Tamoxifen
Historically, the selective estrogen receptor modifier (SERM) tamoxifen was the standard first-line treatment option for hormone receptor-positive MBC in postmenopausal patients, especially in those presenting with advanced disease or in whom adjuvant endocrine therapy had not been given. In 86 clinical studies involving 5,353 patients, the objective response rate (ORR including complete response [CR] + partial response [PR]) of 34% was observed, with an additional 19% of patients achieving stable disease (SD) for at least 6 months (
15). A median duration of response up to 24 months was observed. Response rates (RR) tended to increase with age, with a 27% response in patients younger than 50 years compared with 43% in those over 70. Tamoxifen was generally well tolerated with a low incidence of serious side effects, including a low but significantly increased incidence of endometrial cancer and thromboembolic events due to its partial estrogenic agonist effects (
16).
Tamoxifen was compared with many other endocrine therapies (high-dose estrogens, megestrol acetate (MA), oophorectomy, and other SERMs) in randomized trials, and although these trials are small and lacking statistical power by modern standards, tamoxifen was consistently shown to be at least as effective or better, and often with a better toxicity profile (
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29 and
30). However, the majority of women with
ER +ve breast cancer have already been treated with tamoxifen in the adjuvant setting, and while in the past tamoxifen therapy was often used again if there was a treatment-free period of several years, nowadays alternative endocrine approaches that deprive tumors of circulating estrogens are utilized in preference as first-line therapy.
Aromatase Inhibitors
From the mid 1990s, the potent third-generation oral AIs become the standard first-line treatment option for postmenopausal patients with ER +ve advanced/metastatic breast cancer. Estrogens are normally synthesized in the ovary in premenopausal women; following menopause, mean plasma estradiol (E2) levels fall from about 400-600 pmol/L to around 25-50 pmol/L. These residual estrogens come solely from peripheral aromatase conversion partic-ularly in subcutaneous fat, and plasma E2 levels correlate with body mass index in postmenopausal women (
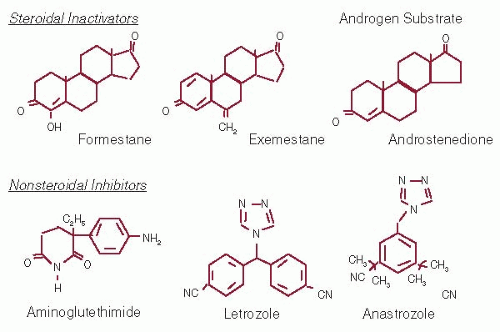
31). The oral AIs anastrozole (Arimidex™), letrozole (Femara™), and exemestane (Aromasin™) all reduce serum estrogen levels in postmenopausal women by preventing the conversion of adrenal androgens into oestrogens (
Fig. 70-3). Anastrozole and letrozole are third-generation nonsteroidal AIs that have similar pharmacokinetics with half-lives of approximately 48 hours, allowing a once-daily schedule (
32,
33). Exemestane is a steroidal aromatase inactivator with a half-life of 27 hours (
Fig. 70-3) (
34). All three compounds are orally active, and based on the clinical trials outlined below (
Table 70-3) these drugs were licensed and approved as first-line endocrine treatment for postmenopausal women with ER+ve advanced breast cancer.
The first published data came from two parallel multi-center double-blind randomized controlled trials in which
anastrozole was compared with tamoxifen as first-line therapy in ER+ve breast cancer. The North American study in 353 women showed that anastrozole significantly prolonged the time to disease progression from 5.6 to 11.1 months
(p = .005)(
35), while in the larger global trial in 668 patients no difference was found between the treatments in terms of median time to progression (TTP) (8.2 months vs. 8.3 months), response rate (RR) (33% both arms), or clinical benefit rate (CBR) (
36). The explanation for the different results may have involved a higher proportion of patients with unknown ER status in the second trial, and a subsequent combined analysis of women with just ER+ve disease from both trials confirmed a significant improvement in disease-free survival (8.5 months vs. 7.0 months) in favor of anastrozole (
37). Short-term side effects such as hot flashes, vaginal dryness, and headaches were infrequent and similar in both trials in comparison with tamoxifen.
The largest single trial was conducted with letrozole in comparison with tamoxifen in over 900 women with advanced breast cancer. Patients treated with letrozole had a significantly higher ORR, CBR, and prolonged TTP (
Table 70-3) (
38). Of particular note in this trial, nearly 20% patients had received prior tamoxifen in the adjuvant setting, although it had ceased a median of 3 years prior to development of metastatic disease—in this subgroup, retreatment with tamoxifen had a low response rate of 8% compared with a 32% response rate with letrozole. The improvements in clinical efficacy for letrozole resulted in an early improvement in survival during the first 2 years, although with longer followup this difference was lost (
39). The explanation for this may relate to the high number (>50%) of patients who prospectively crossed over to the alternate treatment at the time of progression, because significantly more patients benefited from second-line letrozole after progression on tamoxifen than from second-line tamoxifen after letrozole.
Likewise, a European study in 383 patients compared the efficacy and tolerability of the steroidal aromatase inactivator exemestane with tamoxifen as first-line therapy (
40). After a median follow-up of 29 months, there was an improvement in progression-free survival (PFS), together with a higher objective response rate with exemestane than tamoxifen (
Table 70-3). A subsequent meta-analysis of 6 phase III prospective randomized clinical trials involving 2,787 women treated with second- or third-generation AI versus tamoxifen confirmed a significant advantage in ORR, TTP, and clinical benefit (CB = OR + SD), favoring AIs over tamoxifen (
41). However, no difference was found in overall survival. Tamoxifen was associated with a significantly higher incidence of thromboembolic events and vaginal bleeding than the AIs. While some of the smaller trials were not always conducted with the academic rigor of large trials, they all consistently showed small but significant efficacy advantages of the AIs over tamoxifen.
All three drugs (anastrozole, letrozole, exemestane) are thus approved as first-line endocrine therapy options for postmenopausal women with ER+ve advanced breast cancer. It is not clear that one drug is significantly better than any other when direct comparisons have been made, although letrozole achieved greater aromatase inhibition than anastrozole in a crossover pharmaco-dynamic trial (
42). Current clinical evidence suggests that there are unlikely to be major direct clinical differences between the different AIs in MBC (
43). While there are no comparative data for exemestane with anastrozole or letrozole, further clinical responses have been reported for both exemestane and the second-generation steroidal inhibitor formestane in patients relapsing after anastrozole, letrozole, or the other nonsteroidal inhibitors, suggesting some partial non-cross resistance between the two types of inhibitors (
44,
45). In clinical practice, this has meant that exemestane is often used as a second-line option after prior first-line letrozole or anastrozole, as discussed further below.
Fulvestrant (alone or in combination with AI) as First-Line Therapy
Most postmenopausal women with metastatic ER+ve breast cancer have already received either an AI or tamoxifen in the adjuvant setting. Thus, the current clinical challenge is to establish an optimal first-line endocrine therapy for MBC given prior adjuvant endocrine therapy exposure. The ER down-regulator fulvestrant (Faslodex™) is a novel type of antagonist that, unlike tamoxifen, has no known agonist effects (
46,
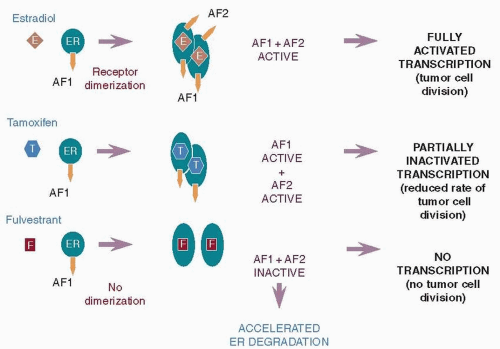
47). Fulvestrant binds to the ER, but due to its steroidal structure and long side-chain, it induces a different conformational shape with the receptor to that achieved by the nonsteroidal antiestrogen tamoxifen. Because of this, fulvestrant prevents ER dimerisation and leads to the rapid degradation of the fulvestrant-ER complex, producing the loss of cellular ER (
Fig. 70-4) (
48). It has been shown that due to its unique mechanism of action, fulvestrant delays the emergence of acquired resistance compared with tamoxifen in an MCF-7 hormone-sensitive xenograft model (
49), and that the lack of agonist effects means fulvestrant does not support the growth of tumors that became resistant to, and subsequently stimulated by, tamoxifen (
50).
Early clinical studies showed that fulvestrant at the initially approved dose of 250 mg monthly by intramuscular injection (i.m.) had similar efficacy to tamoxifen as first-line treatment of hormone receptor MBC, with a median TTP of 6.8 and 8.3 months respectively (HR 1.18, 95% CI, 0.98-1.44;
p = .088) and ORRs of 31.6% and 33.9% respectively (
51). Given the widespread use of prior tamoxifen as adjuvant therapy, two separate phase III first-line trials compared fulvestrant with the AI anastrozole in postmenopausal women with locally advanced or metastatic breast carcinoma who had progressed after prior endocrine therapy (97% with tamoxifen, 56% as adjuvant therapy) (
52,
53). These trials were prospectively designed to allow combined analysis of data, and at a median follow-up of 15.1 months fulvestrant was at least as effective as anastrozole in terms of median time to progression (5.5 months vs. 4.1 months, respectively) and objective response (19% vs. 17%, respectively) (
54). A subsequent survival analysis after a median follow-up of 27 months showed no significant difference in the median time to death between fulvestrant and anastrozole (27.4 months vs. 27.7 months, respectively) (
55).
While these early first-line studies suggested that fulvestrant 250 mg was as effective as either tamoxifen or anastrozole in the first-line setting, more recent first-line studies with fulvestrant have investigated either different loading dose (LD) schedules (LD = 500 mg on day 1, then 250 mg on days 14, 28, and monthly thereafter) or a high dose (HD) schedule (HD = 500 mg on days 1, 14, 28, and monthly thereafter). A phase II trial (FIRST) compared fulvestrant HD with anastrozole as first-line treatment and showed a significantly longer TTP with HD fulvestrant (median TTP not reached versus 12.5 months; HR 0.63; 95% CI, 0.39-1.00;
p = .049) (
56). As discussed later, a randomized comparison of HD versus LD fulvestrant in the second-line setting (CONFIRM trial) has confirmed the better efficacy for the 500 mg HD schedule (
57), resulting in an amendment to fulvestrant’s new drug approval in 2010.
Preclinical evidence from two separate xenograft models suggested that fulvestrant could be significantly more effective when given in a low estrogen environment by combining it with an AI (
58,
59). Two randomized phase III trials tested the combination of anastrozole with fulvestrant (LD) versus anastrozole alone as first-line therapy in postmenopausal MBC patients (
Table 70-3). SWOG S0226 demonstrated that fulvestrant plus anastrozole significantly improved median PFS (15.0 months vs. 13.5 months) and median OS (47.7 months vs. 41.3 months) compared to anastrozole alone (
60). Safety and tolerability were similar between the two treatment arms. Conversely, the FACT trial reported no difference in the median PFS or median OS between the combination and the anastrozole arm (
61). The main difference between these two trials was the proportion of endocrine therapy-naïve patients, 60% and 33% in the SWOG and FACT trial, respectively. Indeed, in an unplanned subgroup analysis of the endocrine-naïve subgroup in the SWOG trial, a significantly higher benefit was observed in the combination arm (median PFS 17.0 months versus 12.6 months), not observed in the smaller number of endocrine-naïve patients in the FACT trial. On the basis of this result, a currently active first-line phase III trial (FALCON, NCT01602380) is establishing whether fulvestrant (HD) and anastrozole is better than anastrozole alone in truly endocrine-therapy-naïve patients.
To date, therefore, the nonsteroidal AIs remain the most effective first-line endocrine option for the majority of postmenopausal patients with MBC. In those with endocrine-sensitive disease, including those who are endocrine-therapy naïve, expected TTP are of the order between 10 to 15 months (
Table 70-3). However, the influence of prior adjuvant endocrine therapy remains an important variable in the likelihood of success. While resistance to AIs inevitably develops, it does not preclude further endocrine responses, and effective second-line options are required for these patients.