Fig. 7.1
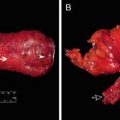
Cadaveric dissection of hemisectioned pelvis . (a) Presacral fascia covers the presacral vein over the sacrum. (b) The fascia picked up by the forceps is the rectal proper fascia enveloping the mesorectum and the rectum
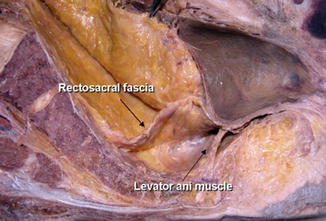
Fig. 7.2
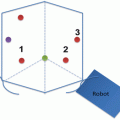
Cadaveric dissection of hemisectioned pelvis ; the retrorectal space. The rectosacral fascia is noted in the retrorectal space at the level of 4th sacrum when dissection proceeds along the rectal proper fascial plane
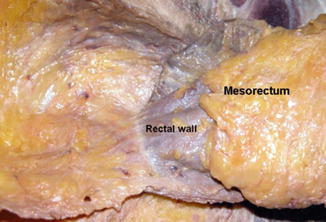
Fig. 7.3
The mesorectum is well developed at the posterolateral side of the rectum . The mesorectum is tapered down, and it ended 2–3 cm above the level of the levator ani muscle
Fig. 7.4
(a) The rectal proper fascia is adhesed to the mesh-like pelvic plexus at the lateral pelvic wall. (b) The fine branches from pelvic plexus enter the rectal wall. The rectum was attached to the lateral pelvic wall by adhesed pelvic plexus
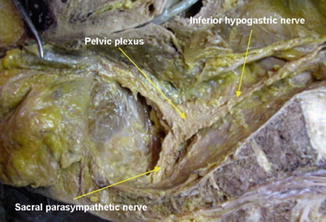
Fig. 7.5
Cadaveric dissection on hemisectioned pelvis shows the inferior hypogastric nerve descend into the pelvic cavity and meet sacral parasympathetic nerve arising from S2th, 3th, 4th foramen nearby the piriformis muscle. The inferior hypogastric nerve forms the pelvic plexus at the lateral pelvic wall after merging the sacral parasympathetic nerves. Nerve bundles from pelvic plexus go to the genitourinary organ along the seminal vesicle in male
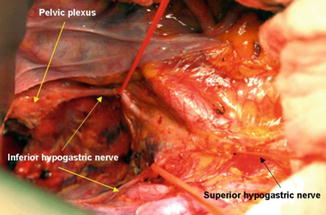
Fig. 7.6
On operative field, bifurcation of the superior hypogastric nerve was noted at the aortic bifurcation. The inferior hypogastric nerve descends along the pelvic side wall. The pelvic plexus forms after merging with the sacral parasympathetic nerve
The ventral and lateral aspects of upper two third of the rectum is covered by peritoneum which then reflects on to the bladder in males (rectovesical pouch) and the uterus in females (rectouterine pouch). The remainder of the rectum including the ventral aspect of the lower third and all of dorsal aspect of rectum is not covered by the peritoneum. However, the rectum and the mesorectum are covered in its entirety by mesorectal fascia. The mesorectal tissue is most obvious and bulky in the dorsal aspect and produces the classically described buttock cheeks on surgical dissection (Figs. 7.7 and 7.8). This fascia separates the mesorectum from the presacral fascia, which covers the sacrum and encloses the hypogastric and splanchnic nerves forming the inferior hypogastric plexus (Figs. 7.1, 7.2, 7.3, and 7.4). The retrorectal space, which is a thin space between the mesorectal and the presacral fascia, constitutes the often described “holy plane” or angel’s hair appearance during TME.
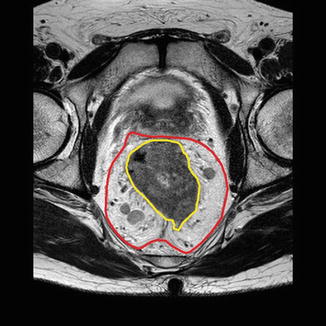


Fig. 7.7
MR pelvis with rectal tumour and mesorectum outlined. The mesorectum is well developed on the posterolateral aspect

Fig. 7.8
Rectal specimen : dissection in TME plane
The retrorectal space is obliterated as dissection is continued posteriorly at the level of fourth sacral vertebrae where it fuses with the presacral fascia and forms the Waldeyers’ fascia. The retrorectal space constitutes an avascular space and is also free of nerves up to this level .
On the ventral aspect, the upper two thirds of the rectum and mesorectal fascia are covered by peritoneum. The mesorectum itself is thin and less distinct. TME plane is accessed by division of the peritoneum between the rectum and the genital organs. This gives access to the recto-genital septum also known as the Denonvilliers’ fascia. It is a thick and distinct fascial structure that can be identified after division of the rectovesical or rectouterine/vaginal peritoneum. The TME plane can be in front of or behind Denonvilliers’ fascia . Dissection below Denonvilliers’ fascia leaving the fascia on the genital organs is protective of branches of the inferior hypogastric plexus which lie underneath the Denonvilliers’ fascia and supply the genital organs. There is some unresolved debate regarding the preferred choice of dissection plane when performing a TME for cancer excision.
Another area of potential nerve injury is on the lateral aspect of the rectum (Fig. 7.4).
Laterally mesorectal fascia is relatively indistinct as it appears to form lateral ligaments. These lateral ligaments are reflections of the mesorectal fascia towards the pelvic sidewall to allow vessels and nerves to reach the rectum. The inferior hypogastric plexus, which is present between the lateral aspect of the presacral fascia and the lateral mesorectal fascia, can be tented in and injured during this lateral dissection (Fig. 7.5). This can be avoided by sharp dissection in this area. If posterior dissection has been completed, sharp circumferential dissection close to the rectal wall will minimise the risk of nerve injury in this area. Occasionally this dissection will encounter the middle rectal vessels. These are branches of the internal iliac artery but are only present in a minority of patients and can be controlled on most occasions by monopolar or bipolar energy devices [4, 5].
Magnetic resonance imaging (MRI) has become an integral part of staging for rectal cancer and helps plan rectal cancer management in the current preferred setting of multidisciplinary team (MDT) management. Current high-resolution MRI techniques help identify all the important anatomical structures around the rectum including mesorectal, presacral and Denonvilliers’ fascia. Anatomic landmarks important to the performance of rectal cancer surgery, in particular the mesorectal fascia, can be defined on MRI, and this is of critical importance in the staging of tumours, assessing resectability, planning surgery and selecting patients for preoperative neoadjuvant therapy (Fig. 7.7) [6].
TME History and Literature
TME appears to have been described by Abel in 1931 [7] well before Heald brought it to worldwide attention. Rectal cancer surgery was considered morbid and difficult surgery with high rates of Abdominoperineal excision of rectum (APER) , high rates of local recurrence and poor long-term survival [8, 9]. Heald first published the Basingstoke experience in 1979 describing proctectomy with an emphasis on sharp dissection and complete removal of the mesorectum and called it TME [10]. Heald went on to publish the first series of TME for rectal cancer with an extremely low local recurrence rate of 2.7% and an overall 5-year survival of 87.5% [11]. The low recurrence rates have since been verified, and similar results have been reported in other series [12–14].
In addition to reducing recurrence rates, TME has also contributed to improved rates of sphincter preservation. Historically the majority of rectal cancers were treated with APER due to the high risk of local recurrence and the difficulty of creating low anastomoses. A distal margin of 5 cm was used as a minimum margin for rectal cancer resection. This has changed significantly with histopathological and surgical studies showing that cancers rarely show distal intramural spread of more than a centimetre [15–19] and furthermore that no improvement existed in local recurrence or survival if the distal margin was more than a centimetre. The development of linear and circular staplers has also contributed significantly to the technical ability to create a low anastomosis after TME. The data available therefore supports the use of low coloanal or colorectal anastomosis if the estimated distal margin of resection is more than a centimetre from the sphincter complex and there are no other contraindications to the procedure. The main contraindication to an anterior resection at this level is the presence of preexisting faecal incontinence or the risk of developing incontinence after surgery. This is discussed in more detail under patient selection.
There are other areas that need to be discussed when reviewing TME in 2017. Neoadjuvant radio and/or chemotherapy now have a prominent place in the current management of rectal cancer . Increasingly it is appreciated that tumour-specific partial mesorectal excision for upper rectal tumours may also be an oncologically safe operation with lower risks of complications including nerve injury .
Unsurprisingly, one of the largest single surgeon series of TME has been published by Heald and colleagues [20] who have reviewed their experience with TME at a single centre over a 20-year period. this series included 519 cases of rectal cancer and included curative and palliative resections. Less than 10% of patients received preoperative radiotherapy. One hundred and two patients were Dukes A, 167 Dukes B, 142 Dukes C, and 108 had Dukes D disease (residual or metastatic disease). Four hundred and sixty five anterior resections were performed. The 5-year survival rate was 81%, and the 10-year survival rate was 80% after a curative TME anterior resection in this series. The local recurrence rate in this group was only 2% for curative anterior resections at 5 and 10 years. The study also reported low clinically evident anastomotic leak rate of 6.5% with a further 5.5% radiological leak rate. In this series radiotherapy was used sparingly and did not appear to change the extremely low recurrence rates [20].
Other large series have also shown low local recurrence rates of 6–8% and good long-term survival around 70% [21]. As a result of these multiple series, TME is now accepted all over the world as standard surgery for rectal cancer and has been shown to improve outcomes. Several countries in Europe established training and audit programmes for TME in the 1990s, and improvements in outcomes of rectal cancer have followed. For example, implementation of TME in Norway resulted in a reduction of local recurrence rates from 12 to 6% and improvement in 4-year survival from 60 to 73%. Radiotherapy was only used in 5% of this group of patients [22]. A similar reduction in local recurrence has also been shown in the Netherlands along with a higher survival rate [23].
The use of radiotherapy in other series has been shown to reduce local recurrence rates. The Dutch Colorectal Cancer Group study investigated the efficacy of preoperative short course radiotherapy in conjunction with TME [24]. Curative rectal cancer surgery was undertaken, and patients were randomised to short course radiotherapy (5 Gy over 5 days) followed by TME or straight to TME. The Dutch TME trial included 1861 patients with 924 patients in the radiotherapy followed by TME group and 937 in the TME alone group. There was a significant difference in local recurrence rate in the two groups (2.4% in the radiation and surgery group vs. 8.2% in the surgery alone group, p < 0.001). This difference in recurrence was most pronounced for low rectal cancers (<5 cm from anal verge). This study showed that further reduction in local recurrence rate can be obtained with addition of radiotherapy to TME and this benefit is most pronounced in low rectal cancers.
The CR07 study which was a UK- and Canadian-based trial further showed that a good plane of surgery combined with short course radiotherapy further reduced the local recurrence rates. The 3-year local recurrence rate in patients who had good quality TME and short course radiotherapy was only 1% in this study [25].
Neoadjuvant long course chemoradiotherapy has also been compared with postoperative chemoradiotherapy in a German trial (CAO/ARO/AIO-94) [26] in patients undergoing TME for locally advanced rectal cancer (T3, T4 or node positive). The neoadjuvant group showed lower local recurrence rates with no difference in complications or survival. Recently 11-year follow-up results of this trial have been published and show continuing benefit of lower local recurrence rates in the preoperative neoadjuvant therapy group compared to postoperative group [27]. This study has not shown any survival benefit, although in a nonrandomised trial Delaney et al. demonstrated a survival benefit in their cohort of T3, node-negative patients who received preoperative radiotherapy [28].
The current challenge is to develop better selection criteria for the use of neoadjuvant therapy so that oncologic benefit can be maximised, while cost and complications are reduced. MRI is increasingly being used to determine the stage of rectal cancer along with the risk of involvement of the radial margin. The identification of so-called threatened margins is being increasingly used to select patients for neoadjuvant treatment [29–31].
Neoadjuvant radiotherapy also offers the possibility of allowing sphincter preservation by reducing the size of the tumour. Reduction in the size of tumour may also allow a TME and low anastomosis mitigating the need of a permanent colostomy. This approach also is controversial as some patients who respond may still have microscopic nests of cancer cells which may lead to an inadequate resection [32].
The incidence of anastomotic leak and neural injury is significant with TME and may be related to the level of anastomosis. Surgeons [33] in Hong Kong reported their results on selective use of PME in 622 rectal cancer patients. Patients with mid or low rectal cancers were treated with TME; rectosigmoid and upper rectal cancers were treated with PME, where the rectum was transected 4–5 cm below the tumour. TME was performed in 396 and PME in 226 patients. TME was associated with longer operative times, higher blood loss, longer hospital stays and a higher incidence of stoma formation. The rate of anastomotic leaks was significantly higher with TME compared with PME (8.1 versus 1.3%). TME was an independent risk factor for anastomotic leak on multivariate analysis. The authors recommend a selective use of TME based on this data which also shows similar oncological outcomes with the two techniques. This data has been replicated in other studies showing similar local recurrence rates in TME and PME [34].
In summary therefore, TME produces superior control of local recurrence as compared with conventional surgical techniques; however, PME in the same plane is appropriate in selected cases. The benefits of TME appears to be additive with the effects of neoadjuvant radiotherapy in most published series .
Complications of TME
It is clear that TME is the standard surgical approach to rectal cancer and has become widely accepted. TME, however, is associated with some complications. As noted earlier, there is a concern that anastomotic leak rate after TME may be significantly higher compared to non-TME dissection. TME leads to a low anastomosis and studies confirm a higher leak rate in lower anastomosis.
The use of neoadjuvant chemoradiotherapy may contribute to devascularisation of the anorectal stump leading to higher leak rates [35]; however, if a defunctioning stoma is used, it does not seem to increase the risk of other complications [36], and several authors recommend formation of a routine diverting stoma after TME after neoadjuvant therapy.
As a follow-up to the Dutch TME trial, the [23] short- and long-term outcomes of patients from the Dutch TME trial [24] were compared with data from the previous trial of cancer recurrence and blood transfusion (CRAB) . Rectal cancer resection involved non-TME dissection in the CRAB trial. The patient population included was older and included more women. Tumour features were however similar between the two studies. The incidence of anastomotic leaks was higher in the TME trial on univariate analysis in this comparison, but this was not significant on multivariate analysis. Anastomotic leak rates of 7–15% and other morbidity of up to 40% have also been described in other studies [37, 38].
Elsewhere, a prospective study of 1958 patients undergoing TME for rectal cancer showed that the risk of leakage was significantly higher in men, in patients undergoing neoadjuvant radiotherapy and in anastomoses that were ≤6 cm from the anal verge [35].
There is also some evidence that leak rates may reduce as surgeon experience builds up [39] and the clinical incidence and detrimental effects of anastomotic leakage are lower if a defunctioning stoma is used [40].
Lower leak rates have also been described in PME for higher rectal cancers, and this is an argument for utilising PME in selected cases [33].
Functional Outcome After TME
TME can lead to increase in bowel frequency, urgency and associated faecal incontinence. This results from the removal of rectal reservoir function and is exacerbated by possible sphincter and neural injury resulting from rectal and pelvic dissection. Anorectal function is compromised after TME with or without the use of preoperative radiotherapy though functional symptoms are worse after neoadjuvant radiotherapy. Anorectal function appears to improve after 12 months especially in the absence of neoadjuvant radiotherapy [41]. In patients who receive preoperative radiotherapy, the effects on anorectal function persist for the longer term. The long-term follow-up of patients from the Dutch TME trial has shown significantly more bowel dysfunction and increased use of incontinence material in patients who received neoadjuvant radiotherapy after a median follow-up of over 14 years [42].
The use of adjuvant radiotherapy can also cause postoperative deterioration of anorectal function. Kollmorgen et al. [43] compared 41 patients who received postoperative radiation to 59 patients who did not. The patients who received radiotherapy had a significantly higher number of incontinent episodes compared to the surgery alone group.
Anastomotic leakage with associated fibrosis also results in poorer function in the long term. Indeed anterior resection syndrome is well recognised and significantly impairs quality of life [44].
Older age and poor baseline anorectal function may lead to poor post TME function. It may be prudent to consider an end stoma in selected patients who are at high risk of poor postoperative functional results, but this has to be a joint decision along with the patient discussing all the risk factors and the effect on their quality of life [45]. This is especially relevant as some studies have shown no difference in post TME bowel function in older (pt > 65 years) compared to younger patients [46, 47].
Preoperative anorectal function may be more relevant compared to age in predicting postoperative anorectal function. Yamana et al. [48] concluded that patients who had a longer anal high-pressure zone, larger maximum tolerable rectal volume and lower rectal sensory threshold had improved postoperative evacuatory function after anterior resection. In summary, a significant number of patients will experience significant reduction in continence scores after surgery alone. This functional damage is further worsened by the addition of radiotherapy [49].
Urinary and Sexual Function After TME
Identification and protection of autonomic nerve fibres is an essential part of TME. The anatomy of nerves is now well described along with an understanding of areas during dissection where there is an increased risk of neural injury. Precise dissection in TME plane, dissection close to the rectum laterally and dissection behind Denonvilliers’ fascia are all protective of urogenital nerves [4, 50]. In anterior tumours, dissection is needed in front of Denonvilliers’ fascia , and nerve protection can be achieved in these patients with sharp dissection through Denonvilliers’ fascia before it fuses with the prostatic fascia [51]. Careful identification and protection of pelvic nerves results in improved postoperative urogenital function after TME. In a study by Junginger et al. [52], complete or partial identification of pelvic nerves was achieved in 82% of patients. Postoperative bladder function was significantly better in patients in whom nerves were identified.
Even though TME may be a more radical procedure compared to non-TME dissection, the sharp dissection with the understanding of anatomy and concerted attempt to preserve the autonomic nerves may lead to less urogenital dysfunction compared to traditional surgery. Maurer et al. compared 29 patients with non-TME dissection to 31 patients after TME and described a lower incidence of postoperative sexual dysfunction in the TME group [53]. Shirouzu et al. reported similar results in a review of 403 patients undergoing proctectomy over a 20-year time span, with some patients having a nerve-sparing approach and some not [54]. Urinary and sexual function was better preserved in the nerve-sparing group. Another study by Kim et al. also compared nerve sparing approach with non-nerve sparing in 69 men and showed a higher rate of preserved sexual and urinary function in the TME group [55].
Neoadjuvant radiotherapy also leads to higher rates of sexual dysfunction and result in a decrease in health-related quality of life (HRQoL) . Marijnen et al. studied the HRQL and sexual function of 990 patients who underwent TME and were randomly assigned to either surgery alone or neoadjuvant short course radiotherapy. Overall HRQoL was similar between the two groups, but neoadjuvant radiotherapy had a negative impact on sexual and urologic function in this study [56].
Bowel Reconstruction After TME
Several techniques are used to restore bowel continuity after TME. Bowel continuity may be restored by a straight (end to end) colorectal/coloanal anastomosis. This may be achieved using a stapler or by a hand-sewn anastomosis. Bowel continuity may also be restored by a side to end anastomosis or by formation of a neorectum by coloplasty or a colo pouch. Colonic pouch formation is shown to result in better functional outcome compared to a straight anastomosis in several studies—at least in the relative short term [57]. The benefit appears to persist in the longer term although the difference in functional outcome is reduced at 12 months post-surgery [58, 59].
Meta-analysis of studies comparing pouch to straight anastomosis has suggested that the pouch option provides better functional outcomes than coloanal anastomosis, including reduced urgency rates and the reduced need for antidiarrheal medications. Leak rates have been shown to be as low as 3% in colonic pouches compared to up to 15% in patients with straight anastomosis [58, 60, 61].
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree