PRESENTATION
In 90% of cases, DCIS presents as an abnormal screening mammogram in an asymptomatic woman. Prior to widespread screening with mammography, DCIS presented with clinical symptoms and was an uncommon entity. In 1983, before screening was widely used, only 4,901 women were diagnosed with DCIS in the United States, accounting for 3.8% of all breast cancer (
6). By 2013, approximately 64,640 new DCIS diagnoses will be made, representing approximately 20% to 25% of all new breast cancers (
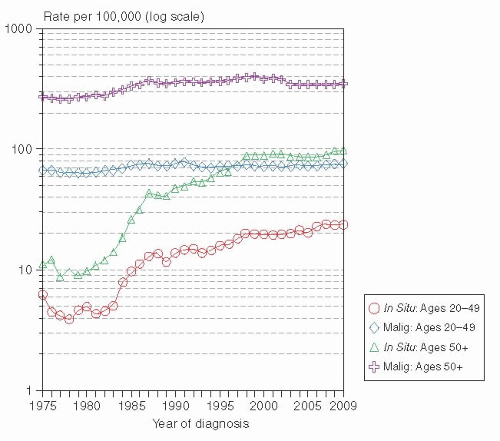
7). The SEER database has documented that the incidence of DCIS rose from 5.83 per 100,000 women in 1975 to 37.25 per 100,000 in 2009 (
8). The increased incidence was observed in all age categories, with the greatest rise among women over 50 years of age (
Fig. 23-1). The incidence of DCIS, like invasive breast cancer, is strongly related to age. DCIS is extremely uncommon before age 35 to 39; with incidences of less than 11 cases per 100,000. After that, the incidence rises steadily to a peak of 102 per 100,000 at ages 65 to 69. In contrast, invasive breast cancer incidence peaks at a later age (75-79 years) with incidence of 433.1 per 100,000 women (
8,
9).
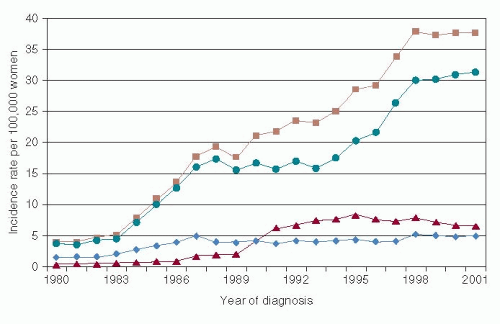
The increase in DCIS diagnosis has not been uniform across histological types (
Fig. 23-2). An earlier analysis of the SEER database from 1980 to 2001 demonstrated that comedo histology rose in incidence from 1980 through 1995 but then stabilized or decreased slightly thereafter, while non-comedo histology continued to rise thorough the end of the study period (
10). The age-adjusted incidence of DCIS was the highest among Caucasian women, followed by African-American and Asian-Pacific Islander women (
11).
Microcalcifications are the predominant finding on screening mammography leading to a diagnosis of DCIS. As an example, in a study of 217 women 50 to 69 years of age with DCIS detected by mammography while participating in the Breast Cancer Screening program in Norway from 1995 through 2007, calcifications were present for 93%. On pathology review, calcifications were associated with grade 1 DCIS in 15%, grade 2 in 11%, and grade 3 in 74% (
12). Calcification morphology on mammography was most frequently described as “fine pleomorphic.” In comparison, in a large surgical series from France of 909 cases of DCIS diagnosed from 1980 to 1999, 76.2% were detected by mammography without clinical symptoms, 12% had a palpable mass, and 12% presented with nipple discharge (
13). Of
those with a palpable mass, 47.1% also had mammographic abnormalities. Microcalcification was the most common finding in 75.5%, with fine pleomorphic calcifications being the most frequently seen (40.4%), followed by amorphous or indistinct calcifications (35.9%). Fine pleomorphic and finelinear branching calcifications were significantly associated with the presence of grade 3 DCIS and necrosis (
13).
DCIS that is diagnosed with a palpable mass or nipple discharge is more likely to be extensive disease. Examination of 50 mastectomy specimens in a population of DCIS patients, of whom 58% were detected with mammography and 42% with clinical mass or nipple symptoms, revealed that presentation with a palpable mass, nipple discharge, or as Paget’s disease was accompanied by a greater incidence of multicentricity and/or microinvasion than was DCIS detected by mammography (
14). In addition, patients with comedo necrosis or micropapillary architecture were more likely to be multicentric than other histologic subtypes. More recent studies suggest that, in most cases, true multicentricity in DCIS is rare. Holland and Hendricks studied 119 mastectomy specimens containing DCIS by a subgross pathologic-mammographic technique (
15). In all but one case, the tumor was confined to a single “segment” of the breast. Clearcut multicentric distribution (defined in this study as foci of DCIS separated by 4 cm or more of uninvolved breast tissue) was seen in only one patient. Faverly et al., using stereomicroscopic three-dimensional analysis to define the growth pattern of DCIS in the mammary duct system, studied 60 mastectomy specimens containing DCIS (
16). There was continuous growth pattern in the ducts for 50% and a discontinuous pattern in 50%, characterized by uninvolved breast tissue “gaps” between foci of DCIS. In most instances, these gaps were small (< 5 mm in 82% of cases). The likelihood of finding such gaps was related to the DCIS
differentiation: 90% of poorly differentiated cases grew in a continuous manner without gaps, while 45% and only 30% of intermediate and well-differentiated lesions, respectively, were continuous. The findings in these two studies indicate that, in most cases, DCIS involves the breast in a segmental distribution, and truly multicentric disease is uncommon.
Mammography is the established method for detection and evaluation of extent of disease, but MRI is being increasingly studied for its clinical utility in DCIS diagnosis (
17). Historically, MRI was considered a poor imaging tool to assess DCIS. The adoption of higher spatial resolution techniques and diagnostic criteria that are different than those used for invasive cancer has led to improved detection of DCIS by MRI. In the International Breast MRI Consortium study of patients with suspicious mammographic or clinical findings, the sensitivity of MRI for the detection of DCIS was 73%; significantly lower than the 91% observed for invasive cancer (
18). The American College of Radiology Imaging Network (ACRIN) compared the diagnostic accuracy of mammography, clinical examination, US, and MRI prospectively in the preoperative assessment of 171 malignant foci, 38 of which were DCIS, in 121 breasts from 111 women. MR imaging identified 34 of 38 (89%) DCIS foci, which was significantly more than was detected with either US
(p < .001) or mammography
(p < .01) (
19). These results were further supported by Kuhl et al. (
20), who reported on 167 cases of DCIS that had, at some point during their workup, undergone both mammography and MRI. All imaging was re-read centrally and MRI studies were read blinded to the mammogram findings. MRI detected 92% of cases as compared to 56% by mammography
(p < .0001). Of the 89 cases of high-grade DCIS, 48% were diagnosed by MRI but missed by mammography. Other studies have similarly demonstrated a 70% to 90% MRI sensitivity for DCIS detection (
21). Additional investigation to clarify the clinical role of MRI for diagnosing DCIS and its prognostic implication are needed.
NATURAL HISTORY
There is significant evidence based on epidemiology, clinical observation, and growing numbers of genetic, molecular, and epigenetic studies that DCIS is a precursor lesion for invasive cancer. However, predicting which individual DCIS case will progress to invasive breast cancer if left untreated remains indefinable.
Epidemiologic studies that examine risk factors for DCIS show that they are remarkably similar to those for invasive breast cancer, including a family history of breast cancer, nulliparity, older age at first childbirth, and breast density (
9). Clinical observation of DCIS progression to invasive cancer is limited as surgical resection is done once a diagnosis of DCIS is made. However, clinical follow-up from benign breast biopsies that received no additional intervention but on re-review many years later were diagnosed as DCIS offers some insight regarding clinical progression. Sanders et al. (
22) identified 28 cases of small, low-grade, non-comedo DCIS during a review of 11,760 consecutive breast biopsies performed from 1950 through 1968. With a median followup of 28 years, 11 of 28 women (39%) developed ipsilateral invasive breast carcinoma, all in the same quadrant from which the original biopsy was taken. Seven (64%) of the 11 recurrences developed within 10 years, and 5 (45%) of the 11 (18% of all 28 cases) died of metastatic disease. Eusebi et al. reported on 80 cases of DCIS retrospectively diagnosed out of 9,446 breast biopsies done between 1964 and 1976, and with a median follow-up of 17.5 years. Pure “clinging carcinoma” (CC) was diagnosed in 41; 9 with pleomorphic nuclei (consistent with DCIS) and 32 with monomorphic nuclei which is currently considered flat epithelial atypia. Five (12%) developed an ipsilateral breast cancer: 2 of the 9 with pleomorphic nuclei developed ipsilateral invasive carcinoma, and 2 of the 32 with monomorphic nuclei developed DCIS. Of the 30 cases of CC associated with cribriform DCIS, LCIS, or both, 5 (17%) had an ipsilateral invasive recurrence. Two of 7 cases with cribriform DCIS developed an ipsilateral DCIS recurrence, and 2 of 2 cases with comedo DCIS developed an invasive recurrence (
23). In a similar study, Rosen et al. (
24), described 30 women with untreated DCIS; complete follow-up was available only for 15. Ipsilateral invasive breast cancers occurred in 7 of the 15 (53%) at a mean of 9.7 years after the diagnosis of DCIS. More recently, Collins et al. (
25), found 13 cases of DCIS (0.7%) out of 1,877 breast biopsies performed from 1973 through 1991 on the Nurses’ Health Study. Nuclear grading of the 13 cases revealed 6 to be intermediate, and 3 were high nuclear grade. A total of 10 cases recurred (77%); 6 (46%) developed invasive breast cancer at a mean of 9 years, and 4 (31%) developed DCIS at a mean of 3.75 years after the initial biopsy was performed.
The clear limitation of these studies is that the completeness of excision by the original biopsy is unknown; however, as a whole, they demonstrate that a significant portion of both low- and high-grade DCIS can progress to invasive breast cancer, supporting its role as a precursor.
Another source of indirect clinical evidence of DCIS as a precursor comes from autopsy studies. Alpers and Wellings (
26) assessed a series of 185 randomly selected breasts from 101 women examined by a subgross sampling technique. One or more foci of DCIS were found in only 11 cases (6%). The lowest prevalence was noted in the oldest women: 3 of 56 (5%) in women younger than age 49; 7 of 70 women (10%) in women ages 50 through 69; and in 1 of 59 women (2%) older than 70 years were found. In a study with similar methodology, Bartow et al. (
27), performed pathologic examination of the breast on 519 autopsied women 14 years of age or older without clinical evidence of breast cancer. Only one case of DCIS was identified in a 40 year old; five occult invasive carcinomas were found in women ages 45 to 87. These findings suggest that DCIS progresses to clinically evident breast cancer given its very low prevalence at autopsy, particularly in the oldest cohort of women.
There has been considerable research over the last several years in understanding the gene expression changes that occur in DCIS relative to what is known in invasive breast cancer. Over the past decade, the successful combination of highly specific tissue microdissection technologies with advanced high-throughput genomic, gene-expression, and proteomic technologies has enabled a better understanding of the pre-invasive stages of breast cancer progression (
28). It is now acknowledged that significant genomic and gene expression parallels exist between the pre-invasive and invasive stages of breast cancer.
Several studies have examined genetic changes in DCIS, i.e., loss of heterozygosity (LOH), at genetic loci (typically considered to be approximate locations of inactivated tumor suppressor genes) known to exhibit high rates of loss in invasive breast cancer. These showed that the frequency of chromosomal losses, specifically regions 16q and 17p, in ADH is similar to that observed in DCIS and invasive carcinoma (
28). O’Connell and colleagues examined 399 pre-malignant breast lesions, studying 15 genetic loci known to show high rates of LOH in invasive breast cancer (
29). In breasts without invasive breast cancer, they found at least one locus of LOH in 42% of ADH, 70% of non-comedo DCIS, and 79% of comedo DCIS. Among specimens harvested from cancerous breasts, LOH was shared with the synchronous cancer in at least one locus in 45% of ADH, 77% of non-comedo
DCIS, and 80% of comedo DCIS lesions. This observation that the majority of both comedo and non-comedo DCIS share their LOH phenotypes with synchronous invasive breast cancer supports the concept that DCIS is a direct precursor of invasive breast cancer.
Ma and associates used laser capture microdissection and DNA microarrays to generate
in situ gene expression profiles in 36 breast tissue specimens that exhibited one or more lesions of ADH, DCIS, and invasive carcinoma (
30). No consistent gene expression alterations unique to ADH, DCIS or invasive carcinoma were found; instead, the greatest alterations in gene expression were seen by histological grades. Similar to what has been observed with invasive breast cancer, distinct gene-expression signatures are present in low- and high-grade DCIS lesions, and are consistent with genetic alterations and phenotypes seen in comparable grade invasive cancer. These data suggest that low-grade DCIS progresses to low-grade invasive and high-grade DCIS to high-grade invasive, with intermediate-grade DCIS representing intermediary behaviors.
There has been increasing focus on the DCIS microenvironment for identification of promoters of tumor progression. Recent gene-expression and epigenetic data strongly suggest that the stromal and myoepithelial microenvironment of preinvasive breast cancer actively participates in the transition from pre-invasive to invasive disease (
28). Allinen et al. (
31) developed a purification procedure that allows the isolation of pure cell populations from normal breast tissue, DCIS, and invasive carcinoma. They demonstrated that genes coding for
CXCL14 and
CXCL12 chemokines were overexpressed in DCIS myoepithelial cells and myofibroblasts, respectively, compared to normal breast tissue. These chemokines can bind to receptors on adjacent epithelial cells and enhance their proliferation, migration, and invasion. Thus, chemokines may have a part in the transition from DCIS to invasive breast cancer by acting as paracrine factors. This illustrates how signaling from the DCIS stromal and myoepithelial microenvironment may play an important role in tumorigenesis.
These genetic and molecular studies give further evidence that ductal pre-invasive stages (ADH and DCIS) are non-obligate precursors to invasive disease with variable clinical behavior.