Postmastectomy Radiation Therapy
Atif Khan
Bruce G. Haffty
INTRODUCTION
The use of postmastectomy radiation therapy (PMRT) is perhaps one of the most intensively studied topics in oncology and yet continues to be a cause of considerable debate. Indeed, some of the first ever prospective randomized trials to be conducted addressed the utility of PMRT. This area has attracted robust scientific inquiry since the initial efforts, and has been the subject of over 20 randomized prospective trials. Despite the scientific scrutiny this area has attracted, important questions still remain to be answered. This chapter will focus on the topic of PMRT and is divided into four sections:
In the section on the Rationale for PMRT, we will review the data supporting the efficacy of PMRT as well as the risks and sequelae of PMRT.
Patient selection.
Reconstruction and PMRT.
Technique of PMRT.
The role of PMRT after neoadjuvant chemotherapy and in locally advanced and inflammatory breast cancer is discussed in Chapters 57, 58, and 59, respectively.
RATIONALE FOR PMRT
The principle that irradiating the chest wall and regional lymph nodes after mastectomy can reduce subsequent localregional recurrences (LRRs) has been well documented by multiple older trials comparing mastectomy alone to mastectomy with postoperative radiation. These trials typically used unsophisticated radiation techniques coupled with outdated radiation treatment machines that produced orthovoltage x-rays, resulting in less precise delivery of radiation to target tissues and increased doses to nontarget normal structures. Naturally, the relevance of these older trials is limited in the context of modern radiation therapy, but they adequately demonstrated two important facts: first, PMRT can effectively reduce the burden of residual local-regional disease, and second, radiation therapy is more comprehensive and more “radical,” in terms of treatment volume, than even the most radical surgery. These trials did not demonstrate improvements in survival; benefits in breast cancer mortality may have been offset by nonbreast cancer-related morbidity and mortality associated with the radiation techniques employed (1).
The potential improvement in local-regional control resulting from adjuvant systemic therapy alone can be studied through the numerous trials of systemic therapy versus nil that have reported patterns of failure (2). Data demonstrating a benefit of systemic cytotoxic chemotherapy on localregional control are somewhat inconsistent, which may be related to the confounding effects of patient selection, surgery and radiation delivery. However, the most recent Early Breast Cancer Trialists’ Collaborative Group (EBCTCG) meta-analysis of systemic therapy trials reported statistically fewer isolated local relapses in patients receiving polychemotherapy (recurrence rate ratio of 0.63 and 0.70 for women younger than 50 and 50-69, respectively) (3). Similarly, adjuvant tamoxifen seems to improve local-regional control as corroborated by the last fully reported EBCTCG meta-analysis, which demonstrated an isolated local recurrence rate ratio of 0.47 with tamoxifen versus without (3). These observations, along with the demonstrable improvement in survival with systemic agents, raise the obvious question of the relative additional benefit of PMRT in patients receiving systemic therapy.
Randomized Trials of Adjuvant Systemic Therapy Alone or in Addition to PMRT
Several trials have studied the efficacy and incremental benefit of PMRT in the presence of systemic therapy (2). The most significant contributions have come from the Danish Breast Cancer Cooperative Group (4, 5) and the British Columbia Cancer Agency (BCCA) (6). The trials conducted
by these two groups, together with the updated findings of the EBCTCG meta-analysis of radiation trials discussed later (7), have decisively altered practice and reaffirmed the role of PMRT in current breast oncology.
by these two groups, together with the updated findings of the EBCTCG meta-analysis of radiation trials discussed later (7), have decisively altered practice and reaffirmed the role of PMRT in current breast oncology.
In protocol 82b, the Danish Breast Cancer Cooperative Group randomized premenopausal women with high-risk breast cancer after modified radical mastectomy (total mastectomy and level I and II axillary dissection) to either nine cycles of cyclophosphamide-methotrexate-fluorouracil (CMF) chemotherapy or to eight cycles of CMF chemotherapy and radiation therapy to the chest wall and regional nodes between the first and second cycles of chemotherapy (4). High-risk status was defined as positive lymph nodes, tumor size greater than 5 cm, or invasion of the skin or pectoralis fascia. Radiation therapy was delivered to a total dose of 50 Gy in 25 fractions or 48 Gy in 22 fractions using anterior electron fields to treat the chest wall and internal mammary nodes (IMNs) and a matched anterior photon field to treat the supraclavicular, infraclavicular, and axillary lymph nodes. A posterior axillary photon field was used in patients with a large anterior-posterior (AP) separation. Over 92% of all patients were treated with megavoltage equipment. The study enrolled 1,708 patients between 1982 and 1989. With a median follow-up of 114 months, the irradiated group demonstrated statistically significant improvements in LRR (32% vs. 9%), disease-free survival (3% vs. 48% at 10 years), and overall survival (45% vs. 54% at 10 years). Over half of all LRRs were on the chest wall.
In the companion trial, protocol 82c (5), postmenopausal women younger than 70 with high-risk breast cancer (defined as in 82b) were randomized after modified radical mastectomy to receive either 30 mg of tamoxifen daily for 1 year beginning 2 to 4 weeks after surgery alone or with concurrent radiation therapy delivered to the chest wall and draining lymph nodes. A total of 1,375 patients were recruited between 1982 and 1990 and followed for a median time of 10 years. As in the 82b study, the irradiated group demonstrated statistically significant improvements in LRR (35% vs. 8%), disease-free survival (24% vs. 36%) and overall survival (36% vs. 45%). As in the 82b study, recurrence at all local-regional subsites was lower with PMRT than without. Although these well-designed efforts by the Danish group are not without flaw (as discussed below) they nonetheless strengthened the theory that, in certain patient subsets, aggressive local-regional control could result in improvements in survival end points.
The smaller British Columbia trial enrolled 318 node-positive premenopausal breast cancer patients and randomized them after modified radical mastectomy to either radiation therapy or no additional local-regional therapy (6). Both groups received adjuvant CMF chemotherapy for 12 (first 80 patients) or 6 months. Radiation therapy was delivered to the chest wall to a dose of 37.5 Gy in 16 daily fractions through opposed tangential photon fields. The supraclavicular and axilla nodes were treated with an AP field and a posterior axillary field, with a target midaxilla dose of 35 Gy. Bilateral IMNs were treated with an additional anterior field to a dose of 37.5 Gy in 16 fractions. All treatments were delivered with cobalt machines, between cycle four and five of chemotherapy. After a median follow-up of 20 years, the 20-year survival free of local-regional disease developing before systemic was 61% in the chemotherapy alone arm and 87% in the irradiated group. The irradiated group had statistically significant improvements in 20-year event-free survival (25% vs. 38%), systemic disease-free survival (31% vs. 48%), breast-cancer specific survival (38% vs. 53%), and overall survival (37% vs. 47%). There were slightly more nonbreast cancer deaths in the irradiated group (9% vs. 4%, p = 0.11). There were three cardiac deaths (2%) in the irradiated group versus one (0.6%) in the control group (p = .62), and 9% of patients in the irradiated group developed arm edema compared with 3% in the control group (p = .035). This study corroborated the Danish experience and again demonstrated some of the most remarkable improvements in survival end points ever reported for any adjuvant therapy.
Taken together, these studies demonstrated that certain patient cohorts have a high risk for LRR that is inadequately addressed by systemic therapy alone. Furthermore, reducing the likelihood of LRR can result in improved survival; presumably, persistent or recurrent local-regional disease can be a source of distant metastases and subsequent death. These studies imply that the benefit of systemic therapy is primarily to lower the competing risk of distant micrometastases, and that adjuvant local-regional therapy and adjuvant systemic therapy independently benefit these patients on the principle of spatial cooperation. There is no definitive randomized data supporting any specific sequencing of systemic therapy and radiation in the postmastectomy setting; for patients receiving both cytotoxic chemotherapy and postmastectomy radiation, the prevailing practice typically sequences the cytotoxic chemotherapy first, followed by radiation. Hormonal therapy, if indicated, may be given concurrently with radiation or following radiation, though some clinicians prefer to sequence tamoxifen after the radiation. Although there is little in the way of long-term followup data and additional studies will likely be forthcoming in the next few years, adjuvant systemic therapy with trastuzumab (typically administered for up to 1 year following chemotherapy) appears to be safe and effective given concurrently with radiation (8).
EBCTCG Meta-Analysis
The EBCTCG has collected primary data from every randomized trial of adjuvant radiotherapy in breast cancer and periodically reports the ongoing analyses on the benefits and risks of radiation therapy in these patients. The most recent full report from 2005 reviewed data on 9,933 patients enrolled in 25 trials of PMRT, all of which were unconfounded by the use of systemic therapy (7). Node-positive patients who had axillary clearance and received radiation therapy after mastectomy had a 5-year LRR rate of 6%, compared to 23% for unirradiated controls (15-year rates were 8% vs. 29%). In every large trial of PMRT in node-positive women, radiation therapy produced a similar proportional reduction in local recurrence, powerfully demonstrating the comparable efficacy of radiotherapy in achieving local control across all time periods. Even more significantly, PMRT also produced comparable proportional reductions in local recurrence in all women irrespective of age or tumor characteristics.
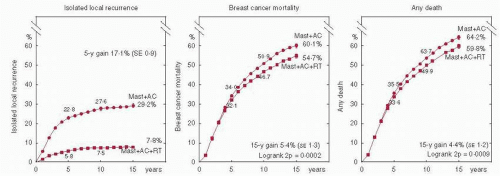
Absolute reductions in local recurrence were dependent on the absolute risk in the control arm (i.e., larger reductions were seen in subsets with greater risk). For patients with a control risk of local recurrence greater than 10%, the addition of radiation therapy (RT) improved local recurrence irrespective of systemic therapy. For women with node-positive disease who were irradiated after mastectomy and axillary clearance, a 17% absolute improvement in 5-year local control translated into a highly statistically significant 5.4% absolute improvement in 15-year breast cancer mortality (60.1% vs. 54.7%, 2p = 0.0002, Fig. 42-1) (7), and a 4.4% absolute improvement in 15-year all-cause mortality (64.2% vs. 59.8%, 2p = 0.0009) over unirradiated controls.
There was an excess cancer incidence in women studied in the EBCTCG report (including women treated with an intact breast), mainly in contralateral breast cancer and lung
cancer, and an excess mortality from heart disease and lung cancer. The averaged detrimental effects were modest, with 15-year absolute loss of 1.8% for contralateral breast cancer and 1.3% for nonbreast cancer mortality. Importantly, the proportional excess of nonbreast cancer deaths was greatest 5 to 14 years and more than 15 years after randomization, and the mean dates of randomization for these two groups was 1975 and 1970, respectively. The authors of the EBCTCG correctly point out that the late hazards evident in their report could well be substantially lower for modern radiation therapy technique and regimens.
cancer, and an excess mortality from heart disease and lung cancer. The averaged detrimental effects were modest, with 15-year absolute loss of 1.8% for contralateral breast cancer and 1.3% for nonbreast cancer mortality. Importantly, the proportional excess of nonbreast cancer deaths was greatest 5 to 14 years and more than 15 years after randomization, and the mean dates of randomization for these two groups was 1975 and 1970, respectively. The authors of the EBCTCG correctly point out that the late hazards evident in their report could well be substantially lower for modern radiation therapy technique and regimens.
The EBCTCG data were presented at the 2007 annual meeting of the American Society of Clinical Oncology (9). Since then further analyses have been carried out and prepared for publication (Sarah Darby, personal communication). Although the data are still preliminary, they represent the first detailed analysis of patients stratified both by extent of axillary dissection (at least level II vs. less extensive and by degree of nodal involvement (1-3 vs. 4+), and several pertinent and new findings have been described.
Among women with node positive disease, radiotherapy reduced the rate of any recurrence both for women who had undergone axillary dissection to at least level II (recurrence rate ratio: 0.75, 2p < 0.00001), and for women who had undergone less extensive axillary dissection (0.59, 2p < 0.00001), although the proportional reduction was larger in the women who had less extensive axillary dissection (2p for difference = 0.003). In addition, the subgroup of patients with axillary dissection to at least level II and one to three positive lymph nodes had a statistically significant improvement in 15-year breast cancer mortality (death rate ratio irradiated vs. unirradiated: 0.80, 15-year gain 7.9%, 50.2 vs. 42.3%, 2p = 0.01) with PMRT. This proportional reduction did not differ significantly according to whether or not the trial policy was to give systemic therapy (usually cmf or, for ER+, tamoxifen) in both trial arms.
The cohort of women with axillary dissection to at least level II and four or more positive nodes also enjoyed significant benefits from PMRT in their risk of any recurrence (recurrence rate ratio: 0.79, 2p = 0.0003) and breast cancer mortality (death rate ratio: 0.87, 2p = 0.04). In contrast to women with node positive disease, women with node negative disease had no benefit from PMRT either in terms of recurrence (rate ratio: 1.06, 2p > 0.1) or breast cancer mortality (rate ratio: 1.18 2p > 0.1). In summary, the EBCTCG update appears to suggest that women with node positive disease are likely to benefit from PMRT, even when they have had axillary dissection to at least level II and probably also in the presence of systemic therapy.
The EBCTCG overview represents one of the most significant contributions to the study of PMRT. However, the relevance of its findings may be limited by the inclusion of older trials that used fractionation schemes, treatment machines, and treatment volumes that are antiquated by current standards, as well as by the usual limitations of meta-analyses. To address these issues, Van de Steene et al. (10) re-examined the EBCTCG data and identified four factors which selected for significant improvement in the odds ratio (OR) for survival in the irradiated versus control populations: start date of the trial (after 1970 [OR 0.935]), number of patients (>600 patients [OR 0.932]), fractionation (conventional [OR 0.896]), and crude survival on the trial (at least 80% [OR 0.799]). Excluding trials that began before 1970 and trials with small sample sizes produced a significant odds reduction of 12.3% ± 4.3% with irradiation (10). Gebski et al. performed a meta-analysis in which they carefully attempted to control for the quality of radiation delivery in PMRT trials. The authors defined optimal dose as being between 40 and 60 Gy delivered in 2 Gy fractions (nonconventional fractionation schemes were converted to 2-Gy equivalents using bioeffective dose calculations) and appropriate treatment volumes as both chest wall and regional lymphatics (11). The authors reanalyzed data from the EBCTCG applying these criteria. The proportional reduction in local-regional recurrence was greater for trials with optimal dose and volume (80%), compared to those with suboptimal dose (70%) or field design (64%). An improvement in breast cancer mortality was restricted to those trials that used appropriate doses and fields for irradiation (6.4% absolute increase in survival, p <.001).
The most concerning risk of PMRT for radiation oncologists is the risk of radiation induced cardiac morbidity. As described above, the EBCTCG meta-analysis as well as other registry data have detected increased risks of cardiac
mortality in irradiated patients (7, 12, 13). In contrast, an analysis of the Danish postmastectomy trials patients by Hojris et al. (14) found, using a technique of RT that avoided cardiac irradiation, equal rates of ischemic heart disease and acute myocardial infarction in the irradiated and unirradiated group. Approximately 3% of patients in both groups had ischemia-related morbidity at a median follow-up of 117 months and less than 1% of patients in both arms had death due to cardiac causes. There was no difference in this study when comparing left- versus right-sided irradiation. However, these numbers may underestimate the true burden of radiation-related cardiac morbidity due to the competing risk of breast-cancer death in this high-risk population, and also because this study was an unplanned retrospective report on a prospectively studied patient cohort.
mortality in irradiated patients (7, 12, 13). In contrast, an analysis of the Danish postmastectomy trials patients by Hojris et al. (14) found, using a technique of RT that avoided cardiac irradiation, equal rates of ischemic heart disease and acute myocardial infarction in the irradiated and unirradiated group. Approximately 3% of patients in both groups had ischemia-related morbidity at a median follow-up of 117 months and less than 1% of patients in both arms had death due to cardiac causes. There was no difference in this study when comparing left- versus right-sided irradiation. However, these numbers may underestimate the true burden of radiation-related cardiac morbidity due to the competing risk of breast-cancer death in this high-risk population, and also because this study was an unplanned retrospective report on a prospectively studied patient cohort.
Gyenes et al. (15) reviewed 960 patients treated on the first Stockholm Breast Cancer trial (modified radical mastectomy alone vs. preoperative vs. postoperative RT accrued 1971-1976) and reported 58 acute myocardial infarctions (MI) in the study population for a crude rate of 6%. There were no differences in acute MI or death due to cardiovascular disease (n = 63/960) between irradiated and unirradiated patients. Importantly, the authors showed that only patients in the high-dose-volume group had an excess hazard ratio (HR) of cardiovascular death (HR 2, 95% CI, 1.0-3.9, p = .04). A retrospective study by Harris et al. (16) examined cardiac events in a series of 961 women irradiated to the intact breast and reported no interaction between left-sided versus right-sided RT on cardiac mortality or congestive heart disease. A significant interaction was noted between leftsided RT in the subsequent development of coronary artery disease (20-year actuarial risk 25% vs. 10% for right-sided, p <.001) and MI (15% vs. 5%, p <.002). Coexistent hypertension was an independent hazard for the development of coronary artery disease.
A study of the Surveillance, Epidemiology and End Results (SEER) database conducted by Giordano et al. (17) compared 15-year cardiac mortality rates in left- versus right-sided breast cancer as a function of the year of diagnosis in patients who received RT. Presumably, patients with left-sided lesions received more heart irradiation than those with right-sided lesions. Although the authors demonstrated excess cardiac mortality in left-sided breast cancer patients diagnosed between 1973 and 1979 (13% vs. 10%, p = .02), they found no significant difference in patients irradiated in the most recent time periods (˜9% for both groups in the 1980-1984 cohort, and 5% to 6% in the 1985-1989 cohort). Beginning in 1979, the hazard of death from ischemic heart disease in left-sided breast cancer patients (vs. right-sided) declined by an average of 6% per year.
In a similar study, Henson et al. (18) evaluated the relative risk (RR) of cardiac disease in women irradiated for leftversus right-sided breast cancer and the relative risk of lung cancer in the ipsilateral versus contralateral lung in women irradiated for breast cancer using the SEER public-use data set. They found that the RR of breast cancer continued to increase, reaching 1.9 (1.52-2.37) 20 years after diagnosis. Similarly, the RR of lung cancer increased continuously in time, peaking at 3.87 (2.19-6.82) for women 20 years after diagnosis. As noted by the study authors, many women in this analysis were treated during an age in which IMN nodal RT was much more common, thus, potentially increasing the toxicity risks compared to contemporary treatment cohorts. Furthermore, current techniques that enhance treatment conformity probably decrease cardiac and lung doses compared to the study cohorts, even when the IMs are treated.
Darby et al. (19) reported a well-executed populationbased case-control study of the risks of cardiac irradiation in patients treated for breast cancer. The mean dose to the whole heart was 5 Gy in the control cohort, and each excess Gy in mean dose conferred a 7% RR decrement. Importantly, no threshold dose for risk was detected. Taken together, these data stress the potential for cardiac morbidity and mortality with breast irradiation but are reassuring that routine contouring of the heart and improvements in imagebased simulation and treatment delivery can substantially reduce these risks.
Little data exists on the cumulative effects of anthracyclines and radiation therapy on cardiac morbidity and function. Perhaps the best data on this topic comes from Fumoleau et al. (20) who reported long-term cardiac function in 3,577 assessable patients randomized on eight French trials of adjuvant therapy, 2,553 of whom received epirubicin-based chemotherapy. Ninety-seven percent of women on the epirubicin cohort had adjuvant radiation (to the intact breast or postmastectomy) and 94% on the nonepirubicin cohort received RT (with about two-thirds of these receiving RT to the IMNs). The 7-year risk of left-ventricular dysfunction was 1.36% in the epirubicin arm and 0.2% in the nonanthracycline patients. Age 65 or greater and body mass index > 27 kg/m2 were additional significant risk factors.
Additional nonlife-threatening late risks of postmastectomy irradiation can include arm edema, fibrosis, shoulder stiffness, and brachial plexopathy. In an instructive report, the Danish postmastectomy investigators invited patients irradiated at Aarhus University Hospital who were alive and without evidence of disease to participate in a study of the late effects of PMRT (21). Eighty-four patients accepted the invitation and were eligible for analysis, and these patients were carefully assessed for late toxicity based primarily on LENT-SOMA criteria. More women in the irradiated group had lymphedema (17% vs. 9%) and impaired shoulder movement (16% vs. 2%) that interfered with work or daily activities. Irradiated patients also had more arm parasthesias (21% vs. 7%) and more arm weakness (14% vs. 2%). Only the shoulder function comparison was statistically significant. Symptomatic pulmonary complications were equal in irradiated and unirradiated patients. In a separate report of 161 patients with neurological follow-up who were irradiated on the Danish 82 protocols, 5% of patients had disabling and 8% had mild radiation-induced brachial plexopathies (22). Kuhnt et al. (23) reported acute and chronic reactions in 194 patients receiving PMRT. Twenty-two percent of patients had any incidence of chronic effects, mostly from arm edema (28 of 43). Five patients had telangiectasia and one patient had plexopathy.
In conclusion, randomized trials as well as data from meta-analyses provide a strong rationale for PMRT in patients with a high likelihood of local-regional residual disease, despite the use of systemic therapy in these patients. Additional local-regional therapy in the form of RT reduces LRR rates by a factor of approximately two-thirds, and one breast-cancer death is averted for every four LRR prevented by RT. The risks of PMRT are modest but demonstrable, and cardiac effects may largely be attributable to older technique. The cardiac and pulmonary toxicities of modern day PMRT continue to be evaluated and are likely minimal with careful three-dimensional planning and treatment techniques.
PATIENT SELECTION FOR PMRT
Node-Positive Patients
Node positivity in the axilla is the most significant predictor of LRR after mastectomy. It should be borne in mind, however, that approximately two-third of LRR occur on the
chest wall, and that axillary failures are far less common (24, 25, 26 and 27). Accordingly, the degree of node positivity should be viewed as an adverse feature that confers a higher risk for overall LRR (i.e., not limited to failure at regional sites).
chest wall, and that axillary failures are far less common (24, 25, 26 and 27). Accordingly, the degree of node positivity should be viewed as an adverse feature that confers a higher risk for overall LRR (i.e., not limited to failure at regional sites).
The Danish and Canadian PMRT trials demonstrated stable relative risk reductions for all events in all groups of node-positive patients. However, the conclusion that all node-positive patients warrant PMRT has been challenged. There are two general criticisms of these studies which limit the generalizing of these findings to all node-positive patients: first, the adequacy of the systemic therapy in the control arms of these studies; and second, the issue of the “background risk” in the relevant study populations.
The most recent EBCTCG meta-analysis of systemic therapy showed a significant but minor improvement for anthracycline containing polychemotherapy regimens over CMF regimens (3). Whether this incremental benefit improves localregional control as well is unknown and is probably unlikely in patients with high risk for local-regional microscopic residual. Furthermore, neither the addition of taxanes nor increases in the intensity or density of chemotherapy have had demonstrable impacts on local-regional control in node-positive patients, although they do improve survival end points presumably by addressing micrometases (28, 29, 30, 31, 32 and 33). In sum, it seems unlikely that present-day chemotherapy regimens would significantly alter the findings of the postmastectomy trials. In contrast, the Danish 82c trial treated postmenopausal patients (untested for estrogen-receptor/progesterone-receptor [ER/PR] status) with 1 year of tamoxifen (5), and it is unknown how a longer duration of hormonal therapy in a population known to be hormone-receptor positive would modulate the risk of LRR and thus the benefit of PMRT.
A more significant factor that limits interpretation of the Danish and British Columbia trials is that node-positive patients on the control arm of these trials had higher LRR rates than commonly reported for patients treated in the United States and elsewhere (4, 5 and 6, 24). This difference is especially obvious in patients with one to three positive lymph nodes, who represented about 60% of patients on these studies. In the unirradiated Danish population, the 18-year probability of local-regional recurrence (as first site of failure) was 59% for patients with four or more positive nodes, and 37% for those with one to three positive nodes (34). In the unirradiated Canadian population, the 20-year isolated LRR rate was 41% for patients with four or more positive nodes, and 21% for patients with one to three positive nodes (6). LRR developing any time before distant failure (i.e., cumulative LRR as first failure) was not reported as a function of the number of positive lymph nodes, but was 39% for the entire unirradiated group. In contrast, several large series of patients treated in the United States and elsewhere have reported LRR rates in the range of 6% to 13% for patients with one to three positive nodes (25, 26, 35, 36) (Table 42-1). This seems to indicate that the background risk for LRR in the Danish and BC trials was higher than average, and this may have exaggerated the benefit of PMRT in this population.
Differences in the extent of axillary surgery may partially explain the differences in the risk of LRR in patients with one to three positive nodes. Full level I and II axillary dissections were not performed; a median of seven lymph nodes were removed in the Danish studies and a median of 11 lymph nodes were examined in patients on the Canadian trial (4, 5 and 6). As such, many of the patients scored as having one to three positive lymph nodes may have actually had four or more positive nodes had full axillary dissections been performed. Tellingly, failure in the axilla either alone or as a component of LRR represented 43% of all LRR in the Danish studies (24), compared to 14% in the data cited above (25).
TABLE 42-1 LRR Rates in Patients Not Treated with Radiation after Mastectomy in Randomized Clinical Trials | ||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree