Cause
Frequency among patients with malignant ascites
Peritoneal carcinomatosis (%)
53.3
Extensive liver metastasis causing portal hypertension (%)
13.3
Peritoneal carcinomatosis and extensive liver metastasis (%)
13.3
Hepatocellular carcinoma and cirrhosis (%)
13.3
Chylous ascites (%)
6.7
Budd-Chiari syndrome
Rare
Another invasive treatment for malignant ascites besides PVS includes placement of percutaneous catheters to allow intermittent paracentesis. These catheters can be placed surgically or by the interventional radiology team via computed tomography [46] or ultrasound guidance [47]. Multiple types of catheters have been used, including a pigtail catheter, peritoneal dialysis catheters (e.g., Tenckhoff catheter ), pleural cavity catheters (e.g., Pleurx® catheter ), fenestrated port-a-catheters, and even a Foley catheter [48–52]. The patient and/or caregiver can be trained to drain excess peritoneal fluid when the patient is symptomatic. The success rates for permanent intraperitoneal catheters functioning until death are quite high (90%) [47, 48]. There is low procedural morbidity. The major complications are obstruction and infection and are around 17% [47, 48], although catheter sepsis has been reported to be 35% [53].
Hyperthermic intraperitoneal chemotherapy (HIPEC) with cytoreductive surgery has been associated with increased survival for patients with carcinomatosis from different types of cancers; however, it has limited application and significant morbidity risk in the palliative setting. HIPEC, without cytoreduction, is an accepted approach for palliation of malignant ascites with good early data. In addition, this procedure can be performed laparoscopically in an attempt to minimize complications [54]. Laparoscopic HIPEC appears to be safe and highly effective for the treatment of malignant ascites. The morbidity, although low, suggests this procedure is best utilized in settings of ascites refractory to less aggressive treatments [55, 56]. The improvement in ascites and lack of a need for catheters or follow-up procedures are encouraging, but it should be noted that none of the studies of palliative laparoscopic HIPEC have included symptom assessment or HRQOL outcome measures. HIPEC has also been shown to be accomplished via ultrasound guidance, further making this technique possible for patients with advanced disease [57].
Bleeding
Tumor-related bleeding or radiation proctitis bleeding may be a difficult problem for the rectal cancer patient. In addition, patients may have a coagulopathy related to illness or treatments. Interventions are infrequently required, as there is likely a high morbidity, and intestinal stomas would likely be necessary. If surgery is deemed necessary, the optimal approach will depend mainly on the location of the tumor, along with the least morbid and most efficacious procedure possible. If tumor is amenable, transanal resection can be accomplished. This can be via transanal microsurgery [6, 7]. These procedures may be technically difficult or not possible in this setting.
Nonsurgical options include radiation therapy, arterial embolization, and endoscopic procedures. Endoscopic laser technologies are efficacious [27]. Other endoscopic treatment for bleeding includes argon plasma coagulation, photodynamic therapy, electrocoagulation, and injection therapy (e.g., 3% polidocanol) [58–60]. While most complications are minor, all of these techniques carry small risks of perforation, but for low to mid rectal tumors, this risk is limited.
Targeted radiation therapy is a reasonable option for slow, unremittent bleeding from rectal tumors [61, 62]. External beam radiation has an efficacy of approximately 75% [63]. Endocavitary radiation therapy can treat bleeding 35–45% [64]. Of course, multiple sessions may be required. While these techniques may have varying degrees of success, they may be utilized in attempts to avoid more invasive and morbid procedures .
Pain
Pain related with malignant disease is a complex symptom, which interferes significantly with most aspects of patients’ lives, including performance of daily activities, social interaction, psychological and emotional status, and ultimately quality of life. Pain is also associated with poor prognosis among patients with advanced rectal cancer [65]. It is estimated that the prevalence of chronic pain in patients with advanced cancer is over 70% [66]. Therefore, all patients with advanced rectal cancer should be screened for pain, so that they can receive a comprehensive assessment and treatment plan. Many patients with advanced rectal cancer experience pain, which can be acute, following invasive procedures or complications (bowel obstruction, pathological fractures), or chronic, usually related to local tissue injury, when the neoplasm invades pain-sensitive structures [67]. Being a subjective experience, pain evaluation involves a thorough history of the pain characteristics, extent of the underlying malignancy, and treatment received. Moreover, a detailed physical examination should be performed to identify the physical findings relevant to the patient’s condition. These findings, in addition with objective data from laboratory and imaging studies, are helpful tools to determine the pain etiology. The constellation of symptoms and signs of cancer pain can in most instances suggest a cancer pain syndrome [68].
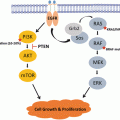
Pain management in patients with advanced cancer is considered a domain of palliative care [69]. In the context of palliative care, pain management is a major component of the interdisciplinary care that focuses on preserving function, helping in adaptation to changes in disease course, and in maintaining quality of life. In general, the first-line approach of management of pain is the use of analgesics. The World Health Organization (WHO) published a stepwise and systematic approach for cancer pain management called “analgesic ladder,” which has influenced clinical practice worldwide [70] (Fig. 22.1). The basic principal behind the analgesic ladder is a stepwise approach to pain control based on the patient’s severity of pain. Several clinical guidelines were developed based on the premises of the analgesic ladder [71–73].
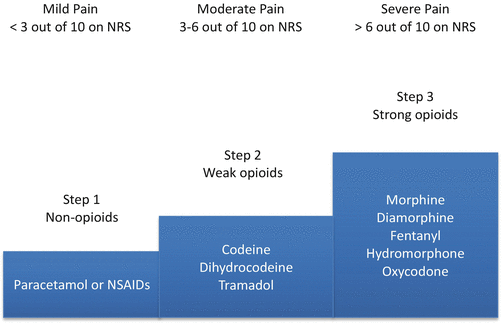

Fig. 22.1
Analgesic ladder. NRS numerical rating scales, NSAIDs nonsteroidal anti-inflammatory drugs. Adapted from: World Health Organization (WHO) Cancer pain ladder for adults. Available from http://www.who.int/cancer/palliative/painladder/en/. Accessed December 1, 2014
Besides the use of analgesics, specific treatments that focus on the etiology of pain are important and fundamental adjuvants for pain management. Therefore, identifying a specific pain syndrome or pain etiology should be an integral part of the assessment of the patient with advanced cancer. Regarding patients with advanced rectal cancer, possible pain syndromes and etiologies include pain caused by bowel obstruction, bone pain, and pathologic fractures due to skeletal metastases, chemotherapy-induced neuropathy, radiation proctitis, and plexopathies.
The prevalence of bone metastasis in patients with colorectal cancer ranges between 5.5% and 10.4% [74, 75]. A recent analysis of the natural history of bone metastasis secondary to colorectal cancer suggests that there is a very aggressive disease course in the bone metastasis, which can result in debilitating symptoms within a short period of time [76]. The complications related to bone metastasis include pain, pathological fractures, and spinal cord compression. Bone pain that failed the use of analgesics and is limited to a single or limited number of sites, external beam radiation therapy can provide pain relief in 60–85% of the cases [77]. The American Society for Radiation Oncology (ASTRO) recommends a single fraction of radiation using a dose of 8Gy to palliate pain from bone metastases. Patients with one or more bone lesions should be given bisphosphonates, in order to prevent pathological fractures, which can cause significant pain, morbidity, and decrease overall survival [78]. More recently, zoledronic acid was shown to be effective for prevention of skeletal-related events (SREs) in patients with bone metastasis from colorectal cancer [76]. Another agent approved for treatment of bone metastases from solid tumors which delays development of SREs is the monoclonal antibody Denosumab [79].
The goals of therapy for bone metastasis include pain relief, preservation of function, as well as skeletal integrity. Therefore, on impending or complete fractures of long bones surgical fixation is warranted [80]. Vertebral metastatic lesions which cause instability of the spine or cord compression should be fixated by surgery or percutaneous repair [81]. It is important to remember that pain caused by an unstable spine will not respond to radiation therapy or spinal bracing [82].
Radiation proctitis which is characterized by bleeding, rectal pain, and tenesmus occurs in 15–32% of the patients who undergo radiation for rectal cancer [83, 84]. Patients with severe tenesmus or pain can be treated with sucralfate (2 g twice daily) or glucocorticoid enemas (hydrocortisone 100 mg twice daily or prednisolone 20 mg twice daily) [85, 86]. Advanced rectal cancer patients can develop lumbosacral plexopathy due to direct tumor invasion or due to the effects from radiation therapy. Lumbosacral plexopathy causes severe, progressive, and debilitating pain. Patients typically present with pain, followed by numbness, paresthesias, and weakness [87]. The plexopathy caused by tumor invasion is more rapidly progressive and debilitating than the one caused by radiation [88]. An earlier diagnosis is associated with better response to treatment and less neurologic deficits. Treatment should be individualized based on patients’ general condition, wishes, comorbidities, and life expectancy. Therapeutic options include: surgical resection in selected cases, radiotherapy, interventional pain management procedures, and systemic therapy including chemotherapy and biologic therapy [88, 89]. Cameron et al., in a systematic review, demonstrated that palliative radiotherapy was effective in improving advanced rectal cancer symptoms , pain being the more prevalent symptom, in 71–81% of the cases [61]. However, the same study reported they encountered a great inter-study variability, making it impossible to perform a meta-analysis, highlighting the need for prospective studies including patient-defined target symptoms.
Conclusions
Specialized care for the terminally ill requires careful consideration of the complex issues they face. As treatments evolve, so will care issues. This includes the long-term effects of cancer care, as well as the effects of living with metastatic disease. Clearly this will mean that early palliative care is indicated, interdisciplinary team approach should be utilized, and each issue must take individuated attention. It is imperative that palliative care providers are familiar with the approaches to the issues that affect rectal cancer patients in the palliative setting and involve appropriate specialists when indicated, and the benefits outweigh the risks of proposed interventions. The patient, family, and treating teams should establish realistic goals and understanding of the likelihood of success, impact on HRQOL, and potential for harm. Continued team follow-up with early referral to palliative services is an essential element for the specialized care of the terminally ill cancer patient.
References
1.
Schneider ME. Medicare inches closer to advance care planning payment. ACS Surgery News. November 7, 2014. .Available at: http://www.acssurgerynews.com/specialty-focus/palliative-care/single-article-page/medicare-inches-closer-to-advance-care-planning-payment/ea63a605984eed18852dab586240807d.html. Accessed January 12, 2015.
2.
3.
4.
5.
Alawadi Z, Phatak UR, Hu CY, et al. Comparative effectiveness of primary tumor resection in patients with stage IV colon cancer. Cancer. Epub 2016 Aug 1; doi:10.1002/cncr.30230. 2017;123(7):1124–33.
6.
7.
8.
Anthony T, Baron T, Mercadante S, et al. Report of the clinical protocol committee: development of randomized trials for malignant bowel obstruction. J Pain Symptom Manag. 2007;34(1 Suppl):S49–59.Crossref
9.
10.
Hwang M, Pirrello R, Pu M, Messer K, Roeland E. Octreotide prescribing patterns in the palliation of symptomatic inoperable malignant bowel obstruction patients at a single US academic hospital. Supp Care Cancer. 2013;21:2817–24.Crossref
11.
Ripamonti C. Management of bowel obstruction in advanced cancer. Curr Opin Oncol. 1994;6:351–7.CrossrefPubMed
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree