but also improved survival (3, 4). In the late 1970s and the 1980s, several preoperative trials assessed the benefit of chemotherapy in women with LABC, and, in addition to improving the rates of operability, most suggested a survival benefit as well (5, 6). Based on these results and the lack of effective alternative strategies, the administration of preoperative chemotherapy with or without radiation therapy prior to surgery thus became the standard for initially inoperable, nonmetastatic breast cancer.
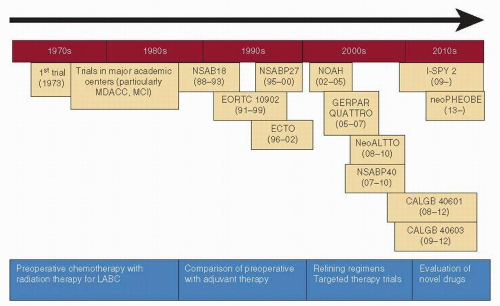 FIGURE 54-1 Evolution of preoperative chemotherapy over the past 30 years. MDACC, MD Anderson Cancer Center, USA; MCI, Milan Cancer Institute, Italy; LABC, locally advanced breast cancer. |
TABLE 54-1 Commonly Used Neoadjuvant Chemotherapy Therapy Regimens | ||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||
group, the AC-D group had better clinical responses (75.2% vs. 85%; p <.001), radiographic responses (68.6% vs. 78.6%; p <.001), breast conservation surgery rate (58.1% vs. 63.4%; p = .05), and pCR rate (7% vs. 14.3%; p <.001). The Hoosier Oncology Group trial also demonstrated the superiority of the sequential adriamycin-docetaxel regimen over the combination regimen (22).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree







