Introduction
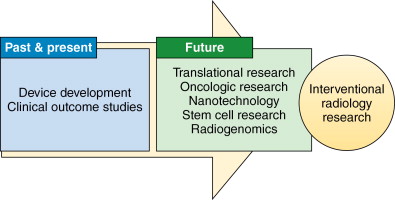
Research in interventional radiology has historically been driven by innovation and technological development leading to the conceptualization, investigation, validation, and application of a myriad of novel procedures into clinical practice. The development of new techniques and therapies is critical to ensuring the continued success and relevance of interventional radiology as a subspecialty. This has been reflected in the vision statement of the Society of Interventional Radiology Interventional Oncology Task Force, which states that there should be enhanced integration of image-guided oncologic technologies by 2015, along with new developments in genomics, proteomics, molecular imaging, and nanotechnology. The rapid evolution of such transformative technologies has necessitated an expansion in the scope of scientific knowledge and technical skills required of interventional physician-scientists. This chapter summarizes the background and current developments in these areas and outlines potential future research of particular relevance to interventional oncology ( Figure 6-1 ).
Nanotechnology
Nanotechnology refers to the design, creation, and manipulation of structures on the submicroscopic scale, usually below 100 nm in size. The use of nanotechnology in medicine, “nanomedicine” has demonstrated exciting potential for clinical use. The majority of current research is focused toward (1) novel methods of imaging and diagnostic techniques, (2) antimicrobial therapy, (3) micro-robotics, (4) cell repair, and (5) delivery of therapy through minimally invasive means. More specifically, potential applications of nanotechnology in interventional radiology (IR) have been described in the areas of drug delivery, thermotherapy, vascular interventions, and implantable devices.
Nanotechnology for systemic and local drug delivery
Nanoparticles for drug delivery are engineered systems in the nanometer size range, and drugs can be attached to the surface of such particles or entrapped or encapsulated within them. The advantages associated with nanoparticle use lie in its ability to increase drug efficiency and improve the therapeutic index of currently available drugs, reducing drug toxicity to improve side-effect profiles, , and controlled release to achieve therapeutic levels for an extended period of time. They can also improve drug stability and solubility and allow targeted drug delivery. The potential to accommodate multiple types of drug molecules may allow for the development of systems for combined therapeutic and imaging applications. , The need for targeted drug delivery is particularly evident in interventional oncology, where it would be preferable for tumor to be exposed to high drug concentrations, while reducing systemic toxicity. As nanoparticles are able to pass through the fenestrations of the highly permeable tumor vasculature and are limited by tumor lymphatic systems, preferential collection of nanoparticles within the tumor interstitium may be achieved, and has been termed the enhanced permeability and retention (EPR) effect. , ,
The development of controlled or triggered intratumoral drug release in systemic chemotherapy is desirable as it can result in optimum drug delivery for longer periods, thus increasing efficacy of treatment. Such targeting has extended beyond the use of the passive EPR effect, and active targeting based on molecular recognition of tumor has been described. In terms of local drug delivery, direct intratumoral injection of chemotherapy-loaded nanoparticles in tumor-bearing mouse models has been observed to increase efficiency in impeding tumor development. Local intratumoral injection of radiosensitizers to enhance radiotherapy such as gadolinium has similarly shown promise in inhibiting tumor growth and increasing life expectancy. In the area of local thrombolysis of particular relevance to IR, nanoparticles modified with streptokinase and targeted to fibrin have been described. , Marsh et al. developed fibrin-targeted nanoparticles surface conjugated to streptokinase in vitro and found that they were able to lyse 90% of clots within 60 minutes of exposure, with substantially more potent fibrinolytic activity and the same degree of thrombolysis as a 1000–fold greater dose of free streptokinase.
Ultrasound-mediated drug delivery
When used in combination with systemically administered encapsulated therapies such as drug-loaded microbubbles or nanoparticles, locally applied ultrasound has been shown to enhance drug transport through tissues and across cell membranes. , As a drug delivery method, it is noninvasive and may be controlled and focused locally, thereby limiting the drug interactions to the target tissue and sparing surrounding normal tissues from unwanted side effects. An added major advantage is the targeting of deep tissues and the ability to be used through intraluminal, endoscopic, or percutaneous means to reach almost any site. Dayton et al. found in an in vitro study that ultrasonography, in combination with nanodroplets carrying the cytotoxic drug paclitaxel, resulted in significant cell death. In vivo studies using low-frequency ultrasonographic and polymeric micelles and liposomes encapsulating doxorubicin or fluorouracil have found statistically significant decreases in tumor size and increased effects of drug on tumor growth. Rapoport et al. described the potential for multifunctional nanoparticles to function both as drug carriers and ultrasound contrast agents for enhanced visualization. Long-lasting contrast provided enhanced tumor visualization in conjunction with ultrasonography-mediated enhanced release of encapsulated doxorubicin and intracellular uptake of drug from nanoparticles.
Nanotechnology and thermal therapy
The use of thermal therapeutics such as radiofrequency ablation has become established clinical practice for certain tumors including solitary renal masses. The minimally invasive nature of thermotherapy allows for treatment of tumors in sites where surgical resection is clinically impractical. However, this requires sufficient depth and penetration of tissues by the heating element. Current heating techniques do not discriminate between tumor and normal tissue, giving rise to potential damage to surrounding structures. In addition, heating tissue to different degrees has different effects—hyperthermia (40°C–42°C) may render cells more susceptible to the cytotoxic effects of chemotherapy or radiation and can induce some degree of apoptosis, , whereas thermal ablation (>46°C) has a direct cytotoxic effect on cells, and can be used as a monotherapy for destruction of tumor cells. A combination of hyperthermic effect of thermal ablation and use of nano-carrier of cytotoxic drugs may have enhanced therapeutic effects on the locoregional treatment of solid tumors, including hepatocellular carcinoma (HCC).
Magnetic thermotherapy
The capacity of magnetic nanoparticles to absorb energy from an alternating magnetic field and convert it to heat, forms the basis of magnetic thermotherapy. The feasibility of this technique has been demonstrated in patients with locally recurrent prostate cancer. , Iron oxide nanoparticles in suspension were injected transperineally into the prostate gland of patients under transrectal ultrasound, and an alternating magnetic field was applied. Because of retention of particles within the gland, serial heat treatments could be performed after a single injection, with the achievement of hyperthermic temperatures achieved at relatively low field strengths. The technique could potentially be used as a combination therapy along with irradiation and implantation of iodine-125 ( I) seeds, as the thermal dose achieved during the study would prove effective in such a setting. The intraarterial route has also been examined as a means for selective magnetic hyperthermia of tumors. In a rabbit model for renal carcinoma, Takamatsu et al. investigated the possibility of intraarterial selective hyperthermia using a transcatheter arterial embolization technique with a mixture of commercially available nano-sized magnetic particles (Ferucarbotran) and the embolic material lipiodol. Injection of this mixture into the renal artery under fluoroscopic guidance and exposure to an alternating-current magnetic field increased intratumoral temperatures to 45°C, which were sufficient for hyperthermia but not ablation. However, given the relative hypovascularity of the tumor, the authors hypothesized that much stronger selective hyperthermia might be achievable in a hypervascular tumor such as HCC.
Radiofrequency ablation
Studies in the use of RFA in combination with chemotherapy for malignant tumors have shown improved efficacy as compared to either treatment alone. , Intravenous administration of a single dose of adjuvant liposomal doxorubicin in the rat breast cancer model increased the extent of coagulation necrosis induced with RFA compared with RFA alone. Similar increases in tumor destruction have been seen in the use of liposomal doxorubicin in combination with RFA in a variety of focal hepatic tumors. Ahmed et al. studied the independent effects of three different chemotherapeutic agents, nanoparticle size, and liposomal circulation time on the same combinational RFA therapy in a rat breast tumor model. It was found that all combinations of RFA with liposomal chemotherapy resulted in significantly greater tumor necrosis than RFA alone. The extent of tumor destruction was greatly influenced by nanoparticle size, with liposomes 100 nm in diameter achieving optimal results. In addition, the use of a pegylated liposomal preparation with prolonged circulation time was demonstrated to significantly influence intratumoral drug accumulation and coagulation necrosis. Thus, increasing drug circulation time and modifying nanoparticle properties may further enhance the efficacy of existing RFA and chemotherapeutic combinations.
Nanotechnology in vascular interventions
Conventional drug-eluting stents function via disruption and delay of the natural healing response and limiting the growth of neointima, making late in-stent thrombosis a major concern particularly in more complex lesions such as those in peripheral arterial disease. Thus, the development of endovascular stents with improved clinical characteristics remains imperative. Nanoparticle-eluting stents have been described and could provide a novel platform for delivery of therapeutic agents to the vessel wall. By utilizing electrodeposition coating to create a thin, uniform layer of biodegradable polymeric nanoparticles on stent surfaces, multiple agents within various nanoparticles may be delivered using a single stent. Intracellular uptake of nanoparticles and retention within the cytoplasm or extracellular space may allow for sustained drug delivery within cells. Such a stent has been tested in a porcine coronary artery model, where it demonstrated good biocompatibility and efficient drug delivery, and could potentially be used in the delivery of proteins or genes that inhibit inflammation, smooth muscle cell proliferation, and thrombosis. Polymer-based drug-eluting stents remain in contact with the arterial wall indefinitely, and the polymer is thought to induce local inflammation and potentially lead to late stent thrombosis and restenosis. , This could potentially be solved by the development of a biocompatible, noninflammatory medium for drug delivery to replace the standard polymer, and one study has demonstrated the efficacy and biocompatibility of a paclitaxel-eluting porous carbon-carbon nanoparticle-coated nonpolymeric cobalt chromium stent in a porcine model.
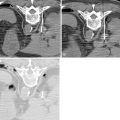
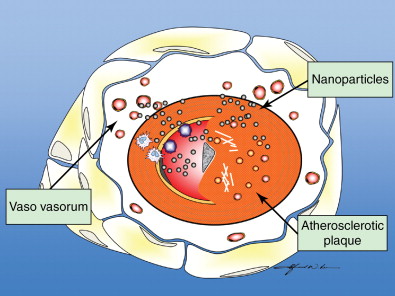
The use of local and systemic intraarterial drug-laden nanoparticles has been reported to have good outcomes in tumor treatment. , Concurrent use of balloon angioplasty has been demonstrated to cause a marked increase in vascular permeability, thereby allowing nanoparticles to selectively enter the arterial wall at specific sites. Doxorubicin-encapsulated nanoparticles administered intravenously have been shown to accumulate at sites of balloon stretch injury, with resultant inhibition of vascular smooth muscle proliferation and dose-dependent suppression of neointimal growth. Such an effect was not observed with administration of doxorubicin alone, and the approach shows potential for increased penetration and localization of therapy. Combining ionic or antibody targeting with local delivery of drug-laden nanoparticles to the vessel wall may deliver sufficient drug concentrations to prevent restenosis while avoiding systemic toxicity. , A study involving the modification of nanoparticles for a resultant positive surface charge has demonstrated enhanced local uptake of such nanoparticles into the arterial wall, possibly because of increased ionic interactions with the negatively charged glycosaminoglycan-rich arterial wall. In addition, in vitro studies by Lanza et al. showed that tissue factor-targeted perfluorocarbon nanoparticles containing doxorubicin or paclitaxel significantly inhibited the proliferation of vascular smooth muscle cells in culture, with the functionalized nanoparticles showing enhanced potency compared with nontargeted nanoparticles or free drug. In addition, these nanoparticles could be formulated with gadolinium and were readily detectable on T1–weighted spin echo imaging, whereas quantification of the nanoparticles was possible using 19F spectroscopy. It follows that targeted therapeutic nanoparticles could potentially provide visualizable and quantifiable therapy to prevent restenosis ( Figure 6-2 ).
Nanotechnology in implantable devices and hemostasis
The use of nanoparticles loaded with dexamethasone as a drug-delivery coating for implantable devices has been shown to reduce local postimplantation inflammatory response and may potentially improve biocompatibility. Several studies have examined the surface modification of nanoparticles with heparin without loss of the anticoagulant properties of heparin, and delivery of such nanoparticles has demonstrated prolonged clotting time of more than 200 seconds, as compared to 16.5 seconds with unmodified nanoparticles. Such an approach could potentially be used in the coating of interventional vascular devices, and hydrophilic surface modification of silicon catheters using a polymeric phospholipid monolayer has been shown to cause decreased platelet adhesion. The potential for nanotechnology to improve hemostasis has been shown through the development of a novel self-assembling peptide that establishes a nanofiber barrier when applied and achieves local hemostasis within 15 seconds, allowing the sealing of wounds in any wet ionic environment in the body. Its efficacy in achieving rapid hemostasis in brain, spinal cord, liver, skin, and even transection of the femoral artery has been demonstrated in a rat model such that bleeding could be stopped without the use of any conventional hemostatic methods such as pressure or cauterization. Additionally, the nontoxic, nonimmunogenic solution is also broken down into amino acids to serve as building blocks for tissue repair.
Ultrasound-mediated vascular therapy
The application of focused ultrasound has been demonstrated to enhance clot lysis when used in conjunction with thrombolytic agents. In addition, the use of microbubble-based contrast agents has also been shown to be effective for ultrasound-enhanced clot lysis, even in the absence of a thrombolytic agent. , Tiukinhoy-Laing et al. showed that the use of echogenic liposomes in conjunction with ultrasound produced effective thrombolysis with the thrombolytic agent tissue plasminogen activator (tPA), with retention of fibrin-specific properties of tPA and liposomal binding to fibrin. The application of ultrasound was observed to release the agent and mechanically disrupt the clot, thereby facilitating thrombolysis, , with improved thrombolytic effect compared with the liposomal drug alone or free tPA. In addition, the echogenicity of the liposomes allowed them to serve as a contrast agent for improved visualization of the clot, and could potentially facilitate focused ultrasound therapy and directed drug release. ,
Stem cell regenerative medicine
The rapid progress in stem cell research in recent years has caused stem cell therapy to garner considerable attention, particularly for diseases such as diabetes or multiple sclerosis that are difficult to treat or considered incurable. The role of the interventionalist in stem cell therapy lies mostly in targeted stem cell delivery via percutaneous, selective intravenous or intraarterial techniques, although this could potentially expand to include procedures prior to stem cell implantation such as the creation of engraftment territories or improvement of engraftment conditions via thermal ablation.
Stem cells are capable of dividing and self-renewing indefinitely as well as differentiating into one or more cell types. Embryonic stem cells (obtained from the inner cell mass of the blastocyst) are pluripotent, while adult stem cells (found in adult somatic tissue) are multipotent cells with more limited differentiation ability and the capacity to develop into cells of a specific organ, tissue, or organ system to potentially fulfill corresponding functions. Adult stem cells may be harvested from bone marrow, adipose tissue, and umbilical cord blood as autologous cells and are generally free of ethical controversy. Numerous studies have demonstrated the ability of stem cells to repair damaged tissue and restore function that would otherwise be irreversibly lost, , fueling further research for the use of such cells in lieu of organ transplantation or in the absence of alternative treatments. However, it has been noted that the type and extent of experimental data varies considerably between differing fields of clinical and experimental stem cell applications and across specific entities. Since discrepancies in results within the field have mostly been attributed to methodologic differences such as variations in stem cell delivery route and timing, further in-depth knowledge in the areas of stem cell properties, harvesting, cell trafficking, engraftment bed receptiveness, engraftment efficiency and subsequent engraftment monitoring has been posited to be critical for future success in stem cell therapy.
A noteworthy but poorly examined area of stem cell research is the utilization of stem cells as a medium for gene and drug delivery, based on the fact that viable stem cells have the ability to adjust, multiply, migrate, and communicate with adjacent cells. For example, Shah et al. found that injected mouse neural precursor cells were able to migrate to the contralateral brain hemisphere and deliver cytotoxic tumor therapy to glioma foci and hence slow tumor growth. ,
The role of interventional radiology in stem cell therapy
Successful stem cell transplantation generally involves adequate stem cell harvesting, trafficking to the target area (including cell homing and interstitial migration), full differentiation, and significant, ideally sustainable, contribution to organ function. Targeted stem cell delivery via interventional techniques may help overcome challenges currently associated with stem cell homing, which involves directed blood dispersion of stem cells. In the setting of liver cirrhosis and fibrosis, selective and direct stem cell release to the target organ by transcatheter intraarterial delivery may overcome potential mechanical barriers that have been postulated to exist for nonselective stem cell delivery techniques. In particular, the recommendation by a renowned stem cell expert of recipient organ perfusion with donor cells “via a radiologically placed catheter” has been noted. Further clinical studies have shown that hepatic regeneration may be enhanced with portal venous infusion of bone marrow-derived stem cells into portal vein branches prior to portal vein embolization and partial hepatectomy. Furthermore, because nonselective systemic routes of administration have been implicated in some of the previously mentioned inconsistencies in experimental results due to differentiation of stem cells, the relevance of interventional radiology in performing selective delivery techniques (such as transportal administration) has been highlighted.
Cell-based therapy for diabetes mellitus
Although pancreatic transplant been used as a therapeutic option for the treatment of medically refractory diabetes mellitus, its limited donor supply, significant morbidity, and associated lifelong immunosuppression confer severe risks and disadvantages for the patient. In contrast, cell-based therapy is minimally invasive and more cost-effective, although the initial use of islet cells from brain-dead donors still possessed the problem of limited donor supply. In evaluating the possible delivery routes of stem cell injection (including percutaneous intrasplenic, subcapsular renal, intraomental, subcutaneous, and into the celiac artery), intraportal venous delivery for cell engraftment has been described as technically most feasible, efficient, safe, and least complicated because of easy access. This has particular implications in IR since percutaneous access to the portal vein for islet cell transplantation has replaced previous invasive surgical laparotomy-based cannulation, , with the preferred method being ultrasonographic guidance for puncture with the adjunct of fluoroscopic guidance. It has been shown that intraportal venous infusion of islet cells may achieve effective control of blood glucose in type 1 diabetics for 1 year before disease recurrence. Possible complications such as the occurrence of tract hemorrhage and portal venous thrombosis have been ascribed to the intraprocedural increase in portal venous pressure. ,
The use of stem cell-based therapy has been examined in animal models, but has encountered problems because of the lack of a clearly identifiable type of pancreatic stem cell. Although embryonic stem cells have been transformed into cells with β cell properties, it is difficult to ascertain if these cells are capable of producing insulin or simply absorb it. , However, studies using rodent models have successfully shown success in curing or improving diabetes , and allowing the differentiation of hematopoietic and bone marrow-derived stem cells into functional islet cells. , As with other organs, pre-cell-transplantation in vitro differentiation of toti- or pluripotent cells into endocrine pancreatic cells appears most efficient. The use of percutaneous intraportal venous delivery, as was observed in islet cell transplantation, appears to best integrate technical feasibility and low complication rates with engraftment efficiency.
Peripheral arterial disease
Peripheral arterial disease has an estimated prevalence of 4.2%–35% and progresses to critical limb ischemia in 4.3%–9.6% of cases, conveying quality of life indexes similar to terminal cancer patients and the eventual undesirable outcome of amputation. For the investigation of treatment of critical limb ischemia, endothelial progenitor cells harvested from peripheral blood or bone marrow have been tested in numerous clinical trials. The initial study in 2002 in patients with chronic limb ischemia demonstrated the safety and efficacy of intramuscular injection of bone marrow cells and the achievement of significantly improved ankle-brachial pressure indexes, transcutaneous oxygen pressures, and pain-free walking times, with reduced rest pain at follow-up. Numerous further studies confirmed these results, with several showing improvement of endothelial function and increased blood perfusion as assessed with 99mTc tetrofosmin perfusion scintigraphy. However, current data for stem cell utilization in peripheral vascular disease is limited by the lack of randomized controlled studies and longer-term clinical follow-up, and although the majority of studies chose the intramuscular route for delivery, the most efficient mode of stem cell delivery, optimal dose, and cell type and subtype for treatment of critical limb ischemia remain uncharacterized and should be the focus of future studies.
Future prospects for stem cell research
The distinct trends in stem cell research may define the future role of the interventional radiology in the area of stem cell therapy, with the most relevant areas of research being stem cell engraftment kinetics, optimal administration timing, and optimal delivery routes via minimally invasive techniques. In addition, the use of thermal ablation and devascularization of malignant or dysfunctional tissue by RFA may potentially increase stem cell engraftment bed fertility, with periablational hyperemia providing the vascular environment for optimal stem cell trafficking to target areas and subsequent stem cell engraftment. An additional area for future investigation is the role of transarterial catheter-directed embolization for devascularization of dysfunctional tissue, with subsequent targeted transarterial cell delivery to improve stem cell trafficking efficiency compared to current peripheral intravenous cell administration techniques.
RNA interference and small interfering RNA
RNA interference (RNAi) is a sequence-specific, posttranscriptional gene silencing mechanism mediated by double-stranded RNA (dsRNA) that reduces the expression of endogenously expressed proteins. Such a mechanism may be harnessed for highly specific silencing of mutant, exogenous, or aberrant genes, and to develop novel drugs for the treatment of a wide range of diseases. The development of RNAi for therapy is based on knowledge of small RNA biogenesis pathways, and the two main types of small RNAs involved in gene silencing are microRNAs (miRNAs) and small interfering RNAs (siRNAs). siRNA-based drugs have distinct advantages over conventional small-molecule or protein-based drugs, including high specificity, higher potency, and reduced toxicity. However, barriers to the translational application of such drugs and the overall attainment of efficient RNAi such as specificity for the target gene, delivery to cell or tissues, durability of RNAi activity, and stability of the target mRNA and encoded protein have become increasingly evident. Further important considerations for therapeutic RNAi are that gene silencing approaches rarely remove 100% of the transcript, off-target silencing may occur, and that each organ, cell type, and target transcript presents unique challenges.
Rnai applications in oncology
In the treatment of cancer, siRNAs could possibly be used to inhibit expression of not only receptor tyrosine kinases but also antiapoptotic genes or other survival factors, tumor-promoting growth factors and the key participants in their signaling pathways, or genes essential for cell proliferation, including cyclins. RNAi technology may be used to suppress oncogenic transcription factors that regulate key cell growth-promoting pathways and are not considered viable conventional drug targets, with the potential for these new drug targets to be designed to eliminate cancer stem cells within a tumor or within chemotherapy- or radiotherapy-resistant tumors. Although siRNA drugs are highly potent and not susceptible to drug-efflux, duration of gene silencing is limited by the dilution of siRNAs that occurs with each cell division, and in proliferating tumor cells, gene silencing lasts for less than a week. However, this may be long enough to suppress key oncogenes, antiapoptotic genes, or drug resistance genes to cause irreversible tumor cell death.
RNAi applications in targeting hepatic cancers
The liver was one of the first organs targeted in the development of RNAi-based therapies for cancer. Stable nucleic acid-lipid particles (SNALPs), a form of liposome, have been used to deliver siRNAs to suppress ApoB expression in the liver of both mouse and nonhuman primates, leading to impressive decreases in blood cholesterol levels. The use of SNALPs to target PLK1 (a cell cycle protein crucial for the activating phosphorylation of many cell cycle proteins) led to cell cycle arrest and tumor cell apoptosis. SNALP-formulated PLK1 siRNA treatment administered in mice with hepatic tumors showed significant improvements in survival. An additional hepatic cancer application has been described using SNALPs to simultaneously deliver siRNAs to kinesin spindle protein (KSP) and vascular endothelial growth factor (VEGF). KSP is required for cell division, and VEGF is required for tumor cell growth. A Phase I trial using this approach has reported evidence of RNAi activity in biopsied tissue, and these initial studies, along with the first report of RNAi activity from exogenously applied siRNA complexes, serve as important milestones in the development of RNAi delivery systems as cancer therapeutics.
Obstacles to RNAi-based therapeutics in oncology
Although RNAi appears promising for use in cancer therapy, several barriers to its clinical use have been described. Extracellular fluids contain RNA-degrading ribonucleases that cause siRNA breakdown, and this has been noted to be the key obstacle in many delivery approaches. Although endocytosis via cell-surface receptors serves as a first step for entry into cells, a viable strategy for the release of RNA from the endosome needs to be developed. By changing the nucleotide linkages at the ends to phosphorothioates that are resistant to exonuclease cleavage and using chemical modifications similar to those that reduce off-target effects, the half-life of siRNA in extracellular fluids and serum may be extended to several days without significantly affecting gene silencing activity. ,
An additional major barrier to RNAi-based therapeutics is intracellular delivery, since siRNA does not cross cell plasma membranes and is not taken up by cells, thereby presenting a problem in terms of systemic intracellular delivery, particularly for disseminated disease. Multiple delivery strategies have been investigated with varying success, including chemical conjugation of RNA to small molecules capable of binding cell surface receptors heavily expressed in cancer, incorporating siRNA into nanoparticles or liposomes that may be modified for cell-specific targeting, and binding siRNA to peptides that facilitate cell membrane penetration, and fusing the small RNAs to aptamers (structured pieces of RNA that recognize cell surface receptors). , , Although RNAi may not be able to achieve efficient gene silencing in all tumor cells in a given population, RNAi-based therapeutics may be able to cause differentiation or death of the highly malignant subpopulation of tumor-initiating cells that may be responsible for tumor resistance, relapse, and possibly metastasis that are often resistant to conventional cytotoxic therapies. Given the complementary nature of these therapies, it follows that combining RNAi-based therapeutics and conventional cytotoxic drugs has the potential to achieve more effective outcomes.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree