The goal of breast cancer screening is early detection of disease, to be followed by appropriate treatment. Screening tests differ from diagnostic tests insofar as screening identifies subgroups of people who have a high probability of
asymptomatic disease, while diagnostic tests are obtained in symptomatic patients. A positive screening result in an individual rarely provides direct evidence of disease; screening tests must be followed by diagnostic tests to determine whether disease is truly present.
When an apparently healthy population undergoes regular screening, medical professionals have an obligation to show that the benefits of screening outweigh the costs. As we discuss in more detail later in this chapter, a positive screening test result and a diagnosis of breast cancer brings anxiety and treatments with associated morbidities and costs. Screening tests should therefore be safe, with minimal side effects. The minimum requirements for establishing a safe, ethical, and cost-effective screening program involve three areas: the disease targeted by the program, the screening tests needed to detect the disease, and the features of the health care system needed to support the program (
1). All the requirements for each of these areas are reviewed in further detail in the following material. If these requirements are not at least partially met, population-wide screening may be ineffective.
Disease Requirements
First, the disease must be serious, with significant morbidity or mortality. Second, an effective therapy for the disease must be available if it is detected as screening would obviously have no value if subsequent treatment would not be beneficial. Third, the natural history of the disease must be understood clearly enough to identify a significant window of opportunity during which the disease is detectable and detection would probably lead to a cure, or at least an effective treatment with less morbidity than the disease itself. Finally, the disease must not be too rare; if it is rare, we can expect an excess of false positive test results, which increases the cost and effort necessary to detect true positives.
Screening Test Requirements and Characteristics
First, a screening test must be reasonably easy and inexpensive to perform. Otherwise, the costs of large-scale screening in terms of time, effort, and money will be prohibitive. This is an important point to remember when considering the relative utility of the many screening modalities now available, as we will discuss in the next section. Second, the screening test must be safe and acceptable both to the individuals undergoing the screening and to their physicians. Finally, the level of accuracy of the screening test must be known and acceptable to the health care system, the physician, and the patient. Its sensitivity, specificity, positive predictive value, and other operating characteristics require careful assessment.
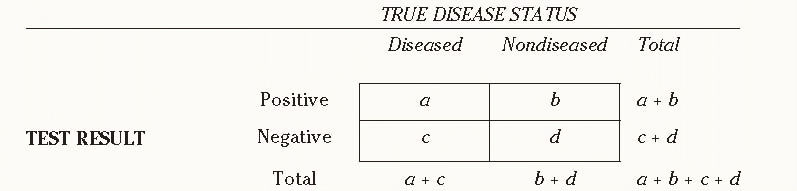
It is critical to understand the characteristics of a given screening test, as well as the interplay of its characteristics with those of the population screened and the clinicians who perform and interpret the test. We present a standard 2 × 2 table (
Table 10-1) comparing the results of screening tests with the disease status of the individuals screened, along with a series of formulas to measure the sensitivity, specificity, and other performance features of the test. The next three paragraphs explain
Table 10-1 in more detail.
A positive test result for a person who does not have the disease assessed by a test is called a false positive result while a negative result for a person who actually has the disease is called a false negative result.
Sensitivity refers to the ability of a screening test to detect a disease when it is present and is calculated as a/(a + c).
If a test is not sensitive, it will fail to detect disease in some people who actually have the disease; they appear in cell c.
Specificity refers to the ability of a screening test to indicate the absence of disease when no disease is present and is calculated as d/(b + d). If a test is not specific, it will falsely indicate the presence of disease in some people who do not have the disease; they appear in cell b.
Another important parameter of a screening test is its predictive value, which may be either positive or negative. If a test result is positive, what is the probability that the person tested actually has the disease (i.e., true positive)? If the result is negative, what is the probability that the person does not have the disease (i.e., true negative)? The answers to these questions depend on the sensitivity and specificity of the screening test, as well as on the prevalence of the disease in the underlying population that undergoes screening. Positive predictive value (PPV) is calculated as a/(a + b). Negative predictive value (NPV) is calculated as d/(c + d), indicating the proportion of people with negative test results who are truly free of disease.
Health Care System Requirements
A screening program divides results into positives and negatives. Follow-up within a health care system must be available for everyone who has a positive result to confirm or rule out the presence of disease. Some follow-up testing can be expensive, time-consuming, and painful; it may even entail a degree of risk for the people who receive it. For example, estimates indicate that for every $100 U.S. dollars spent on breast cancer screening, an additional $33 are spent on subsequent diagnostic evaluations stemming from false positive results (
2).
Before screening is undertaken, treatment should be available, accessible, and acceptable to people with disease. If a country’s resources are too limited to provide treatment in an equitable manner, or if no effective treatment for a given disease is available, it makes no sense, either ethically or in terms of cost-effectiveness, to encourage screening when people in whom disease is actually detected must go untreated.