Ductal Carcinoma In Situ and Other Intraductal Lesions: Pathology, Immunohistochemistry, and Molecular Alterations
Adriana D. Corben
Edi Brogi
Intraductal proliferative lesions are epithelial proliferations confined to the mammary ductal-lobular system. Based on architectural and cytologic features we classify these lesions as usual ductal hyperplasia (UDH), flat epithelial atypia (FEA), atypical ductal hyperplasia (ADH), and ductal carcinoma in situ (DCIS). DCIS, ADH, and FEA are established non-obligate morphologic precursors of breast carcinoma, albeit of very different biologic potential, whereas UDH is a benign proliferation that enters in the differential diagnosis of DCIS and ADH.
NORMAL BREAST
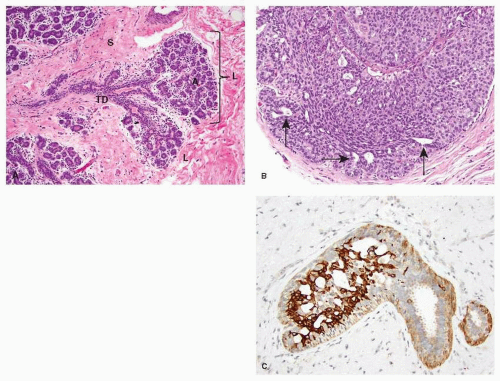
The mammary lobule is the milk-producing unit of the breast. It consists of a grape-like aggregate of acini surrounded by specialized mammary stroma. The acini drain into a terminal ductule, part of which is intralobular, and part extralobular. Few lobules and the terminal ductule that drains them form together the terminal duct lobular unit (TDLU) (Fig. 21-1A). Subgross pathology studies have shown that most of the epithelial changes occurring in the breast, including DCIS, originate in the TDLU (1).
The cellular lining of the mammary lobules and ducts consists of an inner (luminal) epithelial layer and an outer myoepithelial cell (MEC) layer. The luminal epithelium lining the glandular lumen has a polarized morphology, with the nucleus at one pole of the cell and an apical cytoplasmic compartment at the other end. Normal luminal epithelial cells show continuous linear membranous positivity for E-cadherin, a transmembrane adhesion molecule encoded by the CDH1 gene, which is located at 16q22.1. Monostratified normal luminal cells usually are negative for CK5/6. A continuous layer of MECs surrounds the luminal epithelium. The morphology of MECs ranges from inconspicuous, with compressed nuclei and scant cytoplasm, to epithelioid with abundant clear cytoplasm. MECs can be readily demonstrated with immunohistochemical stains for cytoplasmic contractile proteins (i.e., calponin, smooth muscle actin, and smooth muscle myosin heavy chain) or p63—a p53 homologue that decorates the nucleus. E-cadherin reactivity in MECs has membranous linear distribution with a characteristic granular quality.
Estrogen and progesterone play a central role in regulating the growth and differentiation of normal breast tissue. Nuclear expression of estrogen receptor (ER)-α is present in normal ductal and lobular luminal cells, but it is limited to a small and sparse percentage of the cells, and varies with the phases of the menstrual cycle. ER-β is expressed more diffusely in normal breast tissue and is present in the epithelial cells of ducts and lobules, in MECs, endothelium, and stromal cells. The expression of ER-β does not vary during the menstrual cycle but is reduced in UDH, ADH, and DCIS. Some investigators have speculated that the
relative levels of ER-β and ER-α may be important in determining the risk of breast cancer development and higher levels of ER-β relative to ER-α are protective against neoplastic progression. The expression of progesterone receptor (PR) in the ductal and lobular epithelium does not seem to vary with the menstrual cycle.
relative levels of ER-β and ER-α may be important in determining the risk of breast cancer development and higher levels of ER-β relative to ER-α are protective against neoplastic progression. The expression of progesterone receptor (PR) in the ductal and lobular epithelium does not seem to vary with the menstrual cycle.
Carcinomas and its precursors are composed of transformed epithelial cells. Whether all breast epithelium has the potential to transform or this capability is limited to epithelial stem cells or progenitor cells is a topic of research and debate. Few authors have documented silent chromosomal alterations in morphologically normal epithelial cells, and suggested that they may predispose to premalignant or malignant transformation (2). However, these changes have low frequency and are more often seen in morphologically normal cells adjacent to carcinoma than away from it (2). Alterations affecting the p16 tumor suppressor gene increased epithelial proliferation and elevated expression of cyclooxygenase-2 (COX-2) have been documented in morphologically normal mammary epithelium, especially in women at high risk of breast carcinoma (3).
Usual Ductal Hyperplasia
The term UDH refers to a non-neoplastic epithelial proliferation. It can range from mild, consisting of just two to four cell layers, to florid, when it entirely fills and distends the ducts. UDH arising in a radial scar or in a papilloma can show focal necrosis, raising the differential diagnosis of DCIS. The cells comprising UDH are cytologically benign, vary in size, shape and orientation, have poorly defined borders, and are haphazardly arranged (Fig. 21-1B). Fenestrations lined by non-polarized cells are usually present within UDH, and often have circumferential distribution along the periphery of a duct.
Low and heterogenous expression of ER occurs in 30% to 40% of UDH cells, mainly at the periphery of the lesion. The proliferation rate is low (2%-5%). UDH shows a mosaic staining pattern for the basal keratins CK5/6 (see Fig. 21-1C) and 34BE12 (4). The pattern of immunoreactivity helps distinguish UDH from ADH and focal DCIS, as the latter two lesions are usually negative for these antigens with very few exceptions. Although some high-grade DCIS is CK5/6-positive, as discussed later in this chapter, nuclear atypia and pleomorphism distinguish it from UDH. UDH is associated with a 1.5- to 2-folds increase in the risk of breast cancer, which may occur in either breast. The risk is slightly higher in women with UDH who also have a first-degree relative with breast carcinoma (5).
Few and inconsistent genetic alterations have been documented in UDH, but no substantial shared genetic abnormalities with ADH, DCIS, or invasive breast cancer have been
identified (6). Studies have shown that chromosomal loss of heterozygosity (LOH) is lower in UDH (4.5%-13%) than in ADH and low-grade DCIS (7). Comparative genomic hybridization (CGH) has identified few unbalanced chromosomal aberrations in UDH in some studies (8), but not in others (9). Some of the studies reporting chromosomal alterations in UDH used whole genome amplification methods (8) which may be more susceptible to artifacts. Overall, the chromosomal aberrations found in most UDH lesions are not similar to those observed in invasive carcinoma, effectively ruling out the possibility that UDH might represent a morphologic precursor of breast carcinoma (6).
identified (6). Studies have shown that chromosomal loss of heterozygosity (LOH) is lower in UDH (4.5%-13%) than in ADH and low-grade DCIS (7). Comparative genomic hybridization (CGH) has identified few unbalanced chromosomal aberrations in UDH in some studies (8), but not in others (9). Some of the studies reporting chromosomal alterations in UDH used whole genome amplification methods (8) which may be more susceptible to artifacts. Overall, the chromosomal aberrations found in most UDH lesions are not similar to those observed in invasive carcinoma, effectively ruling out the possibility that UDH might represent a morphologic precursor of breast carcinoma (6).
Flat Epithelial Atypia
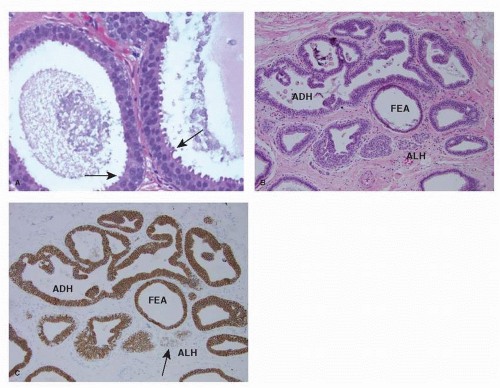
FEA consists of enlarged TDLUs in which the native epithelial cells are replaced by “one to several layers of a single cell type showing low-grade (monomorphic) cytologic atypia” (10) (see Fig. 21-2A). The involved TDLUs have variably dilated acini with rounded contour. The nuclei of FEA cells are monomorphic, round to ovoid, and resemble those of ADH and low-grade DCIS. The proliferation is architecturally “flat” and devoid of any complex pattern (i.e., micropapillae, focal rigid bridges, bars and arcades, or sieve-like fenestrations) seen in ADH and low-grade DCIS. Lesions currently classified as FEA have been previously referred to by a wide range of terms, most notably “clinging carcinoma of the monomorphic type” and “columnar cell change with atypia.” Columnar cell change, columnar cell hyperplasia, and FEA often coexist in adjacent lobules and even within the same TDLU. These diagnoses are not mutually exclusive, but only FEA has cytologic atypia.
The cells of FEA are positive for low-molecular-weight CKs, such as CK8, CK18, and CK19 (9). Even though FEA cells lack expression of high-molecular-weight CKs, such as CK5/6 (9, 11), immunohistochemical stains for CK5/6 do not help to differentiate FEA from monostratified normal ductal epithelium, as the latter is also CK5/6 negative. In FEA strong positivity for ER is present in about 85% of the cells (9, 12) and for PR in about 50% (9, 12). The cells are characterized by strong cytoplasmic expression of bcl-2, and show minimal to no apoptosis (12). The proliferation index of FEA is significantly higher than in morphologically normal TDLUs (6% vs. 2%, respectively) (12).
FEA commonly coexists with ADH (see Fig. 21-2B), lowgrade DCIS and tubular carcinoma, and shares close cytologic and immunophenotypic similarities with these lesions (1, 13, 14). Few investigators have also noted an association between columnar cell lesions/FEA and lobular carcinoma in situ (LCIS) and atypical lobular hyperplasia (ALH) (see Fig. 21-2B-C) (13, 15).
Molecular analysis shows that FEA has recurrent chromosomal alterations consistent with a clonal population (9, 16). Recurrent copy number alterations include loss of 16q and gains of 15q, 16p, 17q, and 19q. Allelic imbalances are most frequently seen at 3p, 9q, 10q, 11q, 16q, 17p, and 17q (16).
Based on these findings FEA is part of the spectrum of low-grade mammary epithelial lesions (Fig. 21-3A), which include ADH, low-grade DCIS, tubular and tubulolobular carcinoma, invasive cribriform carcinoma, low-grade invasive ductal carcinoma, ALH, classic LCIS and invasive lobular carcinoma, classic type. It has been proposed that FEA represents the first morphologically recognizable precursor of low-grade mammary neoplasia (9, 14). The risk of subsequent carcinoma associated with FEA has not been fully determined, but it is believed to be lower than for ADH (10). At present, no additional treatment or special screening modalities are recommended for patients with only FEA (10).
Atypical Ductal Hyperplasia
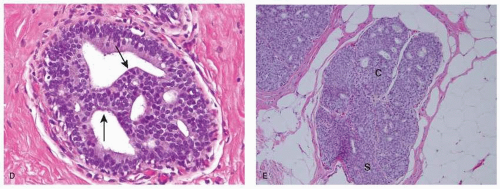
ADH is a very focal neoplastic epithelial proliferation confined to the mammary ductal-lobular system. The cells composing ADH are relatively small and monomorphic, with round to avoid nuclei, fine chromatin and inconspicuous nucleoli. They tend to be evenly spaced, show well-defined cell borders and focally form rigid arches and bridges (see Fig. 21-2D), trabecular bars of uniform thickness, or club-shaped micropapillae. They can also display solid foci or focal incomplete cribriform pattern, which results from the orderly arrangement of polarized cells around a neoformed glandular lumen. These cells are morphologically similar to those composing low-grade DCIS, but they are not as homogeneous. Extent of the lesion is an important criterion in differentiating ADH from low-grade DCIS, although there is no universally accepted size cutoff to distinguish between the two. Usually DCIS is diagnosed when the neoplastic proliferation involves at least two separate ducts or spans at least 2 mm, and any smaller lesion is classified as ADH. The diagnosis of ADH applies only to lesions for which the differential diagnosis of low-grade DCIS is considered, but that do not show the full range of diagnostic features. Despite undeniable limitations, the use of size criteria fosters interobserver reproducibility in the interpretation of small borderline ductal lesions.
The cells comprising ADH typically have strong and nearly uniform (90%-100%) positivity for ER and PR, but no cytoplasmic reactivity for CK5/6 (11) and 34BE12 (4). The use of these markers is of no practical value in the differential diagnosis with low-grade DCIS, but can be useful in the differential diagnosis with UDH, that is positive with a checkerboard pattern (see Fig. 21-1C). ADH has a low proliferative rate (4%-5%).
Recurrent chromosomal alterations including losses at 16q and 17p and gains at 1q (9) have been identified in ADH, similar to the changes found in low-grade DCIS as well as in the lesions part of the low-grade mammary epithelial neoplasia family (see Fig. 21-3A).
ADH is associated with a four to five-fold increase in the risk of subsequent breast cancer, with approximately equal frequency in both breasts.
Apocrine Lesions
The normal breast often shows apocrine metaplasia. Apocrine metaplastic cells are enlarged, have abundant finely granular, and eosinophilic cytoplasm, large round vesicular nuclei, prominent eosinophilic nucleoli and intracytoplasmic vacuoles (17). The degree of nuclear enlargement and prominence of the nucleoli can be worrisome and misleading, unless the apocrine nature of the process is recognized. Usually apocrine metaplasia is an incidental finding and coexists with benign or malignant lesions.
Apocrine atypical cells are characterized by enlarged nuclei with at least three-fold variation in size (17). The distinction between atypical apocrine adenosis and apocrine DCIS can be challenging, especially when the apocrine proliferation involves sclerosing adenosis or a sclerosing lesion. In equivocal cases a conservative diagnosis of atypical apocrine adenosis is usually preferable. Atypical apocrine adenosis in a core needle biopsy mandates excision.
When atypical apocrine epithelium is present in sclerosing adenosis, the combination of epithelial cells with enlarged nuclei and prominent nucleoli and glandular
distortion produces a pattern that can mimic invasive apocrine carcinoma. Immunostains for MEC markers are useful in the differential diagnosis with invasive carcinoma.
distortion produces a pattern that can mimic invasive apocrine carcinoma. Immunostains for MEC markers are useful in the differential diagnosis with invasive carcinoma.
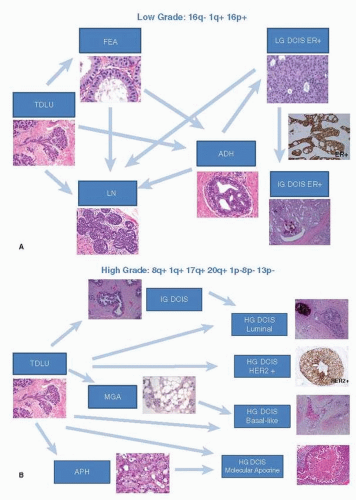 FIGURE 21-3 (A) “Low-grade breast neoplasia family.” Similar genomic aberrations have been identified in FEA, ADH, low-grade DCIS, and lobular neoplasia (ALH/LCIS). FEA is the earliest morphologically recognizable precursor of this group of lesions. (B) High-grade (HG) DCIS. This heterogenous group of lesions lacks ER, PR and expresses HER2. A basaltype of DCIS is also present. The high-grade DCIS harbor numerous genomic chromosomal alterations. Connectors drawn with discontinuous lines (microglandular adenosis and atypical apocrine hyperplasia) represent hypothetical links yet to be demonstrated modified from diagram of Lopez-Garcia et al. (6). |
Intraductal apocrine lesions are typically androgen receptor (AR) positive and frequently lack ER and PR expression. HER2 expression has not been reported in benign apocrine lesions, but it has been described in up to 50% of in situ and invasive apocrine carcinomas (18).
Genetic studies of apocrine metaplasia have shown that at least a proportion carries genetic changes, suggesting that some lesions are clonal and may represent non-obligate morphologic precursors of carcinoma (19, 20). Selim et al. (20) assessed LOH in 41 cases of benign apocrine lesions with different degrees of architectural complexity and found that 20% of cases showed allelic alterations in at least one chromosomal locus. Jones et al. (19) used CGH to compare 10 cases of apocrine micropapillary hyperplasia, 10 apocrine DCIS, and 4 invasive apocrine carcinomas, and found that the number of unbalanced genetic aberrations was directly related to the complexity and atypia of the lesion. In particular, the mean number of chromosomal alterations in apocrine hyperplasia was 4.1 compared to 10.2 in apocrine DCIS and 14.8 in invasive apocrine carcinoma. The most common alterations in apocrine hyperplasia included
gains of 2q, 13q, and 1p, and losses of 1p, 17q, 22q, 2p, 10q, and 16q (19). Apocrine in situ and invasive carcinomas most commonly showed gains of 1q, 2q, and 1p, and losses of 1p, 22q, 17q, 12q, and 16q (19).
gains of 2q, 13q, and 1p, and losses of 1p, 17q, 22q, 2p, 10q, and 16q (19). Apocrine in situ and invasive carcinomas most commonly showed gains of 1q, 2q, and 1p, and losses of 1p, 22q, 17q, 12q, and 16q (19).
The breast cancer risk associated with apocrine adenosis and atypical apocrine adenosis has not been well defined. In one study, none of 47 patients with atypical apocrine adenosis developed cancer at a mean follow-up of 35 months (21). A study of 37 patients with mean follow-up of almost 9 years documented a relative risk of 5.5 (17), but the authors acknowledged that some of their index cases may have represented apocrine DCIS involving sclerosing adenosis rather than atypical apocrine adenosis. A recent study (22) of 37 patients with atypical apocrine adenosis and average followup of 14 years identified 3 out of 37 women (8.1%) who developed breast carcinoma. In one patient DCIS was diagnosed in the contralateral breast after a 12 year follow-up. Two patients developed ipsilateral invasive carcinoma after 4 and 18 years. No apocrine atypia was present in the background breast parenchyma and the tumors showed no evidence of apocrine differentiation. Despite the limited number of study patients, the authors concluded that atypical apocrine adenosis does not appear to be an aggressive lesion and it should not be regarded as a direct precursor of breast carcinoma (22).
DUCTAL CARCINOMA IN SITU
The term DCIS is defined by the World Health Organization (WHO) as “a neoplastic proliferation of epithelial cells confined to the mammary ductal-lobular system and characterized by subtle to marked cytologic atypia and an inherent but not necessarily obligate tendency to progression to invasive breast cancer” (10). DCIS encompasses a heterogeneous group of lesions that differ significantly with regard to clinical presentation, morphologic features, biomarker profile, genetic abnormalities and biologic potential.
At present, DCIS accounts for about 20% to 25% of all newly diagnosed breast cancers, compared to less than 5% in the pre-screening mammography era. Overall, the incidence of DCIS rose from 1.87 cases per 100,000 women in 1973-1975 to 32.5 cases per 100,000 women in 1999-2004. Much of this increase is attributable to the widespread adoption of screening mammography and better detection of lower grade lesions. In general, the rate of noncomedo DCIS has increased across all age groups, whereas the rate of comedo DCIS has been constant or decreased.
In current clinical practice, 80% to 85% of cases with DCIS are detected because of associated mammographic calcifications (Ca2+), which are usually rod shaped or linear branching in high-grade DCIS or granular and segmental in low-grade DCIS. Up to 20%-30% of DCIS may present as a soft tissue density with or without associated Ca2+ or as an area of architectural distortion. Rarely DCIS presents as a palpable mass, nipple discharge, Paget’s disease of the nipple, or constitutes an incidental microscopic finding in breast tissue removed for another abnormality.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree