In 1997, the American Society of Therapeutic Radiology and Oncology (ASTRO) convened a Consensus Panel to develop a unified definition of BF, and the original definition included the following guidelines:
BF is not justification per se to initiate additional treatment and is not equivalent to CF. BF is an appropriate early end point for clinical trials.
Three consecutive increases in PSA is a reasonable definition of BF after RT. For clinical trials, the date of failure should be backdated to the midpoint between the post-RT nadir PSA and the first of the three consecutive rises.
No definition of PSA failure has, as yet, been shown to be a surrogate for clinical progression or survival.
Nadir PSA (nPSA) is a strong prognostic factor, but no absolute level is a valid cut point for separating successful and unsuccessful treatments.
as measured by the sensitivity, specificity, positive and negative predictive values, and hazard of CF after BF: two rises of at least 0.5 ng/mL backdated, PSA level at or greater than the absolute nadir + 2 ng/mL at the call date, and PSA level at or greater than the current nadir + 2 or 3 ng/mL at the call date. Backdating the failure time introduced bias into the estimate of freedom from BF, which was increasingly overestimated at shorter median follow-up times. The authors concluded that alternate definitions of BF were superior as assessed by various quantitative measures of concordance of biochemical and ultimate CF (18,19). Using this same dataset, Horwitz et al. determined the sensitivity and specificity of several BF definitions using distant failure (DF) alone or CF, defined as local failure and/or DF. The sensitivity and specificity of the ASTRO definition to predict DF alone was 55% and 68%, respectively. The sensitivity and specificity of the ASTRO definition to predict CF was 60% and 72%, respectively. Three definitions had higher sensitivity and specificity: (a) PSA > current nadir + 3 ng/mL (sensitivity 66% and specificity 77%), dated at the call; (b) PSA > absolute nadir + 2 ng/mL (sensitivity 64% and specificity 74%), dated at the call; and (c) two consecutive increases of at least 0.5 ng/mL, back dated (sensitivity 67% and specificity 78%) (20).
TABLE 13A.1 SENSITIVITY AND SPECIFICITY OF MULTIPLE BF DEFINITIONS COMPARED TO THE ORIGINAL ASTRO DEFINITION | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
implants and external beam RT. In this series, a transient rise in PSA was found in 35% of the patients. The bounce was defined as an increase of 0.1 ng/mL or greater above the preceding level followed by a subsequent decrease below that level. The median time to the bounce was 18 months and more than 90% occurred within 3 years. The median bounce height was 0.4 ng/mL (0.1-15.8 ng/mL). No independent predictors of bounce were identified and no clinical or biochemical correlation was made with the bounce in this early series (29).
patients may need additional treatment (43,44). Zelefsky et al. detailed the influence of posttreatment PSA nadir response at 2 years after external beam RT on distant metastases (DM) and cause-specific mortality (CSM) using data from 844 patients with localized prostate cancer treated with 3DCRT with long follow-up. Multivariate analysis demonstrated that nadir PSA ≤ 1.5 ng/mL was an independent predictor of progression-free survival after adjusting for T stage, GS, pre-RT PSA value, and RT dose (p = 0.03). The 5- and 10-year cumulative incidences of DM were 2.4% and 7.9%, respectively in those with nadir PSA levels ≤1.5 ng/mL, and were 10.3% and 17.5%, respectively, in patients with higher nadir values. Multivariate analysis showed that the higher nadir PSA value (p = 0.002), higher GSs (p < 0.001), and increasing T stage (p = 0.03) were predictors of DM after adjusting for pre-RT PSA values and RT dose. Multivariate analysis also showed that higher GSs (p = 0.002), and higher nadir PSA values (p = 0.03) were risk factors associated with CSM after adjusting for T stage and pre-RT PSA value. The authors concluded that nadir PSA values of ≤1.5 ng/mL at 2 years after RT for prostate cancer predict for long-term DM and CSM outcomes. The authors recommended that patients with higher absolute nadir levels at 2 years after treatment should be evaluated for the presence of nonresponding disease in the context of considering earlier salvage treatment interventions (45).
generalizations can be made. First, the procedure should be reserved for patients in excellent health with a life expectancy of at least 10 years. Second, patients must have no evidence of metastatic disease and no evidence of lymph node involvement before RT if an initial pelvic lymph node dissection was performed. Salvage surgery should be offered only to those whose cancer (both initial and recurrent) is clinically organ confined and potentially curable. Third, patients should have no evidence of severe radiation cystitis or proctitis. In all of the studies referenced above, candidates for salvage surgery were advised of, and willing to accept, the higher morbidity associated with this approach.
surgical techniques (21). Salvage RP can be safely performed after failed external beam RT, brachytherapy (open or ultrasound-guided), or combinations of these techniques. The majority of patients can be treated via a retropubic approach, which is familiar to most urologic surgeons. Rarely is a combined abdominoperineal approach required.
TABLE 13B.1 COMPLICATIONS OF SALVAGE RADICAL PROSTATECTOMY | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
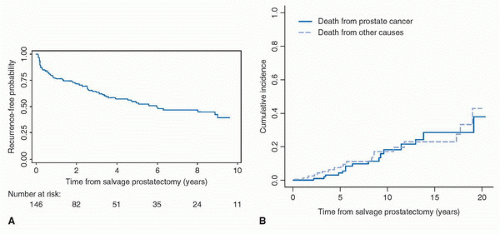
TABLE 13B.2 OUTCOMES AFTER SALVAGE RADICAL PROSTATECTOMY IN SELECTED SERIES OF PATIENTS | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
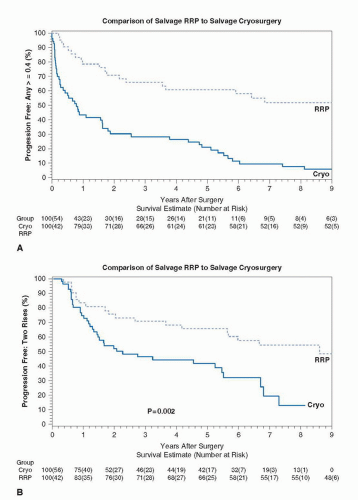
are generally well tolerated, there are concerns that locally ablative therapies provide incomplete and inadequate treatment. The primary goal of any salvage local therapy for radiationrecurrent prostate cancer is to provide a durable cure; the prevention of symptomatic local and systemic progression is a secondary objective. As such, the oncologic efficacy of any treatment is judged by the ability of that treatment to accomplish these goals. It is difficult to assess the outcomes of most currently available salvage therapies because of a lack of long-term outcome data and/or the small sample sizes reported in published series (27). While each of these ablative salvage therapies (HIFU [28,29], brachytherapy [30-32], cryotherapy [33,34]) can be safely delivered, information regarding the benefit they provide in terms of cancer control is limited.
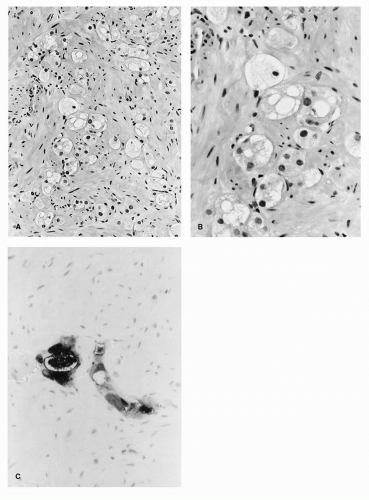
prostate biopsy. The pathology slides should be reviewed by a pathologist with experience interpreting postradiation prostate biopsies. Biopsy specimens showing persistent prostate cancer with no radiation effect or those with persistent cancer in the setting of radiation effect should be considered positive. Following pathologic confirmation of local failure, the extent of disease workup includes a thorough history and physical examination followed by routine laboratory studies, pelvic CT scan, whole body bone scan, and PSA determination. In addition, seminal vesicle biopsies may be performed to rule out more extensive local spread of disease.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree