Metastases
Osseous Metastases
Skeletal metastases are the most common variety of bone tumors and should always be considered in the differential diagnosis, particularly in older patients (47,65). In a series of 1,000 consecutive autopsies of patients who died of carcinoma, metastases to bone were found in 27% (2). Approximately 70% of all malignant bone tumors are metastatic in origin (128). The incidence varies according to the type of primary neoplasm and the duration of disease. Some malignant tumors demonstrate a far greater predilection for osseous involvement than others. Cancers of the breast, prostate, lung, and kidney account for 80% of all metastatic cancers to bone (67). In men, carcinoma of the prostate is reported to account for almost 60% and carcinoma of the lung for 25% of all bone metastases. In women, carcinoma of the breast is responsible for almost 70% of all metastatic lesions, the remaining 30% being mainly due to carcinomas of the thyroid, uterus, and kidney (1,87). Other primary tumors responsible for bone metastases include carcinomas of the stomach, colon, urinary bladder, melanoma, and some neurogenic tumors (121). Some sarcomas, such as osteosarcoma and Ewing sarcoma, may occasionally metastasize to bones. In children aged 5 years and younger, neuroblastoma is usually the primary tumor responsible for metastatic disease. Because of their frequency, cancers of the breast, lung, and prostate are responsible for the majority of bone metastases.
In the past it was thought that the usual route of metastasis is via tumor emboli, mostly carried through the lymphatic system and the ductus thoracicus to the central blood circulation, and then by the arterial system into the periphery (11). This belief was in contrast to the ancient hypothesis of the British surgeon Stephan Paget (96), who, based on a study of more than 900 autopsy records, proposed that metastasis is not a random process, but the tumor cells (which he regarded as “seeds”) need a special environment (the “soil”) to grow and form metastatic lesions. In recent years this theory has gained wide acceptance and was substantiated by experimental data (for additional information see references 33, 83, 84, 106, and 115). Tumor cells seem to acquire a special “genetic signature” that enables them to metastasize (104). In addition, the microenvironment in bone, especially marrow stem cells, supports cancer cells in homing, differentiation, and survival. Conversely, cancer cells influence osteoblasts and osteoclasts by secreted factors such as parathyroid hormone–related peptide
(PTHrP) or endothelin I (17,84,129). This leads to osteolytic or osteoblastic metastases in bone; however, even osteoblastic metastases are accompanied by increased bone resorption, as is clinically evident by the treatment response to bisphosphonates in osteoblastic metastases of prostate cancer. Furthermore, bone contains an abundance of growth factors (e.g., transforming growth factor β, insulin-like growth factors I and II, fibroblast growth factors, and bone morphogenetic proteins) that are released into the microenvironment during the resorption process (55). Stimulated tumor cells release factors that induce osteoblasts to secrete RANK (receptor activator of nuclear factor kappa b)–ligand or RANKL, which is a potent factor for osteoclast formation and activity. Osteoclasts, in turn, resorb bone and thus release additional growth factors that enhance the accumulation of cancer cells (17).
(PTHrP) or endothelin I (17,84,129). This leads to osteolytic or osteoblastic metastases in bone; however, even osteoblastic metastases are accompanied by increased bone resorption, as is clinically evident by the treatment response to bisphosphonates in osteoblastic metastases of prostate cancer. Furthermore, bone contains an abundance of growth factors (e.g., transforming growth factor β, insulin-like growth factors I and II, fibroblast growth factors, and bone morphogenetic proteins) that are released into the microenvironment during the resorption process (55). Stimulated tumor cells release factors that induce osteoblasts to secrete RANK (receptor activator of nuclear factor kappa b)–ligand or RANKL, which is a potent factor for osteoclast formation and activity. Osteoclasts, in turn, resorb bone and thus release additional growth factors that enhance the accumulation of cancer cells (17).
Clinical Presentation
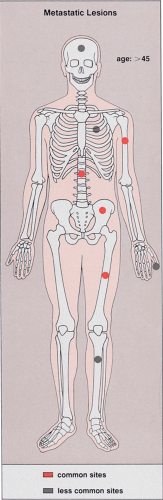
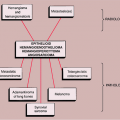
Metastases are relatively rare in patients younger than the age of 40. Therefore, patient age is an important discriminating factor in the diagnosis (81). Unlike primary bone tumors, metastases usually involve the axial skeleton (skull, spine, and pelvis) and the most proximal segments of limb bones. Only in extremely rare cases do metastatic tumors occur distal to the elbows and the knees (82,97). Most metastatic lesions are found in the vertebrae, ribs, pelvis, skull, femur, and humerus (81) (Fig. 8-1).
The majority of skeletal metastases are silent. When metastases are symptomatic, pain is the main clinical feature, although rarely a pathologic fracture through the lesion may focus attention on the disease (98). Clinical and laboratory examinations frequently disclose other signs of malignancy, such as weight loss, anemia, fever, and an elevated erythrocyte sedimentation rate. In addition, serum calcium and alkaline phosphatase levels are usually elevated (125). In the spine, metastases are frequently associated with neurologic symptoms caused by compression of the cord or nerve roots or even by complete obstruction of the thecal sac.
Imaging
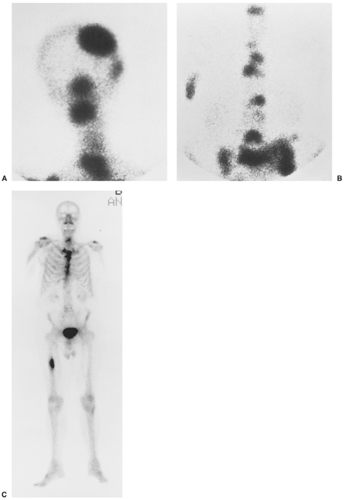
Detection of skeletal metastases is not always possible on routine radiography, because destruction of the bone may not be visible (6,113,127). It has been estimated that 30% to 50% of normal bone mineral must be lost before a bone metastasis becomes visible on a radiograph (9). Radionuclide bone scan, including both planar and single proton emission computed tomography (SPECT) imaging (15), is the best screening method for early detection of metastatic tumors and can detect lytic and sclerotic (blastic) lesions (Fig. 8-2). Computed tomography (CT), magnetic resonance imaging (MRI), and fluorodeoxyglucose (FDG) positron emission tomography (PET) scanning may also be helpful, the latter modality being the most sensitive (23,29,113). On radiography, a metastatic lesion may resemble any of the benign or malignant lesions. There
are no radiographic characteristics of metastasis. The type of bone destruction may be geographic, moth-eaten, or permeative, and the margins may be well or poorly defined. A periosteal reaction and a soft tissue mass may or may not be present, although the latter situation is more common. Metastases can be solitary or multiple, and they can be further subdivided into purely lytic, purely sclerotic, and mixed lesions. This distinction can be of considerable aid in analysis of the site of origin of the primary tumor (27). The primary sources of these lesions are as follows:
are no radiographic characteristics of metastasis. The type of bone destruction may be geographic, moth-eaten, or permeative, and the margins may be well or poorly defined. A periosteal reaction and a soft tissue mass may or may not be present, although the latter situation is more common. Metastases can be solitary or multiple, and they can be further subdivided into purely lytic, purely sclerotic, and mixed lesions. This distinction can be of considerable aid in analysis of the site of origin of the primary tumor (27). The primary sources of these lesions are as follows:
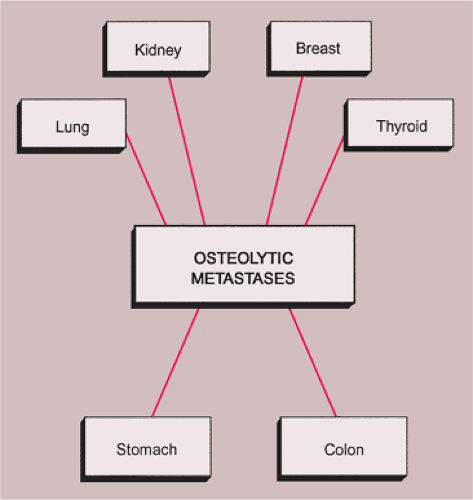
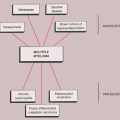
Lytic lesions. Osteolytic metastases are the most common, representing about 75% of all metastatic lesions. The primary source is usually a carcinoma of the kidney, lung, breast, gastrointestinal (GI) tract, or thyroid (35) (Figs. 8-3 and 8-4).
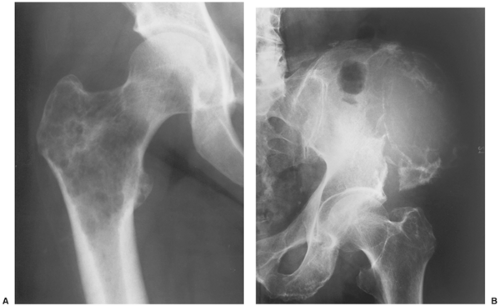
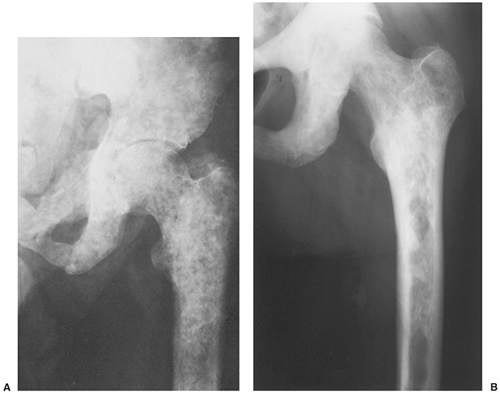
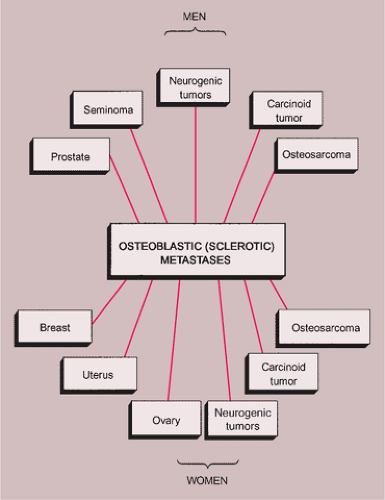
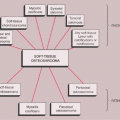
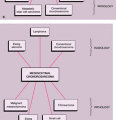
Sclerotic lesions. Osteoblastic metastases represent approximately 15% of all metastatic lesions. In men they are caused mainly by a prostatic gland cancer or a seminoma (21) (Fig. 8-5A). In women the primary source is usually carcinoma of the breast, uterus (particularly cervix), or ovary (93) (Fig. 8-5B). In both genders, metastases may originate from carcinoid tumors (88,100,101), bladder tumors (32), certain neurogenic tumors, including medulloblastoma (121), and osteosarcoma (47,73) (Fig. 8-6).
Mixed lesions. Mixed osteoblastic and osteolytic lesions represent approximately 10% of all metastatic lesions. Any primary tumor can give rise to mixed metastases, the most common primaries being breast and lung tumors. Occasionally, some primary neoplasms may give rise to both lytic and sclerotic metastases (Fig. 8-7).
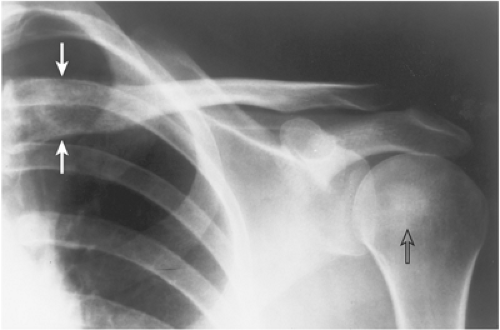
Although metastases in the skeleton may appear similar, regardless of their primary source, in some instances their distribution, location, or morphologic appearance may suggest a site of origin (7,43,56,76,90,112,116). For example, 50% of skeletal metastases distal to the elbows and the knees, although unusual, originate from either bronchogenic or breast carcinoma (85) (Fig. 8-8). Although extremely rare, metastases to the hands and feet, particularly those affecting the distal phalanges (acrometastases), again indicate a primary tumor in the lung, less commonly in the breast or kidney (97) (Fig. 8-9). An extensive osteolysis of the distal phalanx, often accompanied by a soft tissue mass, is the usual presentation of this type of metastasis from bronchogenic carcinoma. Some time ago, characteristic cortical metastases were reported from bronchogenic carcinoma (27,28,48,49,50). These lesions produce a type of cortical destruction termed “cookie-bite” or “cookie-cutter” lesions (Fig. 8-10). The spread of malignant cells to involve the skeleton usually takes place via the hematogenous route. In such instances, the bulk of the tumor lodges in the marrow and spongy bone. Therefore, the initial radiography of metastatic lesions in the skeleton reveals the destruction of cancellous bone; only after further tumor growth is the cortex affected. An anastomosing vascular system in the cortex, originating in the overlying periosteum (118), probably serves as the pathway by which malignant cells from bronchogenic carcinoma reach the compact bone and initiate cortical destruction (39). Primary carcinomas of the kidney and bladder and melanoma may also give rise to cortical metastases (20,36,59). It is of interest that the majority of cortical metastases affect the femur (20,50).
In certain instances, the morphologic appearance of a metastasis may suggest a specific site of origin or may at least narrow the differential diagnostic possibilities (27). For example, bubbly, highly expansive, so-called blow-out metastatic lesions originate from a primary carcinoma of the kidney or thyroid (Fig. 8-11). Multiple round, dense foci or diffuse increases in bone density are often seen in metastatic carcinoma of the prostate (see Fig. 8-5A).
MRI is occasionally useful in determining the origin of the metastatic lesion. Recently, Choi et al. (18) reported a “flow-void” sign apparently characteristic for metastatic renal carcinoma. Reviewing 20 osseous metastatic lesions from renal cell carcinoma, the investigators noted within a mass of intermediate signal intensity the frequent appearance of multiple dot-like or tubular structures exhibiting low signal intensity on T1-weighted sequences. These structures showing the flow-void signal corresponded to dilated blood vessels (arteries supplying the hypervascular tumor and veins draining the lesion). Although the specificity of the flow-void sign for diagnosis of metastatic renal carcinoma is as yet unknown, its presence on MR images might suggest this diagnosis.
In general, metastatic bone disease is characterized by a combination of bone resorption and bone formation. Radiographic imaging of the lesions will reveal the predominant process (12,95). When osteolysis predominates, the lesions appear lytic, and when bone formation is dominant, they appear sclerotic (38,105). Multiple sclerotic metastases may present either in a focal pattern (multiple snowball appearance) or may have a diffuse pattern (generalized radiopacity of bones) (31,60). Bone formation in metastatic disease involves either a stromal or a reactive mechanism. Stromal new bone occurs only in lesions associated with the development of a surrounding fibrous stroma, as in carcinoma of the prostate, and reflects intramembranous ossification of areas within the stroma. In contrast, reactive bone represents an attempt to repair the injury caused by tumor cells. This type of formation occurs to some extent in all metastatic lesions, although it tends to be negligible in highly anaplastic or rapidly growing tumors (105). Bone destruction is always mediated by tumor-induced osteoclastic resorption. Primary tumors responsible for purely osteolytic metastases are usually located in the kidneys, lung, breast, thyroid, or GI tract, whereas primaries responsible for osteoblastic metastases are usually located in the prostate gland. It must be pointed out, however, that after treatment (radiation therapy, chemotherapy, or hormonal therapy), purely lytic lesions may become sclerotic.
Scintigraphy is almost invariably positive in bone metastases (91), and increased uptake is observed in both sclerotic and lytic lesions (16,19,30,44,78,79,94,99) (see Fig. 8-2). This phenomenon is secondary to the
increased bone turnover and reactive repair at the periphery of the lesion (3). Radionuclide bone scan is helpful for distinguishing metastatic disease from multiple myeloma because the latter usually presents with a normal uptake of a tracer (77). Only a few cases have been reported that exhibited negative bone scintigraphy in metastatic disease of the skeleton despite positive radiographic findings (14,68). This can be explained by the fact that bone density, as imaged by conventional radiography, reflects net metabolic activity over an extended period of time and does not necessarily reflect current metabolic activity. Therefore, a radiographically sclerotic lesion with a low metabolic rate can produce a negative bone scan (25). Occasionally, widespread metastatic disease produces a diffusely increased uptake throughout the skeleton rather than discrete hot spots. This so-called superscan appearance is identified by the abnormally intense bone uptake or by the absence of a normally visible activity in the kidneys, bladder, and soft tissues (10). Sometimes metastases cause cold spots (photopenic defects) when there is bone destruction but insignificant reactive bone formation; this may be observed in metastases from lung and breast carcinoma (10,57,61,70).
increased bone turnover and reactive repair at the periphery of the lesion (3). Radionuclide bone scan is helpful for distinguishing metastatic disease from multiple myeloma because the latter usually presents with a normal uptake of a tracer (77). Only a few cases have been reported that exhibited negative bone scintigraphy in metastatic disease of the skeleton despite positive radiographic findings (14,68). This can be explained by the fact that bone density, as imaged by conventional radiography, reflects net metabolic activity over an extended period of time and does not necessarily reflect current metabolic activity. Therefore, a radiographically sclerotic lesion with a low metabolic rate can produce a negative bone scan (25). Occasionally, widespread metastatic disease produces a diffusely increased uptake throughout the skeleton rather than discrete hot spots. This so-called superscan appearance is identified by the abnormally intense bone uptake or by the absence of a normally visible activity in the kidneys, bladder, and soft tissues (10). Sometimes metastases cause cold spots (photopenic defects) when there is bone destruction but insignificant reactive bone formation; this may be observed in metastases from lung and breast carcinoma (10,57,61,70).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree