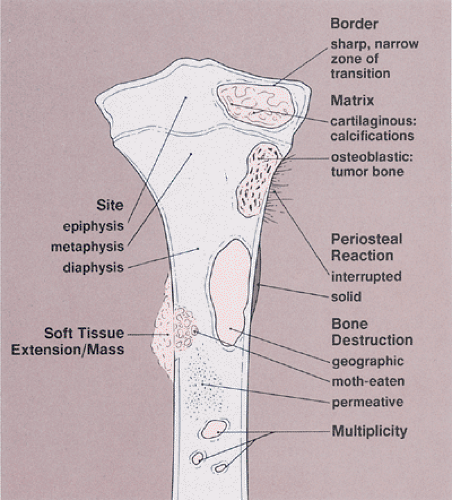
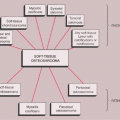
Conventional radiographs continue to provide a wealth of information whose implications can be helpful in the precise diagnosis of a bone tumor (69,96,106,140,192,210). However, radiography does not provide a diagnosis in every case (86). Some types of tumor can be diagnosed with certainty by their radiographic appearance alone, other types can be diagnosed only with various degrees of probability, and still others may have an appearance compatible with that of more than one type of tumor and therefore allow only a differential diagnosis to be made (134,163). The information yielded by radiography includes (Fig. 1-2):
Topography of the lesion (location in the skeleton and in the individual bone)
Borders of the lesion (the so-called zone of transition)
Type of bone destruction
Type of periosteal response to the lesion (periosteal reaction)
Type of matrix of the lesion (composition of the tumor tissue)
Nature and extent of soft tissue involvement
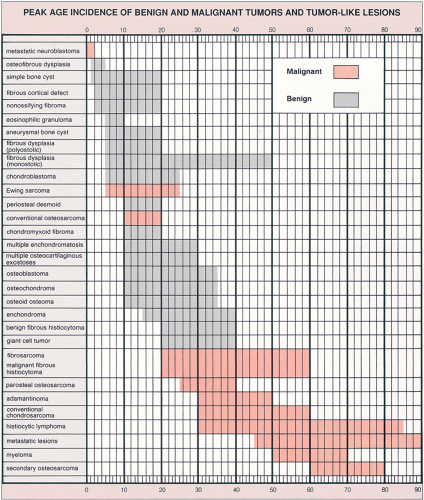
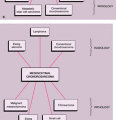
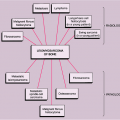
Patient age and determination of whether a lesion is solitary or multiple are the starting approaches in the diagnosis of bone tumors (Fig. 1-3). The age of the patient is the single most important item of clinical data that can be used in conjunction with the radiographic findings to establish a diagnosis (99). Certain tumors occur almost exclusively in a specific age group (Fig. 1-4). For example, aneurysmal bone cyst, chondromyxoid fibroma, and chondroblastoma rarely occur in individuals older than 20 years. Conversely, giant cell tumor of bone almost invariably arises after growth plate closure, and metastatic lesions, myeloma, and conventional chondrosarcoma are rarely encountered in patients younger than 40 years (105,108). Some bone tumors associated with specific age groups may have different radiographic presentations and appear in atypical locations, when they arise outside of their usual age population. Simple bone cysts, for example, are found almost exclusively in the long bones (proximal humerus, proximal femur) before skeletal maturity. After skeletal maturity they may arise in the pelvis, scapula, or calcaneus, among other sites, and may exhibit unconventional radiographic features with progressing age (141).
Determining the number of lesions also has important implications. Benign lesions tend to involve multiple
sites, as in polyostotic fibrous dysplasia, enchondromatosis, multiple osteocartilaginous exostoses, Langerhans cell histiocytosis (eosinophilic granuloma), hemangiomatosis, and fibromatosis. In contrast, primary malignancies, such as osteosarcoma, Ewing sarcoma, fibrosarcoma, and malignant fibrous histiocytoma, rarely present as multifocal disease. Multiple malignant lesions usually indicate metastatic disease, multiple myeloma, or lymphoma.
sites, as in polyostotic fibrous dysplasia, enchondromatosis, multiple osteocartilaginous exostoses, Langerhans cell histiocytosis (eosinophilic granuloma), hemangiomatosis, and fibromatosis. In contrast, primary malignancies, such as osteosarcoma, Ewing sarcoma, fibrosarcoma, and malignant fibrous histiocytoma, rarely present as multifocal disease. Multiple malignant lesions usually indicate metastatic disease, multiple myeloma, or lymphoma.
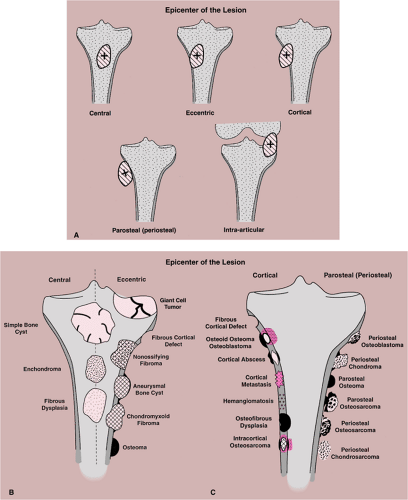
Site of the Lesion
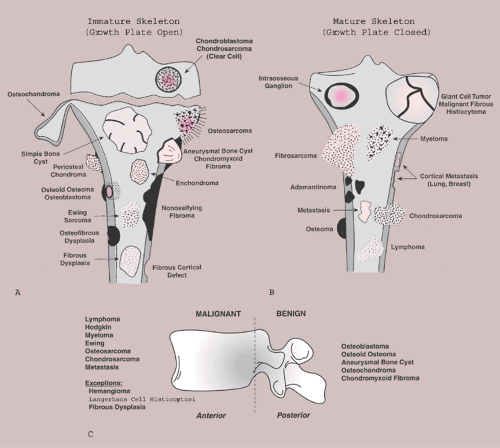
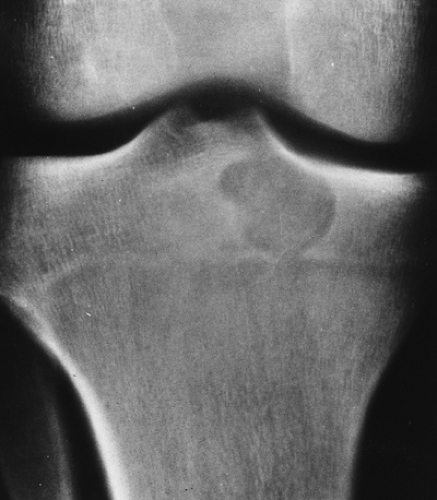
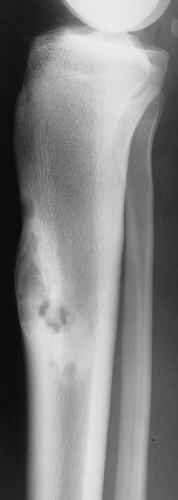
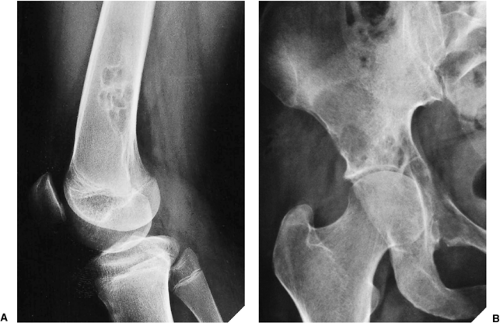
Some tumors have a predilection for specific bones or specific sites in bone (Table 1-1 and Fig. 1-5). This location is determined by the laws of field behavior and developmental anatomy of the affected bone, a concept first popularized by Johnson (84,133). Parosteal osteosarcoma, for example, arises with very rare exceptions in the posterior aspect of the distal femur, a feature so characteristic that it alone can suggest the diagnosis (Fig. 1-6). The same can be said of chondroblastoma, which has a strong preference for the epiphysis of long bones before skeletal maturity (Fig. 1-7). Adamantinoma and osteofibrous dysplasia have a specific predilection for the tibia (Fig. 1-8; see also Figs. 4-49, 4-50 and 7-64). A lesion’s location can also exclude certain entities from the differential diagnosis.
Giant cell tumor, for example, should not be seriously considered if the lesion does not affect the articular end of a bone because very few of these tumors are found anywhere else.
Giant cell tumor, for example, should not be seriously considered if the lesion does not affect the articular end of a bone because very few of these tumors are found anywhere else.
Table 1-1 Predilection of Tumors for Specific Sites in the Skeleton | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||
Equally important in the evaluation of a lesion’s site is its location in relation to the central axis of the bone (Fig. 1-9). This is particularly true when the lesion is located in a long tubular bone, such as humerus, radius, femur, or tibia (71). For example, simple bone cyst, enchondroma, or a focus of fibrous dysplasia always appears centrally located (see Figs. 3-6B, 3-8, 3-9, 4-25, 7-17 and 7-18). In contrast, eccentric location is characteristically observed in aneurysmal bone cyst, chondromyxoid fibroma, and nonossifying fibroma (see Figs. 3-74, 3-75, 4-2B, 4-3, 4-4, 7-31 and 7-33).
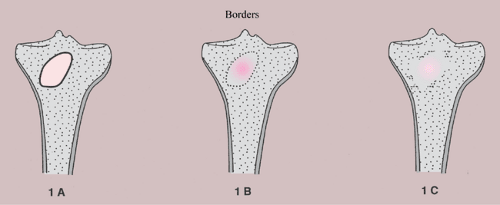
Borders of the Lesion
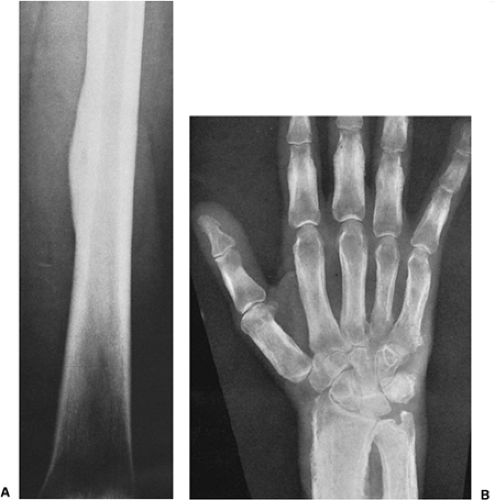
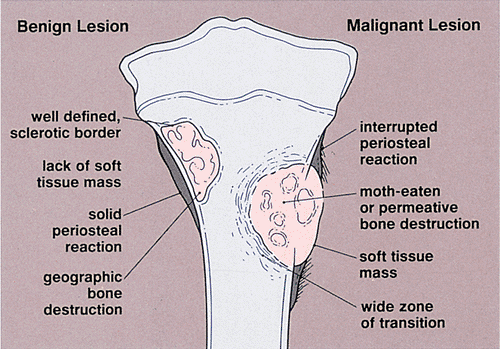
The borders or margins of a lesion are crucial factors in determining the growth rate of a lesion and hence whether it is benign or malignant (113). Three types of lesion margins are encountered: (a) a margin with sharp demarcation by sclerosis between the peripheral aspect of the tumor and the adjacent host bone (IA margin), (b) a margin with sharp demarcation without sclerosis around the periphery of the lesion (IB margin), and (c) a margin with an ill-defined region (either the entire circumference or only a portion of it) at the interface between lesion and host bone (IC margin) (Fig. 1-10). Slow-growing lesions are marked by sharply outlined, sclerotic borders, and this narrow zone of transition usually indicates that a tumor is benign (133). Benign lesions such as nonossifying fibroma, simple bone cyst, and chondromyxoid fibroma almost invariably exhibit sclerosis at their borders (Fig. 1-11A). Indistinct borders (a wide zone of transition), on the other hand, are typical of malignant or aggressive lesions (Fig. 1-11B). Primary malignancies such as fibrosarcoma, malignant fibrous histiocytoma, lymphoma, multiple myeloma (or solitary plasmacytoma), and metastases from primary tumors of the kidney, thyroid, and gastrointestinal tract usually lack a sclerotic border, as does the giant cell tumor of bone. Radio- or chemotherapy of malignant bone tumors can alter their appearance, causing them to exhibit sclerosis and a narrow zone of transition (54).
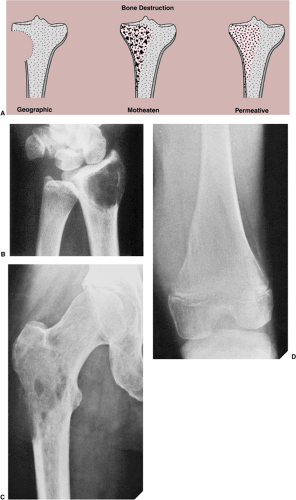
Type of Bone Destruction
Destruction of bone represents not only a direct effect of tumor cells but also reflects a complex mechanism in which normal osteoclasts of the host bone respond to pressure generated by the enlarging mass and by active hyperemia associated with the tumor (134). Cortical bone is destroyed less rapidly than trabecular bone. However, loss of cortical bone appears earlier on radiography because its density is highly homogeneous compared with that of trabecular bone. In the latter, greater amounts of bone must be destroyed (about 70% loss of mineral content) before the loss becomes radiographically evident (134). Like the borders of a lesion, the type of bone destruction caused by a tumor indicates its growth rate. Bone destruction can be described as geographic (type I), moth-eaten (type II), and permeative (type III) (105,107) (Fig. 1-12). Although none of these features are pathognomonic for any specific neoplasm, the type of destruction may suggest a benign or a malignant process. Geographic bone destruction is characterized by a uniformly destroyed area usually within sharply defined borders. It typifies slow-growing, benign lesions, such as simple bone cyst, enchondroma, chondromyxoid fibroma, or giant cell tumor. On the other hand, moth-eaten (i.e., characterized by multiple, small,
often clustered lytic areas) and permeative (i.e., characterized by ill-defined, very small oval radiolucencies or lucent streaks) types of bone destruction mark rapidly growing, infiltrating tumors, such as myeloma, lymphoma, fibrosarcoma, or Ewing sarcoma. However, some nonneoplastic lesions may demonstrate this aggressive pattern. For example, osteomyelitis can exhibit both type II (moth-eaten) and type III (permeative) patterns of destruction (133). Similarly, hyperparathyroidism can cause a permeative pattern (113). The distinction between a moth-eaten and a permeative pattern of destruction may be subtle; often the two patterns coexist in the same lesion.
often clustered lytic areas) and permeative (i.e., characterized by ill-defined, very small oval radiolucencies or lucent streaks) types of bone destruction mark rapidly growing, infiltrating tumors, such as myeloma, lymphoma, fibrosarcoma, or Ewing sarcoma. However, some nonneoplastic lesions may demonstrate this aggressive pattern. For example, osteomyelitis can exhibit both type II (moth-eaten) and type III (permeative) patterns of destruction (133). Similarly, hyperparathyroidism can cause a permeative pattern (113). The distinction between a moth-eaten and a permeative pattern of destruction may be subtle; often the two patterns coexist in the same lesion.
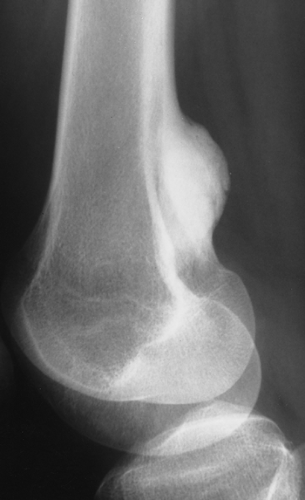 Figure 1-6 Site of the lesion. Parosteal osteosarcoma has a predilection for the posterior aspect of the distal femur. |
Table 1-2 Examples of Nonneoplastic and Neoplastic Processes Categorized by Type of Periosteal Reaction | ||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||
Periosteal Response
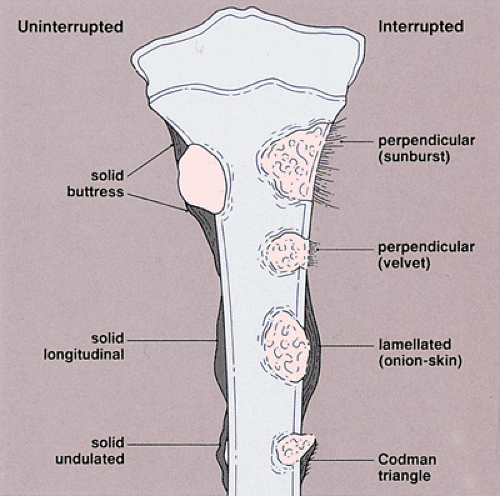
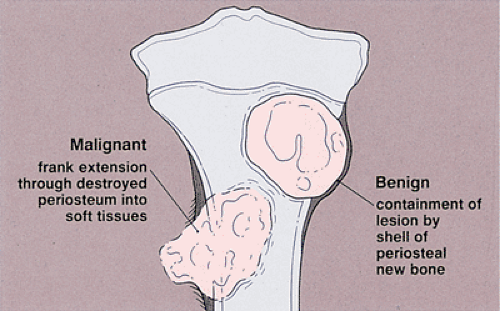
Like the pattern of bone destruction, the pattern of periosteal reaction is an indicator of the biologic activity of a lesion (209). Bone neoplasms elicit periosteal reactions that can be categorized as uninterrupted (continuous) or interrupted (discontinuous) (160) (Fig. 1-13 and Table 1-2). Any widening and irregularity of bone contour may represent periosteal activity. The solid periosteal reaction represents a single solid layer or multiple closely apposed and fused layers of new bone attached to the outer surface of the cortex (160) (Fig. 1-14). The resulting pattern is often referred to as cortical thickening (49). Although no single periosteal response is unique for a given lesion, an uninterrupted periosteal reaction indicates a long-standing (slow-growing), usually indolent, benign process. There are several types of solid periosteal reaction: a solid buttress, such as is frequently seen accompanying aneurysmal bone cyst and chondromyxoid fibroma; a solid smooth or elliptical layer, such as is seen in osteoid osteoma and osteoblastoma; an undulating type, most frequently seen in long-standing varicosities, pulmonary osteoarthropathy, chronic lymphedema, periostitis, and, rarely, with neoplasms (160); and a single lamellar reaction, such as accompanies osteomyelitis,
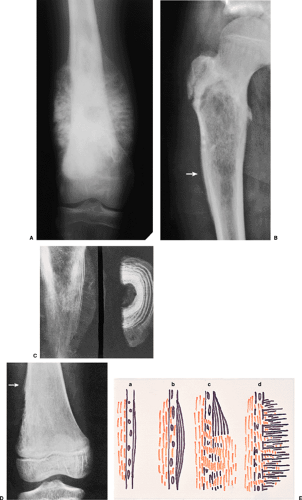
Langerhans cell histiocytosis, and stress fracture. An interrupted periosteal response, on the other hand, is commonly seen in malignant primary tumors and less commonly in some metastatic lesions and highly aggressive nonmalignant processes. In these tumors, the periosteal reaction may appear in a sunburst (“hair-on-end”) (Fig. 1-15A) or onion-skin (lamellated) pattern (Fig. 1-15B, C). When the tumor breaks through the cortex and destroys the newly formed lamellated bone, the remnants of the latter on both ends of the break-through area may remain as a triangular structure known as a Codman triangle (Fig. 1-15D, E).
Langerhans cell histiocytosis, and stress fracture. An interrupted periosteal response, on the other hand, is commonly seen in malignant primary tumors and less commonly in some metastatic lesions and highly aggressive nonmalignant processes. In these tumors, the periosteal reaction may appear in a sunburst (“hair-on-end”) (Fig. 1-15A) or onion-skin (lamellated) pattern (Fig. 1-15B, C). When the tumor breaks through the cortex and destroys the newly formed lamellated bone, the remnants of the latter on both ends of the break-through area may remain as a triangular structure known as a Codman triangle (Fig. 1-15D, E).
Type of Matrix
The matrix represents the intercellular material produced by mesenchymal cells and includes osteoid, bone, chondroid, myxoid, and collagen material (196). Assessment of the type of matrix allows differentiation of some similar-appearing lesions and, in particular, the tumor matrix provides a useful means of differentiating osteoblastic from chondroblastic processes. Although it should be kept in mind that tumor bone is often radiographically indistinguishable from reparative new bone deposited secondary to bone destruction by a reactive sclerosis or callus formation, the presence of irregular, not fully mineralized bone matrix within or adjacent to an area of bone destruction strongly suggests osteosarcoma (Fig. 1-16). Similarly, cottony or cloudlike densities within the medullary cavity and in the adjacent soft tissues are likely to represent tumor bone and hence osteosarcoma.
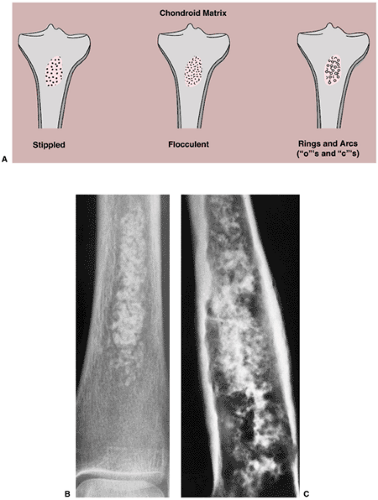
Calcifications in the tumor matrix, on the other hand, point to a chondroblastic process. These calcifications typically appear punctate (stippled), irregularly shaped (flocculent), or curvilinear (annular or comma-shaped, rings and arcs). In benign or well-differentiated malignant tumors they may reflect the process of endochondral ossification (196) (Fig. 1-17A). Differential diagnosis of stippled, flocculent, or ring-and-arc calcifications includes enchondroma (Fig. 1-17B), chondroblastoma, and chondrosarcoma (Fig. 1-17C). Sometimes it is difficult to distinguish osseous matrix from cartilaginous matrix. The former usually appears radiographically more organized and structural (trabecular), whereas the latter is more amorphous, with frequently distinguished characteristic calcifications (described previously).
A completely radiolucent lesion may be either fibrous or cartilaginous in origin, although hollow structures produced by tumor-like lesions, such as simple bone cysts or intraosseous ganglion, can also present as radiolucent areas (Table 1-3).
Table 1-3 Tumors and Pseudotumors That May Present as Radiolucent Lesions | ||||
|---|---|---|---|---|
|
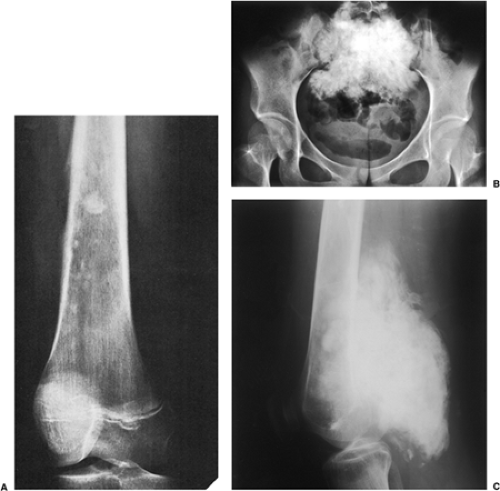
Soft Tissue Mass
With few exceptions—giant cell tumor, aneurysmal bone cyst, and desmoplastic fibroma among the more common examples—benign bone tumors usually do not have an associated soft tissue mass, which is an invariable feature of many advanced malignant and aggressive lesions (94) (Fig. 1-18). Nevertheless, it is important to note that some nonneoplastic conditions also manifest with a soft tissue component (e.g., osteomyelitis). In such cases, however, the associated mass is usually poorly defined and the fatty tissue layers appear obliterated. This is in sharp contrast to the soft tissue extensions typical of malignant processes, in which a defined mass extends through the destroyed cortex but the tissue planes are usually intact.
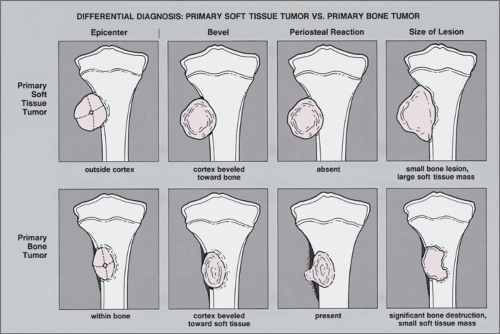
A bone lesion associated with a soft tissue mass should prompt the question of which came first. Is the soft tissue lesion an extension of a primary bone tumor, or is it a primary soft tissue tumor invading bone? Some clues may help to answer this question, but these observations are by no means absolute (Fig. 1-19). A large soft tissue mass with a smaller bone lesion usually indicates secondary bone involvement. That said, it is worth noting that Ewing sarcoma and less commonly other malignant tumors may exhibit a large soft tissue mass accompanying a small primary bone malignancy. Another clue to help determine the primary malignancy may be found in the periosteal response. Primary soft tissue tumors adjacent to bone usually destroy the neighboring periosteum without eliciting a periosteal response. Primary bone malignancies, however, typically prompt a periosteal
reaction when they grow into the cortex and extend into adjacent soft tissues (69,133,160).
reaction when they grow into the cortex and extend into adjacent soft tissues (69,133,160).
Benign Versus Malignant Nature
Although it is sometimes very difficult to establish a lesion as benign or malignant by radiography alone, the clusters of features that can be gathered from radiographs can help in favoring one designation over the other (Fig. 1-20). Benign lesions usually have well-defined sclerotic borders and exhibit a geographic type of bone destruction; the periosteal reaction is solid and uninterrupted, and there is no soft tissue mass. In contrast, malignant tumors often exhibit poorly defined borders with a wide zone of transition; bone destruction appears in a moth-eaten or permeative pattern, and the periosteum shows an interrupted, sunburst, or onion-skin reaction with an adjacent soft tissue mass. It should be kept in mind, however, that some benign lesions may also exhibit aggressive features (Table 1-4).
Table 1-4 Benign Lesions with Aggressive Features | ||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
Scintigraphy (Radionuclide Bone Scan)
The radionuclide bone scan is an indicator of mineral turnover. Because there is usually enhanced deposition of bone-seeking radiopharmaceutical agents in areas undergoing change and repair, a bone scan is useful in localizing tumors and tumor-like lesions in the skeleton (58). This is particularly true in such conditions as fibrous dysplasia, Langerhans cell histiocytosis, and metastatic cancer, in which more than one lesion is encountered. Technetium-99m methyl diphosphonate (MDP) scans are used primarily to determine whether a lesion is monostotic or polyostotic. Such a study is therefore essential in staging a bone neoplasm. It is important to remember that although the degree of abnormal uptake may be related to the aggressiveness of the lesion, this does not correlate well with the histologic grade (Fig. 1-21).
Radionuclide bone scan also plays an important role in localizing small lesions such as osteoid osteoma, which may not always be visible on radiography (Fig. 1-22). Although skeletal scintigraphy is a highly sensitive method for detection of bone neoplasms, its specificity is low. In most instances it cannot distinguish benign lesions from malignant tumors, because increased blood flow with increased isotope deposition and increased osteoblastic activity take place in both benign and malignant conditions. Nevertheless, it can sometimes achieve such differentiation in benign lesions that do not absorb the radioactive isotope. In addition, the radionuclide
bone scan is sometimes useful for differentiating multiple myeloma, which usually shows no significant uptake of the tracer, from metastatic bone cancer, which usually does. Scans using Ga-67 may show uptake in a soft tissue sarcoma and may help to differentiate a sarcoma from a benign soft tissue lesion.
bone scan is sometimes useful for differentiating multiple myeloma, which usually shows no significant uptake of the tracer, from metastatic bone cancer, which usually does. Scans using Ga-67 may show uptake in a soft tissue sarcoma and may help to differentiate a sarcoma from a benign soft tissue lesion.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree